Abstract
We prepare a series of MnOx–CeO2 catalysts with a molar ratio of Mn : Ce = 3 : 7 by coprecipitation and varying the calcination temperature from 300 to 800°C. The catalysts are characterized by powder X-ray diffraction, low-temperature nitrogen adsorption, and X-ray photoelectron spectroscopy, and the catalytic activity of all samples is tested in the CO oxidation reaction. A (Mn,Ce)O2 solid solution with the fluorite structure forms in all catalysts. Based on the studies performed, a catalyst calcined at 600°C is selected for further studies of the effect of topochemical reduction on the catalytic activity in the CO oxidation reaction by X-ray diffraction in the operando mode. The experiment is carried out sequentially in a stepwise mode: stepwise heating/cooling in a reaction mixture of 1% CO + 2% O2 in the mode 150–175–200–175–150°C (stages 1, 3, and 5); reduction of the sample in a mixture of 10% CO + He at 400°C (stage 2); reduction of the sample in a mixture of 10% H2 + He at 400°С (stage 4). The reductive treatment leads to segregation of the initial (Mn,Ce)O2 solid solution and the appearance of dispersed manganese oxides on the surface, while enrichment of the surface with manganese oxide increases its activity in the CO oxidation reaction.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
INTRODUCTION
To date, the preparation of active supported catalysts remains urgent in the development of heterogeneous catalysis. They are mainly obtained by applying the active component via impregnation of an oxide or carbon matrix (carrier). However, a method for obtaining such systems through topochemical reduction (redox exsolution) has recently been proposed [1]. In this method, the catalytically active metal element is first dissolved in the oxide matrix and then separated from the solid solution as catalytically active nanoparticles. The driving force behind this process is usually heat treatment in a reducing environment [2]; the application of an electrochemical potential can enhance the effect [3]. Obtaining nanoparticles by annealing in air was also studied using the nickel-doped electrode material Ce0.9Gd0.1O2 as an example [4], but such treatment requires high temperatures, which can adversely affect the particle size and distribution density on the surface of the support [5]. The resulting nanoparticles are more uniformly distributed over the support surface and have a narrower size distribution compared to the supported catalyst conventionally obtained by vapor deposition or impregnation. These parameters can be controlled within certain limits [6]. Neagu et al. [7] showed that nanoparticles obtained by topochemical reduction have a strong bonding with the oxide substrate, which prevents the deactivation of active sites caused by particle agglomeration. One of the interesting effects is the stability to aging of the catalyst obtained by this method. For example, Nishihata et al. [8], using a palladium catalyst for the complete oxidation of exhaust gases based on perovskite, LaFe0.57Co0.38Pd0.05O3, demonstrated that cycling of the catalyst under redox conditions leads to reversible drift of palladium cations from the perovskite lattice. Due to this effect, the growth of palladium particles over time is suppressed and the activity of the catalyst is maintained at the same level.
Using the (Mn,Zr)O2 solid solution with the fluorite structure as an example, we previously showed that under the conditions of a reducing CO and H2 environment, manganese cations are released from the initial oxide as dispersed MnOx particles [9, 10]. Introducing an additional oxidation stage into the catalyst activation process also increased the catalytic activity in oxidation [11]. Presumably, analogous phenomena should occur in a solid solution based on cerium oxide.
This work is devoted to the operando study of the topochemical reduction of CO oxidation catalysts based on (Mn,Ce)O2 solid solutions with the fluorite structure using X-ray diffraction. The work considers the processes of segregation of solid solutions with the formation of manganese oxides on the surface of a support, occurring both during the reductive treatment of the catalyst in H2 or CO and under oxidizing conditions, including conditions of the catalytic oxidation of CO in excess oxygen.
EXPERIMENTAL
Synthesis
The initial manganese–cerium catalysts were prepared by coprecipitation from a solution of Ce(NO3)3 and Mn(NO3)2 salts. Synthesis was carried out similarly to that described in [9]. The Mn : Ce molar ratio for the series was 3 : 7. The obtained catalysts are denoted as Mn0.3Ce0.7_T, where T is the calcination temperature.
