Abstract—
Quantum-mechanical models for the formation of metal–carbon complexes of Co, Ni, Cu, and Zn ions with С60 fullerene molecules and single-wall С48 carbon nanotubes (SWCNTs) are proposed. The results of calculations show that, in aqueous solutions of electrolytes, Co, Ni, Cu, and Zn ions can be adsorbed into the С60 fullerene and С48 SWCNT surfaces with the formation of stable carbon-nanomaterial—metal (CNM—M) complexes; in this case, the minimum energy of the С60–М complex for Co and Cu ions corresponds to the position above the С6 cell center; for a Ni ion, above the single С–С bond in the С6 cell; and for a Zn ion, above the C atom. The optimized states of the С48–М complexes correspond to the position of metal ions above the С6 cell center.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
In modern metal science, carbon nanomaterials are widely used especially in the role of fillers of composite materials [1, 2]. First, this is related to the unique properties of the obtained materials, because the addition of carbon nanomaterial (CNM) to a metal matrix leads to an increase in its hardness and wear and corrosion resistances [3–8]. However, if a high CNM cost price is taken into account, surface hardening is of great interest. In this case, a metal composite film with carbon nanoparticles as a filler is deposited onto the surfaces of parts. Among the variety of existing methods for obtaining metal composite films, the method of substance condensation on a substrate from vapors or solutions is now widely used; it offers the possibility of controlling metal-film formation and growth within wide limits. This process can occur using pulse currents, ultrasound, magnetic fields, and external laser radiation to intensify and stabilize the processes of metal and CNM codeposition.
The unique physical-chemical properties of electrodeposited metal films depend to a significant extent on the CNM particle concentration in the metal matrix. Therefore, the monitoring and the control of the CNM particle content in metal composite films have attracted particular attention recently. It is impossible to solve this problem without studying the mechanism for the formation of structures of carbon-containing metal composite films. However, the process of combined metal-ion and CNM-particle codeposition onto a substrate remains studied incompletely. First, the mechanism of CNM transport from the solution volume to the substrate is not clarified. The authors of a series of papers suggested that the motion of CNM particles occurs only by a convective flux, which is produced by metal ions in the electrolyte solution [9, 10]; the assumption that CNM particles upon acquiring charge in the electrolyte solution move to the cathode under the action of an electric field generated by a potential difference between the anode and the cathode is also suggested [11, 12]. The mechanism for the formation of metal—carbon complexes can be vary greatly, and CNM particles can acquire charge by different methods.
The authors of [13, 14] theoretically considered the possibility of metal-atom adsorption into CNM particles. The binding energies of neutral atoms of transition metals with CNM (С60 fullerene and С48 SWCNT) particles were calculated using density functional theory. It was shown that the binding energies of atoms adsorbed into the С60 and С48 surfaces varied in the range of 0.05–2 eV (depending on the metal). However, these calculations were carried out for the adsorption of neutral atoms in vacuum rather than for that of positively charged metal ions in an electrolyte solution; they cannot be used to simulate mechanisms for charge acquisition by CNM particles and for the formation of metal—carbon complexes.
To establish the mechanism of the combined codeposition of CNM particles and metal ions, we propose models of the formation of metal—carbon complexes and calculate the binding energies of Co, Ni, Cu, and Zn ions with С60 fullerene and С48 SWCNTs.
EXPERIMENTAL
To calculate the binding energies of metal ions with CNM particles, it is necessary to take into account the following model approximations.
First, adsorption occurs in electrolyte solutions, in particular, in aqueous solutions.
Second, to provide fulfillment of the charge-conservation law, in the calculations, it is necessary to take into account that metal ions must be adsorbed into CNM-particle surfaces, and (as a consequence) the obtained CNM—M complex must have the charge of the initial metal ion. Third, the metal-ion complex joins the CNM particle in turn; i.e., each subsequent metal ion is adsorbed into the CNM particle, which already has a charge obtained during earlier adsorption.
The binding energy of metal ions with CNM particles was calculated within the framework of density functional theory (DFT), which, recently, became one of the most popular methods for calculating the electronic structures of atoms, molecules, clusters, solid bodies, and so on [15, 16]. The growing popularity of DFT is due to, first, the combination of a rather high accuracy of the obtained results, which, in some cases, competes with that of ab initio methods for taking into account the electron correlation, and of very moderate requirements imposed on computing resources making it possible to calculate systems that consist of hundreds of atoms and are of interest for modern nanotechnology. In addition, DFT is used to study the adsorption characteristics of transition metals [17, 18], whose electrocrystallization kinetics is studied in this paper.
