Abstract
In the present study, a dual composite coating consisting of an anodized layer as the inner layer and PCLF (polycaprolactone fumarate)/silicon- and magnesium-codoped fluorapatite (Si-Mg-FA) nanocomposite as the outer layer was fabricated on AZ31 Mg alloy. The thickness of the PCLF/(Si-Mg-FA) nanocomposite coating is 9.72 μm, with the Si-Mg-FA nanoparticles being well distributed in the PCLF matrix. Electrochemical measurements showed that AZ31 Mg alloy with the anodized PCLF/Si-Mg-FA coating has a low corrosion current density (5.137 × 10–6 A/cm2), providing a sufficient protection (Rp = 5888.72 Ω cm2) for Mg alloys. Immersion tests in a simulated body fluid showed that cauliflower-like/cloudy apatite forms on AZ31 Mg alloy with the anodized PCLF/Si-Mg-FA coating and governs good bioactivity. Osteosarcoma cells adhere well to the surface of the coating. Surface modification by the anodized PCLF/Si-Mg-FA coating can be a suitable method for controlling the corrosion degradation and increasing the bioactivity and cell attachment of AZ31 Mg alloy for implant applications.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1. INTRODUCTION
Magnesium, an essential element for bone metabolism, and its alloys are used for temporary fixing of bones due to degradability and specific mechanical features [1]. Low elastic modulus, high specific strength, and low density of these alloys are of special interest for biomedical application in bone replacement and orthopedic utilizations [1–3]. However, the undesirable property of Mg alloys is that they lose the mechanical integrity before bone healing and also the collection of hydrogen gas around the Mg implants [4, 5]. These crucial problems are due to the highly negative equilibrium potential, leading to a rapid corrosion rate [6]. Thus, many efforts have been made to improve the corrosion resistance and slow down the biodegradation rate of Mg alloys [7–9]. Surface modification or formation of a coating on the substrate as a solution to these problems can provide a layer to prevent the substrate from contacting with corrosive media [10]. A bioactive surface can be achieved by using a bioactive coating.
Among different surface modification methods [11–18], micro-arc oxidation (also called micro-plasma oxidation) is one of the most efficient techniques to fabricate the corrosion protection layer on light metals such as magnesium and its alloys for biomedical applications [19–21]. In this technique, a discharge occurs above the dielectric breakdown voltage, and a porous layer consisting of magnesium oxides (ceramic coating) can be formed in situ on the surface [22]. The coating produced by this technique can substantially improve some surface properties of the magnesium substrate, such as resistance to wear, corrosion resistance, and hardness [21]. Nevertheless, as the ceramic coating fabricated by this method is usually porous and includes many microcracks, flaws, and defects, the enhanced corrosion resistance of this coating by itself may be insufficient [23–25]. Thus, subsequent coating deposition is suggested by researchers as a post-treatment for improving corrosion resistance [26, 27]. On the other hand, the ceramic coating with a special porous structure can act as an intermediate layer to promote adhesion of the additional coating by mechanical interlocking [28]. A dip coating technique possessing some interesting characteristics such as low-temperature processing, simple operation, and low cost can act as a sealing technique for the Mg substrate [29, 30]. A polymeric biomaterial layer prepared by the dip-coating technique can increase the corrosion resistance of magnesium alloy substrates by creating a barrier between the metal and corrosive environment [31, 32]. It is worth mentioning that class 0(B), according to ASTM D3359-09, has been categorized for the adhesion strength of a polymer on the Mg surface, indicating a low bonding strength on the substrate [33].
Polycaprolactone fumarate (PCLF), a synthetic polymer, is a promising material for tissue engineering applications because of its biodegradable, biocompatible, and good mechanical properties [34–36]. Nevertheless, there are some significant drawbacks related to this polymer, including fast degradation, poor cell response, and bioactivity, which makes it desirable to fabricate a composite with the incorporation of some bioceramics into the polymer matrix [37–39]. Our previous investigation showed that nanosized silicon and magnesium co-doped fluorapatite obtained by high energy ball milling possesses higher cell response and bioactivity than nanosized fluorapatite [40, 41]. This work is aimed to adjust the corrosion rate and provide the precipitation ability of the apatite layer by using a dual composite coating consisting of an anodized layer as the inner layer and PCLF/Si-Mg-FA nanocomposite as the outer layer on AZ31 Mg alloy. In addition, we compare the corrosion behavior of uncoated, anodized, nanocomposite-coated samples and anodized PCLF/Si-Mg-FA-coated samples.
2. MATERIALS AND MEYHODS
2.1. Preparation of PCLF/Si-Mg-FA Nanocomposite
Based on the previously reported procedure [40], silicon and magnesium co-doped fluorapatite nanopowder was synthesized by milling a blend of silicon oxide (SiO2, Sigma-Aldrich), calcium fluoride (CaF2, Merck), magnesium hydroxide (Mg(OH)2, Merck), calcium hydroxide (Ca(OH)2, Merck), and phosphorous pentoxide (P2O5, Merck) in a high-energy planetary ball mill (Fretch Pulverisette 5) for 12 h. The details of the experimental procedure are given in our previous study [40]. The obtained powder with Si and Mg co-doped fluorapatite was named Si-Mg-FA. Figure 1 shows the transmission electron microscopy (TEM, CM120, Philips) image of Si-Mg-FA nanoparticles exhibiting a spherical morphology with a mean diameter of about 30 nm. In order to prepare PCLF/Si-Mg-FA nanocomposite, firstly, 0.05 g of synthesized Si-Mg-FA nanopowder was mixed with 10 mL of dichloromethane. Then, the slurry was magnetically stirred for 2 h to obtain a homogeneous slurry. Finally, 0.5 g of polycaprolactone fumarate (PCLF, Mw = 10 000) was added to the slurry, and stirring was further continued for 24 h.