Ex Situ and Operando X-Ray Phase Analysis
The X-ray diffraction patterns of the catalysts were obtained using a D8 Advance powder diffractometer (Bruker, Germany) at λ = 1.5418 Å in the 2θ range from 23° to 83° with a step of 0.05° and an exposure time in one position of 5 s. An XRK-900 high-temperature reactor chamber (Anton Paar, Austria) was used for operando research. The heating rate was constant, 12°C/min; the gas-mixture supply rate to the chamber was 300 mL/min for a reaction mixture of 1% CO + 2% O2 + He and 100 mL/min for the mixtures of 10% CO + He and 10% H2 + He. The ICDD PDF-2 database [12] was used for qualitative phase analysis. Quantitative phase analysis and refinement of the lattice parameters were performed by the Rietveld method using the MAUD program [13].
X-Ray Photoelectron Spectroscopy
The chemical composition of the surface of the samples was studied using a SPECS photoelectron spectrometer (SPECS Surface Nano Analysis, Germany). The binding energy (Eb) scale was calibrated using the internal standard method by the Ce3d3/2-U"' peak of cerium, which is part of the support (Eb = 916.7 eV).
Measurement of the Catalyst Surface
The specific surface area was calculated by the Brunauer–Emmett–Teller method using nitrogen-adsorption isotherms measured at liquid-nitrogen temperatures using an ASAP 2400 automatic adsorption meter (Micromeritics, United States).
Catalytic Tests
The activity of the samples in the CO oxidation reaction was determined using a flow setup with a glass reactor (170 mm in length and 10 mm in diameter). The composition of the initial gas mixture was 1% CO and 99% air; the flow rate was varied within 253–487 mL/min to vary the contact time (τ). The CO conversion for each sample was determined at three values of the contact time. The weight of the sample (fraction 0.4–0.8 mm) was 0.2–1.5 g. The reaction temperature was 150°С. The composition of the reaction mixture before and after the reactor was determined using an LKhM-8MD chromatograph (Russia) with a column packed with Ca-A zeolite (3 m) and a thermal-conductivity detector. The activity ACO of the catalyst (cm3 g–1 s–1) was calculated by the equation
where РСО and \({{P}_{{{{{\text{N}}}_{2}}}}}\) are the areas of CO and nitrogen peaks before (A) and after (B) the reaction (the area \({{P}_{{{{{\text{N}}}_{2}}}}}\) was used as the internal standard); mcat is the catalyst weight; and vСО is the volumetric flow rate of CO.
Temperature-Programmed Reduction
Reduction in hydrogen was carried out using a flow setup with a thermal-conductivity detector. The sample was loaded into a quartz reactor, and a reductive mixture of 10 vol % H2 in Ar was passed through it at a rate of 40 mL/min. The heating rate from room temperature to 900°C was 10°C/min.
RESULTS AND DISCUSSION
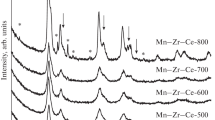
The phase composition and structural parameters of the catalysts of the Mn0.3Ce0.7_T series with a change in the calcination temperature from 300 to 800°C are presented in Table 1. According to X-ray diffraction data, the Mn0.3Ce0.7_T catalysts obtained at 300–600°C were single phase and represented a (Ce,Mn)O2 solid solution based on the CeO2 phase (PDF no. 34-394, space group Fm–3m, a = 5.41134 Å), as evidenced by the noticeably smaller lattice parameters (a = 5.360(1) Å for Mn0.3Ce0.7_300) due to the difference in the ionic radii of the Ce and Mn cations. Judging from the gradual approach of the lattice parameter of the solid solution to the parameter of the pure CeO2 phase, an increase in the calcination temperature causes the diffusion of manganese cations from the structure of the solid solution onto its surface. This process results in the formation of manganese oxide in a quantity sufficient for its detection by X-ray diffraction at a temperature of 800°С (~7 wt % of Mn2O3 for the Mn0.3Ce0.7_800 sample). An increase in the synthesis temperature also leads to particle sintering, which is characterized by an increase in the (Ce,Mn)O2 coherent-scattering region from 6 to 29 nm and a decrease in the specific surface area of the catalysts from 80.6 to 9.2 m2/g.