Numerous studies of the characteristics of molecules and clusters using DFT showed good results in the case of correct choice of the exchange-correlation functional. As shown in [19, 20], the B3LYP three-parameter hybrid functional is the most appropriate for calculations of structural and thermal-chemical characteristics of metal complexes. Using DFT, the authors of [21] studied the structure and energetics of the formation of macrocyclic complexes. It was shown that DFT using the B3LYP hybrid functional gave exact information about the structural, energy, and kinetic characteristics. It is also known that DFT using hybrid exchange-correlation functionals makes it possible to successfully calculate the structural and electronic characteristics of complexes of transition and heavy metals with acceptable computer expenditure [22]. It was shown that when using the B3LYP functional to calculate the characteristics of the structures of transition metals, accuracy is provided on the level of ab initio methods. When optimizing the complex geometry in an aqueous solution, we also took dispersion corrections into account [23, 24]. The influence of the aqueous medium was taken into account by means of the method of a self-consistent reaction field within the framework of the model of the polarization continuum of Tomasi et al. [25].
Then the choice of the basis set of atomic orbitals used for the calculation was based on the fact that the energy and thermodynamic quantities were calculated for metals for which the interaction of valence electrons plays the largest role. The split-valence sets of basis orbitals, in particular, the 6-31g basis set or the extended 6-31-g(d) one containing d-type atomic orbitals for the inclusion of polarizations of the metal electron density, were used to describe such interactions.
We used the GAUSSIAN 09 program package to calculate the energies of molecules, their structures, and oscillation frequencies in the condensed state [26]. The temperature was chosen as 295 K; the pressure was 105 Pa in our calculations.
CALCULATION OF THE BINDING ENERGY
To calculate the binding energy of metal ions with CNM particles, we used the density functional method, in which the energy is determined by the expression
where \(U\) is the potential energy of the interaction of nuclei, \({{T}_{S}}\) is the electron kinetic energy, \({{V}_{{ne}}}\) is the energy of attraction of electrons to nuclei, \(J\) is the classical contribution of the interelectron repulsion to the energy, and \({{E}_{{xc}}}\) is the exchange-correlation functional including the static electron correlation.
The binding energy (\(\Delta W\)) of an adsorbed metal ion with a CNM particle was defined as the difference between the total energy of the CNM complex with the adsorbed metal ion (WC + M) and the sum of energies of its components (WC, WM)
where WC is the CNM-particle energy and WM is the metal-ion energy.
In the case of the by-turn adsorption of metal ions into the CNM surface, the binding energy was calculated as the difference between the total energy of the CNM complex with respect to one (\({{W}_{{{\text{C}} + {\text{M}}}}}\)), two (\({{W}_{{{\text{C}} + 2{\text{M}}}}}\)), and three (\({{W}_{{{\text{C}} + 3{\text{M}}}}}\)) adsorbed metal ions and the energy of components: the CNM-particle energy (\({{W}_{{\text{C}}}}\)) and the energy of one metal ion (\({{W}_{{\text{M}}}}\))
the energy of the CNM particle with one adsorbed metal ion (\({{W}_{{{\text{C}} + {\text{M}}}}}\)) and that of one metal ion (\({{W}_{{\text{M}}}}\))
the energy of the CNM particle with two adsorbed metal ions (\({{W}_{{{\text{C + 2M}}}}}\)) and that of one metal ion (\({{W}_{{\text{M}}}}\))
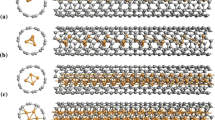
The geometry of the charged complex that consisted of a CNM and an adsorbed metal ion was optimized. The equilibrium geometry of the C60–М and С48–М complexes is shown in Fig. 1. С48 SWCNT dangling bonds were closed by H atoms to decrease the influence of boundary effects (Fig. 1b).
Table 1 contains our obtained results of optimizing the distance from adsorbed metal ions to the CNM particle surface (\(d\)) for an aqueous medium and the calculated results in [13, 14] for vacuum. In our calculations, the quantity \(d\) determines either the length of the perpendicular from the ion to the plane of the С6 or С5 structure ring in the case of the adsorption of ions into the С60 fullerene surface or that of the perpendicular from the ion to the plane of lower four carbon atoms in the С6 cell in the case of ion adsorption into the С48 SWCNT surface.
It was shown that, in the case of copper and cobalt ions, С60–М complexes with the minimum energy corresponded to the structure where the ion is above the С6 cell center. The Ni-ion position above the middle of the C–C bond turned out to be minimum for the C60–Ni complex, while, for the C60–Zn complex, the structure in which the ion is above the C atom was most stable. Similarly, the optimized states of the С48–М complexes corresponded to the metal-ion position above the С6 cell center. The tendency toward an increase in the optimized distance in the series Ni < Cu < Co < Zn was observed for both C60 and С48.
Because the adsorption of one metal ion or more into the CNM-particle surface is theoretically possible, we took into account models in which one nanoparticle can form bonds with at most three metal ions. Taking into account that the system charge must increase by 2e (e is the elementary electric charge) in the case of the addition of each new metal ion, we proposed and optimized models of complexes with one, two or three bound metal ions, which had charges of +2e, +4e, and +6e, respectively.