2.2. Preparation of AZ31 Mg Alloy Substrate and Coating Process
AZ31 Mg alloy plates with the chemical composition listed in Table 1 were cut to a size of 1 × 1 × 0.5 cm (width × length × thickness) and used as specimens in experiments. Some of these specimens were ground with a series of SiC abrasive papers up to 220 mesh followed by ultrasonic washing with distilled water, alcohol, and acetone for 20 min. For the anodizing process, other AZ31 Mg alloy specimens and 316L stainless steel plates (1 × 1 × 0.5 cm) were polished with a series of SiC abrasive papers up to 1200 mesh and used as anode and cathode electrodes, respectively. The electrolytic solution was prepared by dissolving 12 g NaOH, 12 g Na2SiO3, and 2 g KOH in 120 mL distilled water and magnetic stirring for 1 h. The distance between anode and cathode, applied voltage, and time were fixed at 2 cm, 30 V, and 5 min, respectively. The anodized specimens were ultrasonically washed with distilled water and acetone. Both anodized and ground specimens were used as substrates for the coating process. These specimens were coated with the PCLF/Si-Mg-FA nanocomposite solution using the dip-coating procedure and finally dried at 80°C. For this, the substrates were slowly immersed into and withdrawn from a beaker containing the solution three times at a constant speed using a completely automated control system to obtain a uniform coating.
2.3. Characterization and Evaluation
Surface morphology of anodized AZ31 Mg alloy, PCLF/Si-Mg-FA coating, and distribution of Si-Mg-FA nanoparticles within the composite coating matrix were examined by scanning electron microscopy (SEM, Mira 3-XMU) coupled with energy-dispersive X-ray spectroscopy (EDX). Fourier transform infrared (FTIR) spectroscopy and X-ray diffraction (XRD) analysis were used to examine the phase and chemical structure of the samples. FTIR spectrum (65-Perkin Elmer) analysis was carried out in the range of 450–4000 cm–1 at a resolution of 1 cm–1 using the KBr pellet technique. A Philips X’Pert X-ray diffractometer was used to obtain XRD patterns of the samples with CuKα radiation (λ = 0.1542 nm, time per step 1 s, and step size 0.05°).
2.4. Bioactivity Evaluation
The bioactivity behavior of the coated AZ31 Mg alloy specimen was evaluated using Kokubo’s solution [42] as the simulated body fluid (SBF). According to Kokubo’s procedure, this solution was prepared to contain a concentration of ions similar to that of human body plasma. Each sample was placed inside a sterilized polyethylene bottle and immersed in SBF under the physiological condition of pH 7.41 at 37 ± 0.5°C for 28 days. The ratio of SBF solution volume (mL) to specimen surface area (mm2) was fixed at 10 : 1 [42]. Changes in the SBF solution pH value were evaluated at the designated immersion time intervals by a calibrated pH meter. SEM and FTIR were utilized to evaluate the formation of bone-like apatite on the surface of specimens.
2.5. Electrochemical Measurements
Electrochemical tests were carried out at room temperature in SBF solution using a corrosion measurement system (Ivium) with a three-electrode cell configuration. 1 cm2 of the sample surface, platinum and saturated Ag/AgCl electrodes exposed to the solution were the working, reference, and counter electrodes. Before starting polarization scanning and electrochemical impedance spectroscopy (EIS) measurements, the samples were immersed in the solution for 1 h in order to attain a stable open-circuit potential (OCP). The potentiodynamic polarization scans were performed at a scanning rate of 1 mV/s from the initial potential of 250 mV below the corrosion potential. EIS measurements were conducted at open circuit potential in a frequency range between 100 kHz and 10 mHz using 3.5 mV as the amplitude of the perturbation signal.
2.6. Biocompatibility Evaluation
Biocompatibility evaluation of the coated sample was performed in vitro using the SAOS-2 osteosarcoma cell line (cell bank code c453) purchased from the Pastor Institute of Iran. 2.5 × 104 trypsinized cells (0.25% trypsin/0.2% EDTA, Sigma) were counted and seeded in RPMI growth media (RPMI + 10% FBS + 1% penicillin/streptomycin) at 37°C under an atmosphere of 5% CO2 and 95% air in an incubator for 72 h. Cell viability and proliferation were explored by using SEM observation. For this purpose, cultured samples were evaluated using 3-(4,5-dimethylthiazolyl-2)-2,5-diphenyl tetrazolium bromide (MTT, Sigma) colorimetric assay according to the manufacturer’s protocol. After 24 and 72 h of culture, cells were fixed in 2.5% glutaraldehyde for 3 h at room temperature, dehydrated in a graded series of ethanol solution, and finally air-dried. In order to study cell morphology, cultured cell samples were gold coated and studied by SEM (Philips XL30, USA).
