Abstract
A new method of gas-phase diamond deposition with a high-velocity jet used to transport gases activated in microwave plasma to a substrate is developed. Diamond was synthesized from a mixture of hydrogen with the addition of 1% methane. The deposition rate (78 μm/h) was more than an order of magnitude higher than that achieved earlier in experiments with activation of similar initial mixtures in microwave plasma.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
In recent years, CVD diamond synthesis has moved from the research phase to wide application in various technical processes [1, 2]. The use of GHz discharges to activate a mixture of gaseous plasma precursors is the most widespread of the CVD methods. Impressive rates of diamond deposition from mixtures of hydrogen and methane (with the possible addition of other gases) have already been demonstrated in Russia [3–5] and abroad [6–8]. A number of design solutions for creating a plasma “cloud” above a substrate by concentrating microwave radiation have been found. Active components are transported (diffuse) from this cloud to the substrate under the influence of a concentration and temperature gradient with a weak or negligible contribution of convection.
The activation conditions are defined by the microwave radiation intensity and frequency, the composition and flow rate of gases, and their temperature and pressure. The energy exchange (by conduction, convection, and radiation) between plasma and the environment also exerts a certain influence. These conditions govern the deposition rate and the properties of the deposit. The dependence of the deposition rate on the gas composition is an important feature of the process. For example, the deposition rate increases from 3 to 60 μm/h as the concentration of methane in hydrogen varies from 2 to 15% [5]. The deposition rate may increase by a factor of 1.5–2 after the introduction of nitrogen [2, 3, 8]. Argon also enhances the deposition rate [5]. The maximum rate achieved with such mixtures is 165 μm/h [7]. However, the added methane and other gases (N2, Ar) alter the properties of diamond negatively. In the case of high methane concentrations, soot is deposited at a rapid rate on the surrounding surfaces and the growing diamond surface.
In this study, a new method of gas-phase diamond deposition from a high-velocity jet of activated gases is developed. It is a conceptual continuation of the work on jet deposition of polymers [9] and synthesis of diamond-like structures from a high-velocity flow of a thermally activated gas mixture [10]. The schematic design is close to that of electrothermal propulsion units utilizing microwave energy [11].
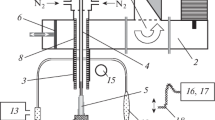
The essence of the method is in switching from the diffusion transport of active fragments to the gas-dynamic transport. Plasma expands from the formation region (forechamber) into a low-pressure region (deposition chamber) with the flow reaching a supersonic velocity. The design of the experimental setup and the first experimental results were presented in [12].
Supersonic expansion provides the opportunity to analyze plasma based on the flow rate and the pressure in the forechamber. We assume that the gas state upstream of the critical section is near-equilibrium. Gas flow rate G through the critical section is then given by
Here, γ is the adiabatic exponent of the gas mixture in plasma, R is the universal gas constant, Т0 is the plasma stagnation temperature upstream of the nozzle, Mr is the molecular mass of the mixture in plasma, and F is the critical section area. The mass flow rate of gas and the pressure in the forechamber can be measured fairly accurately. With the flow rate given and the pressure measured, one may determine the plasma temperature T0 by choosing adiabatic exponent γ and mixture mass Mr by iterations relying on the degree of hydrogen dissociation. The degree of dissociation was chosen in accordance with the data on equilibrium hydrogen dissociation at a given temperature and pressure [13].
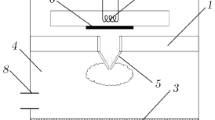
Figure 1 shows the flow of gas through a sonic nozzle as an illustration of the gas-dynamic processes associated with the outflow of plasma to the deposition chamber.
The outflow of hydrogen to the deposition chamber was calculated using the direct statistical simulation method [14]. The hydrogen flow rate was 8000 sccm, and the pressure in the deposition chamber was 20 Torr. Figure 1 shows the spatial distribution of the atomic hydrogen flow nV (m–2 s–1). Axis X is aligned with the jet axis, and r is the radial distance (in meters) from the jet axis. It is worth noting that a fairly narrow jet of atomic hydrogen forms.
The results of one of the experiments with a nozzle 2 mm in diameter, a hydrogen flow rate of 1.36 × 10–2 g/s (10 000 sccm), and a methane flow rate of 1.08 × 10–3 g/s (100 sccm) are presented below. The pressure in the deposition chamber was kept at 100 Torr. A molybdenum substrate kept at a temperature of 1230 K was used for deposition. With the magnetron power at 2.5 kW, the stagnation pressure in the forechamber was 205 Torr. The deposition time was 6 h. Note that with a nozzle 2 mm in diameter, the subsonic flow upstream of the critical section may be considered equilibrium under a pressure on the order of 200 Torr. Therefore, formula (1) may be used to calculate the temperature, which was estimated at ~3500 K for the above conditions. The corresponding degree of hydrogen dissociation was 45%.
