Abstract
In this paper, we decomposed the FeSO4 at the presence of benzoic acid with weight ratio 1 : 1 at two different temperatures 500 and 600°C. The as-synthesized products were characterized by Fourier Transform Infrared (FT-IR) spectroscopy, powder X-ray diffraction (XRD) and transmission electron microscopy (TEM). FT-IR and XRD results confirmed that the as-prepared product at 500°C is a mixture of intermediate compounds, while the as-prepared product at 600°C is obviously pure with high degree of crystallinity of hematite (α-Fe2O3) nanoparticles. TEM images show that shape, size and morphology of the as-prepared products depend on the calcination temperature.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 INTRODUCTION
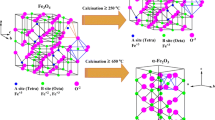
In recent years, iron oxide nanostructures (α-Fe2O3) have attracted interest due to their various properties and applications such as sensors for H2S [1], photocatalytic degradation of Rhoda mine B (RhB) [2, 3], SO3 catalyst formation [4], electrocatalytic methanol oxidation [5] and as anode for Li-ion batteries [6, 7]. All of these properties depend on their unique size and shape [1–9]. Then many groups interested in the design and preparation of different shapes of α-Fe2O3 [10–12]. For example, Liu et al. [10] prepared different shapes such as a quasi-cube, parallel hexahedron, hexagon, nanosheet and irregular sphericity of α‑Fe2O3 from iron oxide red. Li et al., [11] reported the controlled synthesis of α-Fe2O3 hollows by hydrothermal method from β-FeOOH rods. Hematite, as an n-type semiconductor in room temperature, is low-cost and environmentally friendly. It is generally prepared by three main approaches. The first one is direct oxidation of iron foil, the second is the hydrolysis of ferric salts at suitable conditions, and the third is an epitaxial grown from other iron compounds [1–12]. One of the best approaches for the preparation of α-Fe2O3 is thermal decomposition such as hydro- and solvo-thermal and also direct calcination of commercial iron salts or iron complexes [1–3, 5–7].
Herein, we report the temperature effect on the size, morphology and crystalline phase of the as-synthesized hematite via direct calcination of FeSO4 at the presence of benzoic acid at 500 and 600°C.
2 EXPERIMENTAL
1 g FeSO4 as an iron precursor and 1 g benzoic acid were mixed and ground in the crucible for 10 minutes. After that, the mixture was loaded into a furnace and calcined at 500 and 600°C for 3 h. Red-brown precipitates were washed with cold water and dried at room temperature for several days. The as-synthesized products were characterized by FT-IR, XRD, and TEM.
Fourier transform infrared (FT-IR) spectra were recorded as a KBr disk on an FT-IR Perkin–Elmer spectrophotometer. XRD patterns were obtained on Empyrean powder diffractometer of PANalytical in Bragg-Brentano configuration equipped with a flat sample holder and PIXCel3D detector (CuKα radiation, λ = 1.5418 Å). TEM images were recorded on transmission electron microscope Philips CM120 with a LaB6 cathode operating at 120 kV and equipped with CCD camera Olympus Veleta.
3 RESULTS AND DISCUSSION
3.1 FT-IR Spectra
The FT-IR spectra of iron precursor and the as-prepared products at 500 and 600°C are presented in Fig. 1. In the FT-IR spectrum of iron precursor (Fig. 1a), there are peaks at wavenumbers 3357, 3072, 2990, 2835, 2672, 2557, 2688, 1687, 1602, 1583, 1454, 1424, 1326, 1292, 1179 cm–1, and etc. concerning to the vibration modes of FeSO4 and benzoic acid. When iron precursor out in a furnace at 500 and 600°C, the compound was decomposed to iron oxide and various gases such as CO2, SO2 and H2O. FT-IR spectrum of the as-prepared products at 500°C (Fig. 1b), shows that the decomposition of the iron oxide is not completed, because there are various low peaks at 2922, 2852, 1719, 1626, and 1409 cm–1 concerning to benzoic acid. However, the two sharp peaks at 551 and 465 cm–1 confirm the change of FeSO4 to iron oxide (Fe2O3) [2]. There are none impurity peaks in the FT-IR spectrum of the as-prepared products at 600°C (Fig. 1c). It confirms that the decomposition of the iron oxide is completed. The sharp peaks at 543 and 470 cm–1 are assigned to the α-Fe2O3 [2, 7, 10]. The broad and weak peaks at about 3400 cm–1 in the FT-IR spectra of the as-prepared products at 500 and 600°C confirm the adsorption of a water molecule on the surface of the products [13].