3. RESULTS AND DISCUSSION
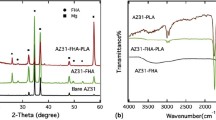
Figure 2a shows the XRD pattern of AZ31 Mg alloy in the as-received condition. It can be seen that strong α-Mg peaks dominate this pattern. Also, there are very weak peaks of the β-Mg17Al12 phase due to the low Al content in the alloy. The presence of the β phase with a more positive standard potential than that of the α-Mg phase resulting in the formation of a micro-galvanic couple is detrimental to corrosion performance of this alloy [32]. The XRD pattern of the anodized layer is shown in Fig. 2b. The SEM image and EDS spectrum of the AZ31 alloy surface after anodizing is shown in Fig. 3. The XRD and EDS results indicate that the anodized layer (reaction layer) is a blend of weak MgSiO3 and Mg2SiO4 phases, as well as strong MgO and Mg phases. Due to discharging channels between the substrate and electrolyte produced by sparks, magnesium can directly react with active oxygen in the electrolyte to form MgO (reaction 1). In an aqueous solution, hydroxylation of SiO3–2 leads to the formation of Si(OH)4 (reaction 2). During anodizing, Si(OH)4 groups are combined at high temperatures to form the SiO2 phase (reaction 3). It was reported that the temperature at the sparking areas on the Mg substrate surface can be high and even exceed 1000°C [43]. Under such conditions, both SiO2 and MgO are present in the molten state leading to the reaction and formation of Mg2SiO4 (reaction 4) according to [32]. Under the effect of sparks, SiO3–2 ions are drawn into the discharging area on the surface of Mg and react with Mg+2 to form MgSiO3 (reaction 5):
The formation of MgO protective layer for the magnesium substrate and Mg2SiO4 (forsterite) bioactive filler indicates that ions in the electrolyte were directly engaged in reactions near the discharging area [44–46]. However, according to XRD and EDX analysis in the present study, MgO as a dominant phase could act as a barrier layer to protect the underlayer against sever corrosion attack when the specimens were exposed to SBF. As can be seen in Fig. 3a, the anodized layer shows a rough surface (2.7 ± 0.3 μm) because of the presence of pores, flaws, and microcracks, as marked by the white arrows on the surface. The formation of these defects is attributed to some factors, including thermal stress in fast solidification of molten oxides when the coating surface contacts the electrolyte solution. This gives rise to tensile stress at the substrate/coating interface and causes trapping and evolution of gas bubbles, such as oxygen and hydrogen followed by aqueous vapor [22, 47]. However, in this study, the anodized layer with a rough structure is not only an anticorrosive and bioactive layer but also acts as an intermediate layer to improve the adhesion between the anodized layer and the next coating layer by mechanical interlocking [28].
The XRD pattern of the PCLF/Si-Mg-FA nanocomposite layer is observed in Fig. 2c. This pattern shows the characteristic peaks of nanoparticles as the additive and PCLF as the matrix of the nanocomposite. Comparison between 2θ diffraction angles of the (110) and (200) crystallographic planes of pure PCLF and PCLF as the matrix in this study shows an increase from 21.49° and 23.83° to 22° and 24°, respectively [48]. The cross-section morphology of the anodized/coated sample (anodized and PCLF/Si-Mg-FA coating) is shown in Fig. 4a. It can be seen from the figure that the coating consists of the inner anodized layer and the outer nanocomposite coating layer. The nanocomposite coating with dense and uniform morphology and a thickness of about 9.72 μm wholly covers the substrate surface. The surface morphology and elemental maps of the nanocomposite coating are presented in Figs. 4b–4h. According to Fig. 4b, Si-Mg-FA nanoparticles are well distributed in the PCLF matrix.
Additionally, the polymer layer exhibits a homogeneous structure free of pores and defects. The uniform distribution of elements in the coating is confirmed by elemental maps (Figs. 4c–4h). The atomic number and concentration of elements determine the density of colored dots in the elemental maps [49, 50]. Because of the atomic number effect, a high and low density of dots can be seen for Ca and F, respectively, in comparison with Si and Mg. The atomic numbers of C, O, F, Mg, Si, and Ca are 6, 8, 9, 12, 14, and 20, respectively. Although the O and C elements have the lowest atomic numbers among other elements, their dot densities are much higher than those of other elements, which are due to their higher concentration in the structure of the nanocomposite.
Figure 5a shows an SEM image of an anodized/nanocomposite-coated sample after 28 days of immersion in SBF solution. It can be seen that there is an apparent distinction between the surface morphology of the samples before and after soaking in SBF. This difference in morphology is due to the dissolution–precipitation process and the formation of needle-like structure from SBF on the surface, indicating the characteristic morphology of apatite.