The data of X-ray photoelectron spectroscopy show similar dependences as those obtained by X-ray phase analysis (Table 1). For catalysts prepared at 300–600°С and representing a single-phase (Ce,Mn)O2 solid solution, the [Mn]/[Mn + Ce] surface ratio is close to the stoichiometric value of 0.30. An increase in the calcination temperature leads to enrichment of the surface with manganese cations, the [Mn]/[Mn + Ce] ratio increases from 0.29 to 0.37, which is associated with the formation of manganese-oxide phases.
To assess the catalytic activity, we tested the samples in the CO oxidation reaction at a temperature of 150°C (Fig. 1). In the calcination temperature range of 300–500°C, we found a slight increase in activity from 5.1 × 10–2 to 5.8 × 10–2 cm3 (СО) g–1 s–1, followed by a sharp decrease to 0.3 × 10–2 cm3 (СО) g–1 s–1 with a further increase in the calcination temperature to 800°C. As for the specific activity (normalized to the specific surface area of the sample), we observed a sharper increase in activity with an annealing temperature increasing from 300 to 500°C. This effect shows the relationship between the catalytic activity and the release of manganese to the surface of the solid solution during annealing, which is essential for understanding the processes occurring at the atomic level. The Mn0.3Ce0.7_500 catalyst demonstrates the highest activity normalized to the surface. The specific activity of the Mn0.3Ce0.7_600 and Mn0.3Ce0.7_400 samples is at the same level, despite an almost twofold decrease in the specific surface. The latter may indicate the formation of new active centers, probably associated with MnOx on the surface of the solid solution, which is also evidenced by an increase in the amount of manganese, as follows from the results of X-ray photoelectron spectroscopy (Table 1). According to the published data , introducing manganese atoms into the CeO2 lattice leads to the formation of oxygen vacancies, which form an active center capable of capturing and activating molecular oxygen [14]. Thus, the (Ce,Mn)O2 solid solution has a higher activity in the CO oxidation reaction in comparison with CeO2. MnOx, in turn, has its own activity in oxidative reactions [15]. By analogy with our work on (Mn,Zr)O2 [11], we decided to test the possibility of increasing the number of such active centers due to the release of manganese cations from the bulk of the solid solution by means of topochemical reduction, using the operando X-ray diffraction method.
To test this approach, we used the Mn0.3Ce0.7_600 catalyst. This selection was due to the maximum annealing temperature at which the sample is single phase according to X-ray diffraction data.
The curve of the temperature-programmed reduction of Mn0.3Ce0.7_600 in hydrogen (Fig. 2) contains three distinct hydrogen absorption peaks at temperatures of 259, 354, and 809°C. CeO2 is characterized by two absorption peaks [16–18]. One peak at 300–500°C is associated with the reduction of surface oxygen and greatly depends on both the shape of the particles (tubes, cubes, rods [16]) and the amount and type of dopant in the complex oxide based on cerium oxide. For example, the doping of CeO2 with iron shifts the first peak to lower temperatures [17], while the first peak of TixCe1 – xO2 is shifted to higher temperatures [18]. Another absorption peak is located in the temperature range of 650–900°C; it is associated with the reduction of oxygen in the bulk of fluorite. It is less susceptible to a form factor or a dopant effect. The reduction of Mn3+ and Mn4+ oxides occurs in the temperature range of 200–600°C and depends both on the structure of the oxide and the size of its particles [19] and on the bonding with the support. For example, an increase in the annealing temperature for TiO2-supported manganese catalysts leads to the disappearance of the low-temperature peak corresponding to the reduction of MnO2 to Mn2O3 due to interaction with the support [20]. For Mn0.3Ce0.7_600, the first two peaks of hydrogen absorption are most likely associated with the reduction of manganese cations either in the bulk of the solid solution or as segregated oxides Mn4+ → Mn3+ and Mn3+ → Mn2+, respectively. The reduction of the surface oxygen of the solid solution can also make a small contribution in the regions of the first two absorption peaks (~2/3 of the high-temperature absorption peak [16]). The high-temperature peak refers to the reduction of lattice oxygen in the pure CeO2 phase. Based on these data, the reduction temperature of 400°C was selected to study the processes of increasing catalytic activity due to topochemical activation.
