Abstract
The results of experimental study of crystallization of chromium-bearing Ba-titanates (redlegeite, lindsleyite, and hawthorneite) in the chromite–rutile/ilmenite system in the presence of the H2O–CO2–BaCO3 fluid at pressures of 1.8, 3.5, and 5.0 GPa and temperatures of 1000–1200°C, simulating the formation of these phases through metasomatism of peridotite of the upper mantle, are reported. Experiments showed the formation of Ba–Cr-titanates in the entire pressure range studied and the possibility of joint crystallization of titanates. However, redledgeite is formed only in the Fe-poor chromite–rutile–H2O–CO2–BaCO3 system, whereas minerals of the magnetoplumbite group are preferably crystallized in the system with ilmenite. Lindsleyite was not detected at a pressure of 1.8 GPa. A positive dependence of the Cr content in titanates on pressure was shown. The Raman spectra of redlegeite, lindsleyite, and hawthorneite are presented.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Mantle metasomatism is the process of transformation of mantle rocks under the influence of external fluids and melts, regardless of their origin and composition [1]. In most cases, this process is expressed in the formation of minerals atypical of mantle rocks, such as amphiboles, phlogopite, apatite, various carbonates and sulfides, titanite, ilmenite, rutile, and other rarer minerals. The consistent and regular formation of assemblages with these minerals indicates that metasomatism of mantle rocks proceeds in several stages with an increasing effect, rather than being a one-stage process. It is primarily expressed in an increase in the activity of K and/or Na, which at the initial stages of the process is due to the active decomposition of Al-rich phases (garnet, spinel) with the formation of phlogopite. Intensification of metasomatism results in further reactions of pyroxenes with the appearance of potassium richterite [2]. The formation of phases in which K and Na (and LILEs) are bound to Cr, Ti, and Fe3+ usually indicates the most advanced stages of metasomatic transformations [2]. The mineral indicators of these stages include chromium-bearing titanates enriched in K and Ba (in some cases, Na and Ca), HFSEs, LREEs, U, and Th. These are the priderite-group minerals of the hollandite supergroup [7], namely the solid solutions K(Ti7Cr)O16 (K–Cr priderite)–K(Ti7Fe3+)O16 (priderite)–Ba(Ti6Cr)O16 (redledgeite)–Ba(Ti6Fe3+)O16 (Ba priderite); crichtonite-group minerals, namely the solid solution K(Ti, Cr, Fe,..)21O38–Ba(Ti, Cr, Fe,..)21O38 (mathiasite–lindsleyite, hereinafter LIMA) and minerals of the magnetoplumbite group, namely the solid solution K(Ti, Cr, Fe,..)12O19–Ba(Ti, Cr, Fe,..)12O19 (yimengite–hawthorneite, hereinafter HAYI). These minerals are described in metasomatized peridotite in association with phlogopite, potassium richterite, and low-alumina clinopyroxene, where garnet is absent (or occurs only as relics), and spinel is characterized by a high Mg# and Cr content (see Table 1 in [3]). Their formation is explained by the reactions of peridotite with fluids or melts with a low SiO2 activity, enriched in alkalis and LILEs [4–6]. The close associations of titanates with chromite, ilmenite, and rutile convince us that these minerals are the sources of Cr and Ti for the formation of titanates. Sometimes various K–Ba titanates coexist or replace each other, depending on the degree of metasomatism.
Experimental data on the stability of K–Ba titanates include a few works [9, 10]. The synthesis of K–Ba priderite containing Fe3+ and Fe2+ from mixtures of oxides and simple titanates showed that this mineral was stable at pressures of 3.5 and 5 GPa up to temperatures of ~1500°С [9]. Experiments on the synthesis of Ba–Cr priderite are not known. Hawthorneite and lindsleyite were synthesized in the TiO2–ZrO2–Cr2O3–Fe2O3–MgO–BaO–K2O system at 7–15 GPa and temperatures of 1300–1500°С [10].