The degree of corrosion attack diminished substantially for the nanocomposite-coated sample compared to the anodized sample (Figs. 5a–5c). The surface of the anodized and bare sample was partially covered with white spherical precipitates on the cracked layer, while the evolution of further white particles was observed on the anodized sample surface. In addition, the presence of microcracks and flaws on the anodized surface facilitated the penetration of SBF into the substrate and boosted its corrosion rate. For the anodized/nanocomposite-coated sample, the ion exchange rate between the coating film and SBF was diminished, enhancing the anticorrosion property of the composite coating and providing a less significant corrosion attack. The EDX analysis results shown in Figs. 5d–5f imply that the precipitated particles are mainly composed of Ca and P. The presence of calcium and phosphorus confirm apatite formation on the sample surface in SBF solution [51]. For the bare and anodized sample, EDX analysis displayed the formation of Mg(OH)2 owing to the atomic ratio of Mg to O (1 : 2) as the main corrosion products on the alloy surface. In addition, the EDX pattern demonstrated the presence of Ca, P, O, and C, indicating the formation of hydroxyapatite. The presence of C might be caused by hydrocarbons absorbed from the environment. Figure 6a displays the change of the SBF pH during 14 days of immersion at the frequent period of 48 h for uncoated, anodized, PCLF/Si-Mg-FA, and anodized-PCLF/Si-Mg-FA coated samples. Surprisingly, the pH shift in the coated samples was lower in comparison with the uncoated ones. It was slightly lower for the PCLF/Si-Mg-FA coated samples and anodized PCLF/Si-Mg-FA coated samples compared with the uncoated and anodized samples. The noticeable boost in pH in the uncoated and anodized samples is explained by a high release amount of OH ions in physiological media [27, 52]. Nevertheless, the pH values of samples with PCLF-containing coatings amplified slightly from 7.4 to 7.69; the precipitated particles were the apatite phase or not. Figure 6b shows FTIR spectra of an anodized/nanocomposite-coated sample before and after soaking in SBF. The FTIR spectrum of the anodized/nanocomposite-coated sample before soaking in SBF represents characteristic structural bands of Si-Mg-FA nanoparticles and PCLF polymer. The adsorption peaks within 3000–2850 and 1250–1450 cm−1 regions attributed to C–H stretching functional groups, the peak at 1734 cm−1 caused by C=O stretching of ester functional groups, and bands within 1150–1250 cm−1 region associated with C–O–C ether group are related to PCLF polymer. P–O and C–O bands observed within 350–850 and 1200–1400 cm−1, respectively, are attributed to Si-Mg-FA nanoparticles. According to the FTIR spectrum of the anodized/nanocomposite-coated sample after soaking in SBF shown in Fig. 6b, the phosphate and carbonate functional group bands are characteristic features of biological apatite [53]. The peak at about 1750 cm–1 corresponds to carbonate groups, indicating the presence of CO3–2 in PO4–3 sites of the apatite lattice [54]. However, EDS and FTIR analysis, as well as the cauliflower-like/cloudy morphology indicate the formation of biological apatite during immersion in SBF [2].
In order to evaluate the corrosion process, potentiodynamic polarization (PDP) and electrochemical impedance spectroscopy (EIS) tests were performed in SBF for the uncoated, anodized, nanocomposite-coated and anodized-PCLF/Si-Mg-FA coated samples. Polarization curves of samples with and without coatings are presented in Fig. 7. The corrosion potential Ecorr, the corrosion current density icorr, anodic/cathodic Tafel slopes βa and βc, and the polarization resistance Rp derived from the polarization curves are summarized in Table 2. The value of Rp calculated by the Stern–Geary equation is inversely proportional to the corrosion rate [55–57]:
A higher Rp indicates a better corrosion resistance. Also, generally, the higher corrosion potential and lower corrosion current density imply a better corrosion resistance in polarization tests [58]. The corrosion current density and the corrosion potential for the anodized-PCLF/Si-Mg-FA coated sample are 5.137 × 10–6 A/cm2 and –1.36 VSCE, respectively (Fig. 7 and Table 2). These values are considerably lower than icorr and higher than Ecorr of the uncoated sample, indicating that the anodized/nanocomposite coating enhances the corrosion resistance of Mg alloy. A higher Ecorr and lower icorr of the anodized/nanocomposite-coated sample in comparison with the nanocomposite-coated sample could be related to the effect of the porous anodized intermediate layer in enhancing the mechanical interlocking ability of the outer nanocomposite layer. The Ecorr and icorr changes listed in Table 2 are almost consistent with the trend of Rp changes. The Rp ranking is as follows: uncoated > anodized > PCLF/Si-Mg-FA coated > anodized-PCLF/Si-Mg-FA coated.
Figure 8a shows the electrochemical AC impedance measurements of uncoated, anodized, PCLF/Si-Mg-FA coated, and anodized-PCLF/Si-Mg-FA coated specimens in SBF solution. The equivalent circuit elaborated by ZWinSimp program was applied to interpret the experimental results. In this equivalent circuit, as shown in Fig. 8b, Rs, RCT, and CPE are the solution resistance, the charge transfer resistance, and the constant phase element, respectively. As can be seen in Fig. 8a, all the samples exhibit one capacitive loop. The capacitive loop diameter at the high-frequency region is proportional to the charge transfer process [59]. The following equation was used to calculate the exchange current density i0 from RCT values [60]:
where F and n are the Faraday constant and the number of transferred charges, respectively. As i0 is inversely proportional to RCT, the higher the RCT, the lower the corrosion rate of the sample. The anodized-PCLF/Si-Mg-FA coated sample exhibits a much larger diameter of the high-frequency capacitive loop than other samples. As the larger diameter, i.e., the larger value of RCT, means the lower corrosion rate, this sample should have better corrosion protection for the Mg alloy substrate than others due to the presence of anodized and nanocomposite coatings simultaneously. The nanocomposite coating with thick homogeneous and defect-free structure as the outer layer seals the defects and porosity of the anodized layer, and thereby stops the penetration of ions of SBF solution into the coating/substrate interface. The anodized layer as the inner layer with a porous structure can also further prevent the penetration of ions and improve the adhecion of the outer layer. It is worth mentioning that RCT equal to 1.1 KΩ cm2 for the uncoated sample increases to 2.8 and 5.2 KΩ cm2 for the PCLF/Si-Mg-FA coated and anodized-PCLF/Si-Mg-FA coated samples, which is inconsistent with the PDP results and confirms the protection efficiency of the PCLF/Si-Mg-FA coating that reduces the corrosion rate of AZ31 Mg alloy.
Figures 9a and 9b show SEM images of cells cultured on the coated sample after 3 days of incubation at different locations. As shown in these figures, the coating is able to support cell growth so that the entire surface is observed to be covered by filopodia extension of cells. In other words, cells adhered to the surface and to each other through neighboring cells. In the end, it can be concluded that PCLF/Si-Mg-FA coated AZ31 Mg alloy has features and properties necessary for biomedical applications.