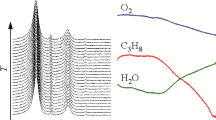
A series of successive experiments were carried out as part of the study of the effect of various treatments on the structural characteristics and catalytic properties of the manganese–cerium catalyst Mn03Ce07_600. The stages were the following: study of the sample under stepwise heating/cooling in a reaction mixture of 1% CO + 2% O2 in the mode 150–175–200–175–150°C keepeng for 2 h at each temperature simultaneously with the recording of reaction products (stages 1, 3, and 5), reduction of the sample in a mixture of 10% CO + He at 400°C for 2 h (stage 2), and reduction of the sample in a mixture of 10% H2 + He at 400°С for 2 h (stage 4). The study of the sample without pretreatment in the CO oxidation reaction (stage 1) showed that heating after reaching a temperature above 175°C improves the CO conversion from 12 to 24% within 2 h; after reaching a temperature of 200°C, the conversion is 45% and stabilizes at 53% in 30 min (Fig. 3). Upon cooling after reaching each temperature, the conversion did not change; however, its values at each temperature exceeded those upon initial heating by ~5%. Segregation of the solid solution after the reaction was not observed (Fig. 4 and Table 2). However, a slight decrease in the lattice parameter from 5.3788(6) to 5.3762(6) Å was found, which may indicate an increase in the oxidation state of manganese. The reduction of the sample at 400°С in a 10% CO + He mixture (stage 2) led to the release of manganese from the solid solution and the formation of the MnO phase (PDF no. 65-638, space group Fm–3m, a = 4.538 Å). Simultaneously with the appearance of the manganese(II) oxide phase, we observed a jump in the lattice parameter of the fluorite phase by over 0.2% and a decrease in the microstress value by 1.5 times. A repeated study of the system during CO oxidation (stage 3) showed the gradual disappearance of reflections corresponding to the MnO phase with increasing temperature. The most probable reason is the oxidation of MnO under the conditions of a catalytic reaction (1% CO + 2% O2) into other phases of manganese oxides in a more dispersed state, for example, Mn2O3 or Mn3O4, since the lattice parameter of the (Mn,Ce)O2 phase, remaining the same, prevents the reverse incorporation of manganese. The activity of the sample increased slightly after reduction. The greatest difference was found at a reaction temperature of 200°C, at which the conversion changed from 53% (stage 1) to 58% (stage 3); this increase is most likely due to manganese oxides on the surface of the solid solution. Reduction in a 10% H2 + He mixture showed similar results to those obtained in a 10% CO + He mixture. According to the X-ray diffraction data, the lattice parameter of the (Mn,Ce)O2 phase changed from 5.3896(4) Å at stage 3 to 5.3911(4) Å and a larger amount of MnO was formed (Table 2); apparently, hydrogen ensured the more manganese to drift from the solid solution.
Summing up, we synthesized a manganese–cerium solid solution with a fluorite structure and demonstrated that reduction treatment leads to the appearance of dispersed manganese oxides on the surface of the initial phase. In turn, this increases the activity of the system under study in the CO oxidation reaction. Nevertheless, no significant increase in the catalytic activity of Mn03Ce07_600 was observed after topochemical reduction. We believe that the main reason is that the diffusion of manganese from the bulk of the solid solution both creates new catalytically active sites associated with manganese-oxide nanoparticles and decreases their number in the solid solution.