Table 2 contains the results of optimizing the distance between the adsorbed Ni ion and the С60 fullerene surface for different complex charges; they were calculated for interaction in vacuum and water. As our calculations showed (Table 2), the distance between the adsorbed metal ion and the CNM-particle surface increases with increasing charge.
Table 3 contains the results of calculating the binding energy (\(\Delta W\)) of an adsorbed metal ion with a CNM particle. It is seen from Table 3 that all considered metal ions form strong bonds with С48 particles, unlike the compounds with С60, because their binding energy exceeds a thermal-motion energy of 0.025 eV significantly.
In the case of the subsequent attachment of several metal ions to one CNM particle, their binding energy decreases, as is seen from the calculated results for the С60 fullerene and the Ni ion given in Table 4. Optimization of the geometry of С60 complexes with several Ni ions led to the following results: four ions were arranged in the form of a square with a fullerene at the center, three ions acquired the form of a rectangular isosceles triangle, and two ions were located along the fullerene diameter. In all cases, the ions were located above the single С–С bond. The interaction of CNM particles with two or more metal ions did not lead to the formation of stable complexes (Table 4), and all further calculations were carried out for a charge of +2е, which corresponded to the valence of one metal ion.
Comparison of the obtained binding energies of the CNM—M complex with the thermal-motion energy showed that, in aqueous solutions of electrolytes, Co, Ni, Cu, Zn ions can be adsorbed into the С60 fullerene and С48 SWCNT surfaces with the formation of stable CNM—M complexes. Consequently, CNM particles can be transported from the volume of the aqueous solution of the electrolyte to the cathode surface, because the CNM—M complex acquires positive charge.
CONCLUSIONS
Our proposed mechanism for the adsorption of metal ions into the CNM particle surface with the further motion of the charged complex in the cathode direction was verified using the computing method. Comparison of the results of calculating the binding energies of the CNM—M complexes with the thermal-motion energies showed that, in an aqueous solution of electrolytes, Co, Ni, Cu, and Zn ions can be adsorbed into the C60 fullerene and C48 SWCNT surfaces with the formation of stable CNM—M complexes. For the С60–М and С48–М complexes, the binding energies increased in the Zn < Co < Ni < Cu sequence. It was shown that complexes adsorbed by one metal ion or more were not stable.
Thus, the transport of CNM particles from the solution volume to the cathode can occur via the stage of the adsorption of deposited-material cations into their surfaces. The metal—carbon complex that acquired the charge is transported to the cathode, where, during the deposition process, it turns out to be surrounded by discharged metal ions.
REFERENCES
C. Gheorghies, D. E. Rusu, A. Bund, S. Condurache-Bota, and L. P. Georgescu, Appl Nanosci. 4, 1021 (2014). https://doi.org/10.1007/s13204-013-0285-y
G. K. Burkat, T. Fujimura, V. Yu. Dolmatov, E. A. Orlova, and M. V. Veretennikova, Diamond Relat. Mater. 14, 1761 (2005). https://doi.org/10.1016/j.diamond.2005.08.004
V. P. Isakov, A. I. Lyamkin, D. N. Nikitin, A. S. Shalimova, and A. V. Solntsev, Prot. Met. Phys. Chem. Surf. 46, 578 (2010). https://doi.org/10.1134/S2070205110050138
Liping Wang, Yan Gao, Qunji Xue, Huiwen Liu, and Tao Xu, Mater. Sci. Eng., A 390, 313 (2005). https://doi.org/10.1016/j.msea.2004.08.033
Zabludovs’kii V. O., Dudkina V. V., Shtapenko E. P., Nauka Progr. Transp. Vestn. Dnepropetr. Nats. Univ. im.V. Lazaryana 47 (5), 70 (2013). https://doi.org/10.15802/stp2013/17968
V. V. Dudkina, V. A. Zabludovskii, and E. F. Shtapenko, Metallofiz. Noveishie Tekhnol. 37, 713 (2015). https://doi.org/10.15407/mfint.37.05.0713
V. V. Titarenko and V. A. Zabludovskii, Metallofiz. Noveishie Tekhnol. 38, 519 (2016). Doi https://doi.org/10.15407/mfint.38.04.0519