Figure 9c demonstrates the cell viability of bare, anodized, PCLF/Si-Mg-FA, and anodized-PCLF/Si-Mg-FA coated samples during 24 and 72 h of culture time. The cell viability of the bare alloy was more than 78% in the entire incubation period, while the cell viability of the PCLF coating containing Si-Mg-FA was higher compared to the anodized and bare sample. Based on the ISO10993-5 standard, the cell viability varying within 75–99% corresponds to grade 1 cytotoxicity of samples, indicating that they meet the biomedical application criteria. Hence, bare samples and those with PCLF coating containing Si-Mg-FA possess great biocompatibility. The entirely coated alloy displayed greater cell viability in comparison with the bare alloy. The reason for this may be that the bare alloy degraded much faster than the coated alloy, resulting in an increased pH of the extraction medium and a substantial release of Mg ions into the medium, both of which were detrimental to cell viability, proliferation, and growth. The result revealed that the addition of Si-Mg-FA into the PCLF matrix increases the biocompatibility of the alloy. It is worth noting that the pH of the medium greatly affected the cell proliferation. Therefore, lower pH values and corrosion rate are regarded to be the key reason for the increase of cell viability. So, as the changes on the Mg alloy surface caused by rapid corrosion had a major impact on cell viability, the formation of PCLF coating containing Si-Mg-FA could provide great protection of the substrate as well as control over the degradation rate and buffering behavior leading to enhancement of cell viability.
4. CONCLUSIONS
A combination of anodized and polymer-based nanocomposite coatings was fabricated by anodizing and the dip coating method on AZ31 Mg alloy. The nanocomposite coating consisted of an anodized coating as the inner layer and PCLF/Si-Mg-FA nanocomposite as the outer layer. The nanocomposite coating with a homogeneous distribution of Si-Mg-FA nanoparticles in the PCLF matrix was dense and free of defects, whereas the anodized layer showed a rough surface due to the presence of pores, flaws, and microcracks. The anodized layer with a rough structure acted not only as a corrosion resistance and bioactive layer, but also as an intermediate layer to improve the adhesion of nanocomposite coating by mechanical interlocking. The results showed that the PCLF/Si-Mg-FA nanocomposite coating has the ability to form bone-like apatite on its surface, revealing good bioactivity. It enhances the corrosion resistance of Mg alloy and thereby considerably decreases the corrosion rate of this alloy in comparison with uncoated or anodized specimens.
REFERENCES
Wang, Y., Guo, J., Shao, Z., Zhuang, J., Jin, M., Wu, C., Wei, D., and Zhou, Y., A Metasilicate-Based Ceramic Coating Formed on Magnesium Alloy by Microarc Oxidation and Its Corrosion in Simulated Body Fluid, Surf. Coat. Technol., 2013, vol. 219, pp. 8–14. https://doi.org/10.1016/j.surfcoat.2012.12.040
Yang, L. and Zhang, E., Biocorrosion Behavior of Magnesium Alloy in Different Simulated Fluids for Biomedical Application, Mater. Sci. Eng. C, 2009, vol. 29, pp. 1691–1696. https://doi.org/10.1016/j.msec.2009.01.014
Staiger, M.P., Pietak, A.M., Huadmai, J., and Dias, G., Magnesium and Its Alloys as Orthopedic Biomaterials: A Review, Biomaterials, 2006, vol. 27, pp. 1728–1734. https://doi.org/10.1016/j.biomaterials.2005.10.003
Gu, X., Zheng, Y., Cheng, Y., Zhong, S., and Xi, T., In Vitro Corrosion and Biocompatibility of Binary Magnesium Alloys, Biomaterials, 2009, vol. 30, pp. 484–498. https://doi.org/10.1016/j.biomaterials.2008.10.021
Hiromoto, S., Shishido, T., Yamamoto, A., Maruyama, N., Somekawa, H., and Mukai, T., Precipitation Control of Calcium Phosphate on Pure Magnesium by Anodization, Corrosion Sci., 2008, vol. 50, pp. 2906–2913. https://doi.org/10.1016/j.corsci.2008.08.013
Chai, L., Yu, X., Yang, Z., Wang, Y., and Okido, M., Anodizing of Magnesium Alloy AZ31 in Alkaline Solutions with Silicate under Continuous Sparking, Corrosion Sci., 2008, vol. 50, pp. 3274–3279. https://doi.org/10.1016/j.corsci.2008.08.038
Jabbarzare, S., Bakhsheshi Rad, H.R., Nourbakhs, A., Ahmadi, T., and Berto, F., Effect of Graphene-Oxide on Corrosion, Mechanical and Biological Properties of Mg-Based Nanocomposite, Int. J. Mineral. Metallurg. Mater., 2020. https://doi.org/10.1007/s12613-020-2201-2
Zeng, R., Dietzel, W., Witte, F., Hort, N., and Blawert, C., Progress and Challenge for Magnesium Alloys as Biomaterials, Adv. Eng. Mater., 2008, vol. 10. https://doi.org/10.1002/adem.200800035
Zreiqat, H., Howlett, C., Zannettino, A., Evans, P., Schulze-Tanzil, G., Knabe, C., and Shakibaei, M., Mechanisms of Magnesium-Stimulated Adhesion of Osteoblastic Cells to Commonly Used Orthopaedic Implants, J. Biomed. Mater. Res. A, 2002, vol. 62, pp. 175–184. https://doi.org/10.1002/jbm.10270