The results of experiments on the formation of K–Cr end members of titanates (K–Cr priderite, matiasite, and yimengite) from chromite–rutile and chromite–ilmenite associations in the presence of H2O–CO2–K2CO3 fluid were reported earlier [3, 8]. This paper presents the results of experiments on joint crystallization of Ba–Cr end members of solid solutions of titanates (redledgeite, hawthorneite, and lindsleyite) upon interaction of these associations with H2O–CO2–BaCO3 fluid at 5 GPa and 1200°С, 3.5 GPa and 1200°С, and 1.8 GPa and 1000°С.
The experiments were performed at the Korzhinskii Institute of Experimental Mineralogy, Russian Academy of Science, using the NL-13T “anvil-with-hole” apparatus at 5 GPa, NL-40 at 3.5 GPa, and a PC-40 piston-cylinder at 1.8 GPa (Table 1). Natural chromite with the composition (Mg0.49–0.54Fe0.50–0.54Mn0.01–0.02Zn0.01–0.02) (Al0.17–0.20Cr1.55–1.61Fe0.10–0.22Ti0.03–0.07)O4 from garnet lherzolite xenolith from the Pionerskaya kimberlite pipe (Arkhangelsk region), ilmenite with the composition Fe0.98Mg0.01Mn0.06Ti0.93Al0.01Nb0.01O3 represented by a xenocrystal from kimberlite of the Udachnaya pipe (Yakutia), and synthetic TiO2 powder were used as the starting materials. The fluid component was prepared from a mixture of synthetic BaCO3 and oxalic acid. The oxygen fugacity was not buffered in the experiments. Following the previous conclusions [3, 8], the Fe3+/Fe2+ ratio in chromite in the experimental products corresponds to Δ log fO2 values, 1.1–1.6 logarithmic units below the FMQ buffer.
The composition of the phases (Tables 2, 3) was analyzed by X-ray spectral microanalysis on a Tescan Vega-II XMU scanning electron microscope equipped with an INCA Energy 450 X-ray detection and sample composition calculation system in the EDS mode at an accelerating voltage of 20 kV, a current of 400 pA, and an electron beam diameter of 157–180 nm (for chemical analysis) or 60 nm (for imaging). The Raman spectra of titanates were obtained using a Renishaw RM1000 Raman spectrometer equipped with a Leica microscope. A diode-pumped solid-state laser with a wavelength of 532 nm and a power of 20 mW was used. The spectra were recorded at 50-fold magnification for 100 s.
In the chromite–rutile–H2O–CO2–BaCO3 system, redledgeite and lindsleyite are formed at 3.5 and 5 GPa and 1200°C (Table 2). They are associated with recrystallized chromite and rutile (Fig. 1a). Redledgeite forms individual anhedral and subhedral (suboctahedral) crystals ranging in size from a few to 40 μm (with a maximum up to 100 μm) (Fig. 1a), as well as inclusions in transformed chromite. Such forms of occurrence of redledgeite are known in natural associations (for example, in chromitite of the Verblyuzh’egorsk Massif, Russia [11]). Lindsleyite forms small anhedral grains up to 10 µm in size, with inclusions in chromite, rutile, and the newly formed redledgeite.
BSE images of run products in the systems: (a) chromite–rutile with H2O–CO2–BaCO3 fluid, run Ba–Ti; (b) chromite–ilmenite with H2O–CO2–BaCO3 fluid, run 3Ba-1 (Table 1). Chr, chromite; Hwt, hawthorneite; Ldy, lindsleyite; Red, redledgeite; Rt, rutile.