V. V. Tytarenko, V. A. Zabludovsky, E. Ph. Shtapenko, and I. V. Tytarenko, Galvanotechnik, No. 4, 648 (2019).
G. A. Chiganova and L. E. Mordvinova, Inorg. Mater. 47, 717 (2011). https://doi.org/10.1134/S0020168511070089
Hiroshi Matsubara, Yoshihiro Abe, Yoshiyuki Chiba, Hiroshi Nishiyama, Nobuo Saito, Kazunori Hodouchi, and Yasunobu Inou, Acta Electrochim. 252, 3047 (2007). https://doi.org/10.1016/j.electacta.2006.09.043
Sam Zhang, Deen Sun, Yongqing Fu, and Hejun Du, Surf. Coat. Technol. 167, 113 (2003). https://doi.org/10.1016/S0257-8972(02)00903-9
J. G. Hou, Xiang Li, Haiqian Wang, and Bing Wang, J. Phys. Chem. Solids 61, 995 (2000). https://doi.org/10.1016/S0022-3697(99)00349-2
H. Valencia, A. Gil, and G. Frapper, J. Phys. Chem. C 114, 14141 (2010).
A. Larsson, D. Simon, J. C. Elliott, G. J. Repp, G. Meyer, and R. Allenspach, Phys. Rev. B: Condens. Matter Mater. Phys. 77, 115434 (2008). Rev B.77.115434https://doi.org/10.1103/Phys
E. F. Shtapenko, V. A. Zabludovskii, and E. O. Voronkov, Poverkhn.: Rentgenovskie, Sinkhrotronnye Neitr. Issled., No. 12, 95 (2010).
W. Koch and M. C. Holthausen, Chemists Guide to Density Functional Theory (Wiley, New York, 2001).
N. Lopez, N. Almora-Barrios, and G. Carchini, Catal. Sci. Technol. 2, 2405 (2012). https://doi.org/10.1039/C2CY20384G
T. C. Allison and Y. Y. Tong, Phys. Chem. Chem. Phys. 13, 12858 (2011). https://doi.org/10.1039/C1CP20376B
G. Schreckenbach, P. J. Hay, and R. L. Martin, Inorg. Chem. 37, 4442 (1998). https://doi.org/10.1002/(SICI)1096-987X(19990115)20:1<70::AID-JCC9>3.0.CO;2-F
B. Miehlich, A. Savin, H. Stoll, and H. Preuss, Chem. Phys. Lett. 157, 200 (1989). https://doi.org/10.1016/0009-2614(89)87234-3
D. A. Keire, Hee Jans Yun, Lin Li, et al., Inorg. Chem. 40, 4310 (2001). https://doi.org/10.1021/ic0010297
J. Andzelm and J. Labanowski, Density Functional Methods in Chemistry (Springer, Heidelberg, 1991). https://doi.org/10.1007/978-1-4612-3136-3
S. Grimme, A. J. Grimme, S. Ehrlich, and H. Krieg, J. Phys. Chem. 132, 154104 (2010). https://doi.org/10.1063/1.3382344
S. Grimme, EhrlichS. Grimme, and L. Goerigk, J. Comput. Chem. 32, 1456 (2011). https://doi.org/10.1002/jcc.21759
J. Tomasi, B. Mennucci, and R. Cammi, Chem. Rev. 105, 2999 (2005). https://doi.org/10.1021/cr9904009
M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, J. A. Montgomery Jr., T. Vreven, K. N. Kudin, J. C. Burant, J. M. Millam, S. S. Iyengar, J. Tomasi, V. Barone, B. Mennucci, M. Cossi, G. Scalmani, N. Rega, G. A. Petersson, H. Nakatsuji, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, M. Klene, X. Li, J. E. Knox, H. P. Hratchian, J. B. Cross, V. Bakken, C. Adamo, J. Jaramillo, R. Gomperts, R. E. Stratmann, O. Yazyev, A. J. Austin, R. Caimni, C. Pomelli, J. W. Ochterski, P. Y. Ayala, K. Morokuma, G. A. Voth, P. Salvador, J. J. Dannenberg, V. G. Zakrzewski, S. Dapprich, A. D. Daniels, M. C. Strain, O. Farkas, D. K. Malick, A. D. Rabuck, K. Raghavachari, J. B. Foresman, J. V. Ortiz, Q. Cui, A. G. Baboul, S. Clifford, J. Cioslowski, B. B. Stefanov, G. Liu, A. Liashenko, P. Piskorz, I. Komaromi, R. L. Martin, D. J. Fox, T. Keith, M. Al’Laham, C. Y. Peng, A. Nanayakkara, M. Challacombe, P. M. W. Gill, B. Johnson, W. Chen, M. W. Wong, C. Gonzalez, and J. A. Pople, Gaussian 03, Revision C.02 (Gaussian, Wallingford, 2004).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Translated by L. Kulman
Rights and permissions
About this article
Cite this article
Tytarenko, V.V., Shtapenko, E.P., Voronkov, E.O. et al. Quantum-Mechanical Modeling of the Interaction between Carbon Nanostructures and Metal Ions. J. Surf. Investig. 15, 866–871 (2021). https://doi.org/10.1134/S102745102104039X
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S102745102104039X