Ali, M., Elsherif, M., Salih, A.E., et al., Surface Modification and Cytotoxicity of Mg-Based Bio-Alloys: An Overview of Recent Advances, J. Alloys Compnd., 2020, vol. 825, pp. 154140. https://doi.org/10.1016/j.jallcom.2020.154140
Sedelnikova, M.B., Sharkeev, Yu.P., Tolkacheva, T.V., Khimich, M.A., Bakina, O.V., et al., Comparative Study of the Structure, Properties, and Corrosion Behavior of Sr-Containing Biocoatings on Mg0.8Ca, Materials, 2020, vol. 13, p. 1942. https://doi.org/10.3390/ma13081942
Panin, A.V., Kazachenok, M.S., Perevalova, O.B., Sinyakova, E.A., Krukovsky, K.V., and Martynov, S.A., Multiscale Deformation of Commercial Titanium and Ti–6Al–4V Alloy Subjected to Electron Beam Surface Treatment, Phys. Mesomech., 2018, vol. 21, no. 5, pp. 441–451. https://doi.org/10.1134/S1029959918050089
Panin, A.V. and Shugurov, A.R., Local Curvature of Internal and External Interfaces in Mass Transfer Responsible for Thin Film Degradation, Phys. Mesomech., 2013, vol. 16, no. 4, pp. 348–354. https://doi.org/10.1134/S1029959913040085
Shugurov, A.R., Akulinkin, A.A., Panin, A.V., Sergeev, V.P., Kalashnikov, M.P., Voronov, A.V., and Cheng, C.-H., Study of Crack Resistance of TiAlN Coatings by Scratch Testing, Phys. Mesomech., 2017, vol. 20, no. 2, pp. 185–192. https://doi.org/10.1134/S1029959917020084
Lyukshin, P.A., Lyukshin, B.A., Matolygina, N.Yu., and Panin, S.V., Stress-Strain State in a Buckled Thermal Barrier Coating on an Elastic Substrate, Phys. Mesomech., 2018, vol. 21, no. 6, pp. 498–507. https://doi.org/10.1134/S1029959918060048
Shugurov, A.R. and Panin, A.V., Effect of Local Curvature of the Coating–Substrate Interface on Deformation and Fracture of Ceramic Coatings under Uniaxial Tension, Phys. Mesomech., 2017, vol. 20, no. 4, pp. 472–479. https://doi.org/10.1134/S1029959917040130
Yankovskii, A.P., Effect of Thermal Action on Hardening of Specimens with Thin Hard Coatings, Phys. Mesomech., 2012, vol. 15, no. 1–2, pp. 112–121. https://doi.org/10.1134/S1029959912010122
Konovalenko, Ig.S., Dmitriev, A.I., Smolin, A.Yu., and Psakhie, S.G., On the Estimation of Strength Properties of Porous Ceramic Coatings, Phys. Mesomech., 2012, vol. 15, no. 1–2, pp. 88–93. https://doi.org/10.1134/S1029959912010092
Muhaffel, F. and Cimenoglu, H., Development of Corrosion and Wear Resistant Micro-Arc Oxidation Coating on a Magnesium Alloy, Surf. Coat. Technol., 2019, vol. 357, pp. 822–832. https://doi.org/10.1016/j.surfcoat.2018.10.089
Lin, X., Tan, L., Zhang, Q., Yang, K., Hu, Z., Qiu, J., and Cai, Y., The in Vitro Degradation Process and Biocompatibility of a ZK60 Magnesium Alloy with a Forsterite-Containing Micro-Arc Oxidation Coating, Acta Biomater., 2013, vol. 9, pp. 8631–8642. https://doi.org/10.1016/j.actbio.2012.12.016
Zhao, L., Cui, C., Wang, Q., and Bu, S., Growth Characteristics and Corrosion Resistance of Micro-Arc Oxidation Coating on Pure Magnesium for Biomedical Applications, Corrosion Sci., 2010, vol. 52, pp. 2228–2234. https://doi.org/10.1016/j.corsci.2010.03.008
Hornberger, H., Virtanen, S., and Boccaccini, A., Biomedical Coatings on Magnesium Alloys—A Review, Acta Biomater., 2012, vol. 8, pp. 2442–2455. https://doi.org/10.1016/j.actbio.2012.04.012
Shi, Z., Song, G., and Atrens, A., The Corrosion Performance of Anodised Magnesium Alloys, Corrosion Sci., 2006, vol. 48, pp. 3531–3546. https://doi.org/10.1016/j.corsci.2006.02.008
Dietzel, W., Klapkiv, M., Nykyforchyn, H., Posuvailo, V., and Blawert, C., Porosity and Corrosion Properties of Electrolyte Plasma Coatings on Magnesium Alloys, Mater. Sci., 2004, vol. 40, pp. 585–590. https://doi.org/10.1007/s11003-005-0085-y
Shi, Z., Song, G., and Atrens, A., Influence of the β Phase on the Corrosion Performance of Anodised Coatings on Magnesium–Aluminium Alloys, Corrosion Sci., 2005, vol. 47, pp. 2760–2777. https://doi.org/10.1016/j.corsci.2004.11.004
Song, G.-L., An Irreversible Dipping Sealing Technique for Anodized ZE41 Mg Alloy, Surf. Coat. Technol., 2009, vol. 203, pp. 3618–3625. https://doi.org/10.1016/j.surfcoat.2009.05.042
Razzaghi, M., Kasiri-Asgarani, M., Bakhsheshi-Rad, H.R., and Ghayour, H., In Vitro Bioactivity and Corrosion of PLGA/Hardystonite Composite-Coated Magnesium-Based Nanocomposite for Implant Applications, Int. J. Mineral. Metallurg. Mater., 2021, vol. 28(1), pp. 168–178. https://doi.org/10.1007/s12613-020-2072-6
Wu, C., Wen, Z., Dai, C., Lu, Y., and Yang, F., Fabrication of Calcium Phosphate/Chitosan Coatings on AZ91D Magnesium Alloy with a Novel Method, Surf. Coat. Technol., 2010, vol. 204, pp. 3336–3347. https://doi.org/10.1016/j.surfcoat.2010.03.045
Gao, Y., Yerokhin, A., and Matthews, A., Mechanical Behaviour of CP-Magnesium with Duplex Hydroxyapatite and PEO Coatings, Mater. Sci. Eng. C, 2015, vol. 49, pp. 190–200. https://doi.org/10.1016/j.msec.2014.12.081
Jia, Z., Li, M., Liu, Q., Xu, X., Cheng, Y., Zheng, Y., Xi, T., and Wei, S., Micro-Arc Oxidization of a Novel Mg–1Ca Alloy in Three Alkaline KF Electrolytes: Corrosion Resistance and Cytotoxicity, Appl. Surf. Sci., 2014, vol. 292, pp. 1030–1039.