Hawthorneite and lindsleyite coexisting with recrystallized chromite, ilmenite, and Nb-bearing rutile are formed in the chromite–ilmenite–H2O–CO2–BaCO3 system at pressures of 3.5 and 5.0 GPa and 1200°C. Hawthorneite crystallizes as euhedral octahedral and subhedral grains up to 40 µm in size, but there are individual anhedral grains larger than 100 µm as well. Lindsleyite forms anhedral inclusions in hawthorneite with a size of <10 μm (Fig. 1b). At 1.8 GPa and 1000°С, redledgeite and hawthorneite were detected in the experimental products in association with chromite, ilmenite, and a phase similar in composition to barium mica, ferrokinositalite [12] (wt %, K2O, 0.62; BaO, 22.55; MgO, 8.71; Al2O3, 16.87; FeO + Fe2O3, 12.54; SiO2, 23.62; Cr2O3, 3.55; H2O, 3.05). Hawthorneite is characterized by angular isometric grains up to 150 µm in size, while redledgeite forms inclusions in recrystallized chromite with a size of <30 µm.
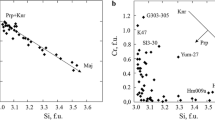
The compositions of the synthetic chromium-bearing Ba-titanates vary slightly in the products of individual runs (Fig. 3), except for hawthorneite synthesized at 5 GPa. This phase shows a Ti + Fe ↔ Cr trend of isomorphism. This trend coincides with the trend towards an increase in the content of Cr in this phase with pressure from 3.5 to 5 GPa (Fig. 3). The Cr content in lindsleyite and redledgeite shows a direct dependence on pressure, especially for the phases synthesized at 3.5 and 5 GPa (Fig. 3). However, in contrast to hawthorneite, an increase in the Cr content in these phases is accompanied by an increase in the Fe content, according to the scheme Ti ↔ Fe + Cr. Thus, with increasing pressure, all Ba titanates tend to be enriched in chromium. These results are consistent with the data [10] for pressures of 7–15 GPa, which show a poor negative dependence of the Ti content on pressure for the phases of the hawthorneite–yimengite series, and negative relationship between Ti + Mg and Cr for the phases of the lindsleyite–mathiasite series, which indirectly indicates a positive correlation of the Cr content in titanates with pressure.
The presence of Ba titanates was confirmed by Raman spectroscopy. Intense peaks in the spectrum of redledgeite (red spectrum in Fig. 2) at 179, 354, and 693 cm–1 and peaks of lower intensity at 276 and 549 cm–1 are close to the peaks in the spectra of synthetic K–Cr priderite [13].
The shift of the first peak in the redledgeite spectrum towards higher wavenumbers may be due to the presence of the heavier Ba cation instead of K. The Raman spectrum of lindsleyite (green line in Fig. 2) is characterized by intense peaks at 213, 275, 384, and 679 cm–1 and a shoulder characteristic of all titanates at ~580 cm–1. Intense peaks at 204, 353, and 685 cm–1 in the spectrum of hawthorneite (blue line in Fig. 2) are comparable to the peaks in the spectra of Al-rich yimengite from xenolith garnet lherzolite from the Obnazhnaya kimberlite pipe, Yakutia [3]. The shift of the latter peak to lower wavenumbers in synthetic hawthorneite compared to natural yimengite is associated with a higher content of Ti.
Thus, experiments in the chromite–rutile/ilmenite–H2O–CO2–BaCO3 systems show that Ba–Cr titanates are formed in a wide pressure range of 1.8–5.0 GPa. Compared with the data obtained for K–Cr titanates [3, 13], the new experiments indicate that the hollandite-group minerals, K–Cr priderite and redledgeite, are formed only in the low-iron systems chromite–rutile–H2O–CO2–BaCO3/K2CO3, while the magnetoplumbite-group minerals crystallize more preferentially in the ilmenite-bearing systems. No crichtonite-group minerals were detected at pressures of <3 GPa. As a result of the experiments, pairs of titanate phases (redledgeite + hawthorneite and hawthorneite + lindsleyite) were obtained, similar to the associations in the chromite–rutile/ilmenite–H2O–CO2–K2CO3 systems [3, 13]. This supports the possibility of joint formation of titanates through metasomatism of peridotite of the upper mantle in the presence of fluids or melts containing K and Ba (and other LILEs).