Figure 2 shows the deposited film imaged with a Hitachi SU8220 scanning electron microscope at the Center for Common Use, Rzhanov Institute of Semiconductor Physics.
Figure 3 presents the Raman spectrum of the sample surface. This spectrum contains one line at 1332 cm–1 with a FWHM of 9 cm–1 and is indicative of the fine quality of the diamond coating.
The thickness of the deposited film in the central region of the substrate was 470 μm. The mean growth rate was 78 μm/h. The deposition rate indicated is more than an order of magnitude higher than that achieved earlier in experiments with activation of hydrogen with the addition of 1% methane in microwave plasma [3, 12]. This record-high (for the specified deposition conditions) result should stimulate further studies into diamond synthesis with plasma jets. Specifically, it raises new questions regarding the identification of the primary components of activated gas involved in diamond synthesis, the influence of the flow density, and the probable influence of the synergistic effect on atomic scales with the interaction of different components.
REFERENCES
R. S. Balmer, J. R. Brandon, S. L. Clewes, et al., J. Phys.: Condens. Matter 21, 364221 (2009). https://doi.org/10.1088/0953-8984/21/36/364221
M. N. R. Ashfold, E. J. D. Mahoney, S. Mushtaq, et al., Chem. Commun. 53, 10482 (2017). https://doi.org/10.1039/C7CC05568D
A. P. Bolshakov, V. G. Ralchenko, A. V. Polskiy, V. I. Konov, E. E. Ashkinazi, A. A. Khomich, G. V. Sharonov, R. A. Khmelnitsky, E. V. Zavedeev, A. V. Khomich, and D. N. Sovyk, Plasma Phys. Rep. 38, 1113 (2012).
A. B. Muchnikov, A. L. Vikharev, A. M. Gorbachev, et al., Diamond Relat. Mater. 19, 432 (2010). https://doi.org/10.1016/j.diamond.2009.11.012
A. P. Bolshakov, V. G. Ralchenko, V. Y. Yurov, et al., Diamond Relat. Mater. 62, 49 (2016). https://doi.org/10.1016/j.diamond.2015.12.001
J. E. Butler, Y. A. Mankelevich, A. Cheesman, et al., J. Phys.: Condens. Matter 21, 364201 (2009). https://doi.org/10.1088/0953-8984/21/36/364201
Q. Liang, C. Y. Chin, J. Lai, et al., Appl. Phys. Lett. 94, 024103 (2009). https://doi.org/10.1063/1.3072352
Y. Horino, A. Chayahara, Y. Mokuno, et al., New Diamond Front. Carbon Technol. 16, 63 (2006).
A. K. Rebrov, R. V. Maltsev, A. I. Safonov, et al., Thin Solid Films 519, 4542 (2011). https://doi.org/10.1016/j.tsf.2011.01.290
V. A. Volodin, A. A. Emel’yanov, A. K. Rebrov, N. I. Timoshenko, and I. B. Yudin, J. Eng. Phys. Thermophys. 85, 101 (2012). https://doi.org/10.1007/s10891-012-0626-9
D. J. Sullivan and M. M. Micci, in Proc. 23rd AIAA/DGLR/AIDAA/JSASS Int. Electric Propulsion Conf., Seattle, United States,1993, p. 337.
A. K. Rebrov, M. S. Bobrov, A. A. Emelyanov, et al., Interfacial Phenom. Heat Transfer 7, 131 (2019). https://doi.org/10.1615/InterfacPhenomHeatTransfer.2019031315
N. B. Vargaftik, Reference Book on Thermophysical Properties of Gases and Liquids, 2nd ed. (Nauka, Moscow, 1972).
G. A. Bird, Molecular Gas Dynamics and the Direct Simulation of Gas Flows (Clarendon, Oxford, 1994).
Funding
This study was performed within a state task (budgetary grant nos. АААА-А17-117030110017-0 and AAAA-A17-117022850029-9) and supported by the Russian Foundation for Basic Research (project no. 18-29-19069).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated by D. Safin
Rights and permissions
About this article
Cite this article
Rebrov, A.K., Emelyanov, A.A., Plotnikov, M.Y. et al. Synthesis of Diamond from a High-Velocity Microwave Plasma Flow. Dokl. Phys. 65, 23–25 (2020). https://doi.org/10.1134/S1028335820010127
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1028335820010127