Jafarzadeh, A., Ahmadi, T., Taghian Dehaghani, M., and Mohemi, K., Synthesis, Corrosion and Bioactivity Evaluation of Gelatin/Silicon and Magnesium Co-Doped Fluorapatite Nanocomposite Coating Applied on AZ31 Mg Alloy, Russ. J. Non-Ferr. Met., 2018, vol. 59, no. 4, pp. 458–464.
Razavi, M., Fathi, M., Savabi, O., Tayebi, L., and Vashaee, D., Biodegradable Magnesium Bone Implants Coated with a Novel Bioceramic Nanocomposite, Materials, 2020, vol. 13, no. 6, p. 1315. https://doi.org/10.3390/ma13061315
Materials, A.S.F.T. Standard Test Methods for Measuring Adhesion by Tape. Test D3359-09, USA: ASTM, 2011.
Runge, M.B., Wang, H., Spinner, R.J., Windebank, A.J., and Yaszemski, M.J., Reformulating Polycaprolactone Fumarate to Eliminate Toxic Diethylene Glycol: Effects of Polymeric Branching and Autoclave Sterilization on Material Properties, Acta Biomater., 2012, vol. 8, pp. 133–143. https://doi.org/10.1016/j.actbio.2011.08.023
Ahmadi, T., Monshi, A., Mortazavi, V., Fathi, M., et al., Fabrication and Characterization of Polycaprolactone Fumarate/Gelatin-Based Nanocomposite Incorporated with Silicon and Magnesium Co-Doped Fluorapatite Nanoparticles Using Electrospinning Method, J. Mater. Sci. Eng. C, 2020, vol. 106, p. 110172. https://doi.org/10.1016/j.msec.2019.110172
Golafshan, N., Gharibi, H., Kharaziha, M., and Fathi, M., A Facile One-Step Strategy for Development of a Double Network Fibrous Scaffold for Nerve Tissue Engineering, Biofabrication, 2017, vol. 9, p. 025008. https://doi.org/10.1088/1758-5090/aa68ed
Bottino, M.C., Thomas, V., and Janowski, G.M., A Novel Spatially Designed and Functionally Graded Electrospun Membrane for Periodontal Regeneration, Acta Biomater., 2011, vol. 7, pp. 216–224. https://doi.org/10.1016/j.actbio.2010.08.019
Yang, F., Both, S.K., Yang, X., Walboomers, X.F., and Jansen, J.A., Development of an Electrospun Nano-Apatite/PCL Composite Membrane for GTR/GBR Application, Acta Biomater., 2009, vol. 5, pp. 3295–3304. https://doi.org/10.1016/j.actbio.2009.05.023
Kharaziha, M., Nikkhah, M., Shin, S.-R., Annabi, N., et al., PGS: Gelatin Nanofibrous Scaffolds with Tunable Mechanical and Structural Properties for Engineering Cardiac Tissues, Biomaterials, 2013, vol. 34, pp. 6355–6366. https://doi.org/10.1016/j.biomaterials.2013.04.045
Ahmadi, T., Monshi, A., Mortazavi, V., Fathi, M., et al., Synthesis and Dissolution Behavior of Nanosized Silicon and Magnesium Co-Doped Fluorapatite Obtained by High Energy Ball Milling, Ceramics Int., 2014, vol. 40, pp. 8341–8349. https://doi.org/10.1016/j.ceramint.2014.01.040
Golafshan, N., Alehosseini, M., Ahmadi, T., et al., Combinatorial Fluorapatite-Based Scaffolds Substituted with Strontium, Magnesium and Silicon Ions for Mending Bone Defects, Mater. Sci. Eng. C, 2021, vol. 120, p. 111611. https://doi.org/10.1016/j.msec.2020.111611
Kokubo, T. and Takadama, H., How Useful is SBF in Predicting In Vivo Bone Bioactivity?, Biomaterials, 2006, vol. 27, pp. 2907–2915. https://doi.org/10.1016/j.biomaterials.2006.01.017
Yahalom, J. and Zahavi, J., Experimental Evaluation of Some Electrolytic Breakdown Hypotheses, Electrochim. Acta, 1971, vol. 16, pp. 603–607. https://doi.org/10.1016/0013-4686(71)85169-1
Krishna, L.R., Somaraju, K., and Sundararajan, G., The Tribological Performance of Ultra-Hard Ceramic Composite Coatings Obtained through Microarc Oxidation, Surf. Coat. Technol., 2003, vol. 163, pp. 484–490. https://doi.org/10.1016/S0257-8972(02)00646-1
Zhang, Y. and Yan, C., Development of Anodic Film on Mg Alloy AZ91D, Surf. Coat. Technol., 2006, vol. 201, pp. 2381–2386. https://doi.org/10.1016/j.surfcoat.2006.04.015
Kharaziha, M. and Fathi, M., Synthesis and Characterization of Bioactive Forsterite Nanopowder, Ceramics Int., 2009, vol. 35, pp. 2449–2454. https://doi.org/10.1016/j.ceramint.2009.02.001
Zhang, Y., Yan, C., Wang, F., and Li, W., Electrochemical Behavior of Anodized Mg Alloy AZ91D in Chloride Containing Aqueous Solution, Corrosion Sci., 2005, vol. 47, pp. 2816–2831. https://doi.org/10.1016/j.corsci.2005.01.010
Johari, N., Fathi, M., and Golozar, M., Fabrication, Characterization and Evaluation of the Mechanical Properties of Poly (ε-Caprolactone)/Nano-Fluoridated Hydroxyapatite Caffold for Bone Tissue Engineering, Composites. B. Eng., 2012, vol. 43, pp. 1671–1675. https://doi.org/10.1016/j.compositesb.2012.01.013
Goodhew, P.J. and Humphreys, J., Electron Microscopy and Analysis, New York: Taylor and Francis, 2001.