The positive dependence of the Cr content in Ba titanates on pressure (Fig. 3) may be considered as a relative depth marker for natural associations including K–Ba titanates. However, among K-titanates, such a dependence was revealed only for magnetoplumbite-group minerals [3, 13]. Therefore, to use this effect, further study of the K and Ba isomorphism and their influence on the isomorphism of Cr, Ti, and Fe in titanates in the presence of H2O–CO2–K2CO3–BaCO3 fluids with a variable K/Ba ratio is required.
REFERENCES
B. Harte, in Continental Basalts and Mantle Xenoliths, Ed. by C. J. Hawkesworth and M. J. Norry (Shiva, 1983), pp. 46–91.
O. G. Safonov and V. G. Butvina, Geochem. Int. 54 (10), 858–873 (2016).
V. G. Butvina, O. G. Safonov, S. S. Vorobey, E. V. Limanov, S. A. Kosova, K. V. Van, G. V. Bondarenko, and V. K. Garanin, Geochem. Int. 59 (8), 757–778 (2021). https://doi.org/10.1134/S0016702921080024
J. Konzett, R. Wirth, Ch. Hauzenberger, and M. Whitehouse, Lithos 182–183, 165–184 (2013).
A. Giuliani, V. S. Kamenetsky, D. Phillips, M. A. Kendrick, B. A. Wyatt, and K. Goemann, Geology 40 (11), 967–970 (2012).
J. Konzett, K. Krenn, D. Rubatto, C. Hauzenberger, and R. Stalder, Geochim. Cosmochim. Acta 147, 1–25 (2014).
C. Biagioni, C. Capalbo, and M. Pasero, Eur. J. Mineral. 25, 85–90 (2013).
V. G. Butvina, S. S. Vorobey, O. G. Safonov, and G. V. Bondarenko, in Advances in Experimental and Genetic Mineralogy: Special Publication to 50th Anniversary of Korzhinskii Institute of Experimental Mineralogy of the Russian Academy of Sciences, Ed. by Y. A. Litvin and O. G. Safonov (Switzerland, 2020), Vol. 9, pp. 201–222. https://doi.org/10.1007/978-3-030-42859-4_9
S. Foley, H. Hofer, and G. Brey, Contrib. Mineral. Petrol. 117, 164–174 (1994).
J. Konzett, H. Yang, and D. J. Frost, J. Petrol. 46 (4), 749–781 (2005).
A. B. Alekseev, in Proc. 7th Int. Sci. Symp. Named after Acad. M. A. Usov “Problems on Geology and Mineral Resources Development” (Tomsk Polytechn. Univ., Tomsk, 2003), pp. 80–82 [in Russian].
S. Guggenheim and H. E. Frimmel, Can. Mineral. 37, 1445–1452 (1999).
V. G. Butvina, S. S. Vorobey, O. G. Safonov, D. A. Varlamov, G. V. Bondarenko, and Yu. B. Shapovalov, Dokl. Earth Sci. 486 (2), 711–716 (2019). https://doi.org/10.1134/S1028334X19060254
Funding
This study was performed as part of a State Assignment FMUF-2022-0001 (1021060708334-5-1.5.2;1.5.6;1.5.4) of the Korzhinskii Institute of Experimental Mineralogy, Russian Academy of Sciences, for 2022–2026.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflicts of interest.
Additional information
Translated by A. Bobrov
Rights and permissions
About this article
Cite this article
Butvina, V.G., Safonov, O.G., Bondarenko, G.V. et al. Experimental Study of the Formation of Ba–Cr Titanates in the Fluid-Bearing Chromite–Rutile/Ilmenite System at Т = 1000–1200°С and Р = 1.8–5.0 GPa. Dokl. Earth Sc. 504, 248–253 (2022). https://doi.org/10.1134/S1028334X22050063
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1028334X22050063