REFERENCES
K. Kousi, C. Tang, I. S. Metcalfe, et al., Small 17, 2006479 (2021). http://www.doi.org/10.1002/smll.202006479.2
D. Neagu, G. Tsekouras, D. N. Miller, et al., Nat. Chem. 5, 916 (2013). http://www.doi.org/10.1038/nchem.1773
M. Chanthanumataporn, J. Hui, X. Yue, et al., Electrochim. Acta 306, 159 (2019). http://www.doi.org/10.1016/j.electacta.2019.03.126
J. Tan, D. Lee, J. Ahn, et al., J. Mater. Chem. A 6, 18133 (2018). http://www.doi.org/10.1039/C8TA05978K
S. -K. Otto, K. Kousi, D. Neagu, et al., ACS Appl. Energy Mater. 2, 7288 (2019). http://www.doi.org/10.1021/acsaem.9b01267
J. Myung, D. Neagu, D. N. Miller, et al., Nature 537, 528 (2016). http://www.doi.org/10.1038/nature19090
D. Neagu, T.-S. Oh, D. N. Miller, et al., Nat. Commun. 6, 8120 (2015). http://www.doi.org/10.1038/ncomms9120
Y. Nishihata, J. Mizuki, T. Akao, et al., Nature 418, 164 (2002). http://www.doi.org/10.1038/nature00893
O. A. Bulavchenko, Z. S. Vinokurov, T. N. Afonasenko, et al., Dalton Trans. 44, 15499 (2015). http://www.doi.org/10.1039/C5DT01440A
O. A. Bulavchenko, Z. S. Vinokurov, T. N. Afonasenko, et al., Mater. Lett. 258, 126768 (2020). http://www.doi.org/10.1016/j.matlet.2019.126768
O. A. Bulavchenko, Z. S. Vinokurov, T. N. Afonasenko, et al., Mater. Lett. 315, 131961 (2022). http://www.doi.org/10.1016/j.matlet.2022.131961
S. Gates-Rector and T. Blanton, Powder Diffr. 34, 352 (2019). http://www.doi.org/10.1017/S0885715619000812
L. Lutterotti, Nucl. Instrum. Methods Phys. Res., Sect. B 268, 334 (2010). http://www.doi.org/10.1016/j.nimb.2009.09.053
G. Qi and R. T. Yang, J. Phys. Chem. B 108, 15738 (2004). http://www.doi.org/10.1021/jp048431h
K. Frey, V. Iablokov, G. Safran, J. Osan, et al., J. Catal. 287, 30 (2012). http://www.doi.org/10.1016/j.jcat.2011.11.014
G. Feng, W. Han, Z. Wang, et al., Catalysts 8, 535 (2018). http://www.doi.org/10.3390/catal8110535
L. Zhang, G. Spezzati, V. Muravev, et al., ACS Catal. 11, 5614 (2021). http://www.doi.org/10.1021/acscatal.1c00564
S. Watanabe, X. Ma, and C. Song, J. Phys. Chem. C 113, 14249 (2009). http://www.doi.org/10.1021/jp8110309
E. R. Stobbe, B. A. de Boer, and J. W. Geus, Catal. Today 47, 161 (1999). http://www.doi.org/10.1016/S0920-5861(98)00296-X
S. M. Lee, K. H. Park, S. S. Kim, et al., J. Air Waste Manage. Assoc. 62, 1085 (2012). http://www.doi.org/10.1080/10962247.2012.696532
ACKNOWLEDGMENTS
The studies were carried out using equipment of the Center for Collective Use National Center for the Study of Catalysts.
Funding
This work was supported by the Russian Science Foundation, grant no. 21-73-10 218.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflicts of interest.
Additional information
Translated by O. Zhukova
Rights and permissions
About this article
Cite this article
Vinokurov, Z.S., Afonasenko, T.N., Mishchenko, D.D. et al. Operando X-ray Diffraction Study of Mn–Ce Catalysts for CO Oxidation. J. Surf. Investig. 17, 694–700 (2023). https://doi.org/10.1134/S1027451023030345
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1027451023030345