Evis, Z. and Webster, T., Nanosize Hydroxyapatite: Doping with Various Ions, Adv. Appl. Ceram., 2011, vol. 110, pp. 311–321.
Xu, L., Zhang, E., Yin, D., Zeng, S., and Yang, K., In Vitro Corrosion Behaviour of Mg Alloys in a Phosphate Buffered Solution for Bone Implant Application, J. Mater. Sci. Mater. Med., 2008, vol. 19, pp. 1017–1025. https://doi.org/10.1007/s10856-007-3219-y
Saberi, A., Bakhsheshi-Rad, H.R., Karamian, E., Kasiri-Asgarani, M., and Ghomi, H., A Study on the Corrosion Behavior and Biological Properties of Polycaprolactone/Bredigite Composite Coating on Biodegradable Mg-Zn-Ca-GNP Nanocomposite, Progr. Organ. Coat., 2020, vol. 147, p. 105822. https://doi.org/10.1016/j.porgcoat.2020.105822
Fathi, M., Meratian, M., and Razavi, M., Novel Magnesium-Nanofluorapatite Metal Matrix Nanocomposite with Improved Biodegradation Behavior, J. Biomed. Nanotechnol., 2011, vol. 7, pp. 441–445. https://doi.org/10.1166/jbn.2011.1310
Razavi, M., Fathi, M., Savabi, O., Vashaee, D., and Tayebi, L., Micro-Arc Oxidation and Electrophoretic Deposition of Nanograin Merwinite (Ca3MgSi2O8) Surface Coating on Magnesium Alloy as Biodegradable Metallic Implant, Surf. Interface Analys., 2014, vol. 46, pp. 387–392. https://doi.org/10.1002/sia.5465
Jones, D., Principles and Prevention of Corrosion, Upper Saddle River, NJ: Prentice Hall, 1996.
Stern, M. and Geary, A.L., Electrochemical Polarization. I. A Theoretical Analysis of the Shape of Polarization Curves, J. Electrochem. Soc., 1957, vol. 104, pp. 56–63.
Stern, M., Electrochemical Polarization. II. Ferrous-Ferric Electrode Kinetics on Stainless Steel, J. Electrochem. Soc., 1957, vol. 104, pp. 559–563.
Cui, X., Li, Y., Li, Q., Jin, G., Ding, M., and Wang, F., Influence of Phytic Acid Concentration on Performance of Phytic Acid Conversion Coatings on the AZ91D Magnesium Alloy, Mater. Chem. Phys., 2008, vol. 111, pp. 503–507. https://doi.org/10.1016/j.matchemphys.2008.05.009
Razavi, M., Fathi, M., Savabi, O., Beni, B.H., Vashaee, D., and Tayebi, L., Surface Microstructure and In Vitro Analysis of Nanostructured Akermanite (Ca2MgSi2O7) Coating on Biodegradable Magnesium Alloy for Biomedical Applications, Colloid. Surf. B. Biointerface, 2014, vol. 117, pp. 432–440. https://doi.org/10.1016/j.colsurfb.2013.12.011
Udhayan, R. and Bhatt, D.P., On the Corrosion Behaviour of Magnesium and Its Alloys Using Electrochemical Techniques, J. Power Source, 1996, vol. 63, pp. 103–107. https://doi.org/10.1016/S0378-7753(96)02456-1
Author information
Authors and Affiliations
Corresponding authors
Additional information
Translated from Fizicheskaya Mezomekhanika, 2021, Vol. 24, No. 3, pp. 82–86.
Rights and permissions
About this article
Cite this article
Mohemi, K., Ahmadi, T., Jafarzadeh, A. et al. Synthesis, Corrosion, and Bioactivity Evaluation of the Hybrid Anodized Polycaprolactone Fumarate/Silicon- and Magnesium-Codoped Fluorapatite Nanocomposite Coating on AZ31 Magnesium Alloy. Phys Mesomech 25, 85–96 (2022). https://doi.org/10.1134/S1029959922010106
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1029959922010106