3.2 XRD Patterns
Figure 2 depicts XRD patterns of the as-prepared nanoparticles which were prepared at different temperatures. The strong and sharp diffraction peaks in Fig. 2b imply good crystallinity of the product prepared at 600°C. All diffraction peaks that appeared at 2θ ≈ 24.1° (012), 33.1° (104), 35.6° (110), 40.8° (113), 49.4° (024). 54.0° (116), 57.5 (018), 62.4° (214), 63.9° (300), 71.9° (1010) and 75.4° (220) can be clearly indexed to the rhombohedral phase of hematite (α-Fe2O3) with JCPDS card number 33-0664 [6, 14–17]. There are none impurity peaks in the pattern. The structure was further refined by Rietveld fit in crystallographic program Jana2006 [18] with calculated lattice constants of a = 5.0356(2) Å and c = 13.7548(9) Å which are in good agreement with the tabulated values. The size of crystallites was calculated using fundamental parameter approach [19] integrated in Jana2006, which removes the instrumental part of the diffraction pattern by means of known geometry of the diffractometer. The average crystallite size determined for the as-prepared product at 600°C is 41 nm. There are many less intense and overlapped diffraction peaks in the diffraction pattern of the sample prepared at 500°C (Fig. 2a). Only small part of the diffraction pattern can be explained as mixture of precursor FeSO4 and product Fe2O3. Majority of the diffraction peaks match to intermediate products which cannot be unambiguously identified.
3.3 TEM Images
Figure 3 shows the TEM images of the as-prepared products at 500 and 600°C. The particles calcined at lower temperature forms clusters, and it is not possible to find single particles. It can be the confirmation of the previous results, that process of calcination is not finished. The particles calcined at higher temperature are almost of elliptic shape with size about tens nm. Although they make clusters, it is possible to observe the individual particles.
4 CONCLUSIONS
The crystalline phase of the products by decomposition of the mixture of FeSO4 and benzoic acid depended on the temperature. The product obtained at a temperature of 500°C is a mixture of the precursor, α-Fe2O3 and impurity. While, the product obtained at a temperature of 600°C is undoubtedly α‑Fe2O3. The particles calcined at lower temperature forms clusters, and at higher temperature are almost of elliptic shape with size about tens nm.
REFERENCES
H. J. Zhang, F. N. Meng, L. Z. Liu, and Y. J. Chen, J. Alloys Compd. 774, 1181 (2019).
M. Qiu, R. Wang, and X. Qi, J. Taiwan Inst. Chem. Eng. 102, 394 (2019).
A. Kusior, K. Michalec, P. Jelen, and M. Radecka, Appl. Surf. Sci. 476, 342 (2019).
X. Wang, J. Zhang, Z. Wang, Y. Wang, M. Vujanovic, P. Li, and H. Tan, J. Enviroment. Manage. 236, 420 (2019).
S. Hu, L. Jiang, B. Wang, and Y. Ma, Int. J. Hydrogen Energy 44, 13214 (2019).
Z. Li, Y. Mao, Q. Tian, W. Zhang, and L. Yang, J. Alloys Compd. 784, 125 (2019).
S. Yao, Z. Shi, and X. Zhang, J. Alloys Compd. 794, 333 (2019).
M. Wang, T. Hou, Z. Shen, X. Zhao, and H. Ji, Sens. Actuators, B 292, 171 (2019).
Z. Huang, L. Qin, Z. Xu, W. Chen, F. Xing, and J. Han, J. Energy Inst. 92, 835 (2019).
J. Liu, H. Yang, and X. Xue, CrystEngComm 21, 1097 (2019).
D. Li, R. Xu, Y. Jia, P. Ning, and K. Li, Chem. Phys. Lett. 731, 1336623 (2019).
J. R. Jesus, R. J. S. Lima, K. O. Moura, J. G. S. Duque, and C. T. Meneses, Ceram. Int. 44, 3585 (2018).
G. Huang, E. He, Z. Wang, H. Fan, J. Shangguan, E. Croiset, and Z. Chen, Ind. Eng. Chem. Res. 54, 8469 (2015).
C. Hao, F. Feng, X. Wang, M. Zhou, Y. Zhao, C. Ge, and K. Wang, RSC Adv. 5, 21161 (2015).
D. M. S. N. Dissanayake, M. M. M. G. P. G. Mantilaka, T. C. Palohawadana, G. T. D. Chandrakumara, R. T. De Silva, H. M. G. A. Pitawala, K. M. Nalin de Silva, and G. A. J. Amaratunga, RSC Adv. 9, 21249 (2019).
H. Cui, Y. Liu, and W. Ren, Adv. Powder Technol. 24, 93 (2013).
X. Zhang, Y. Niu, X. Meng, Y. Li, and J. Zhao, CrystEngComm 15, 8166 (2013).
V. Petricek, M. Dusek, and L. Palatinus, Z. Kristallogr. 229, 345 (2014).
R. W. Cheary and A. A. Coelho, J. Appl. Crystallogr. 31, 862 (1998).
ACKNOWLEDGMENTS
We are grateful to the Golestan University for financial support of this work. XRD and TEM analysis were supported by the project 18-10504S of the Czech Science Foundation using instruments of the ASTRA lab established within the Operation program Prague Competitiveness e project CZ.2.16/3.1.00/2451.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Aliakbar Dehno Khalaji, Mousavi, S.M., Jarosova, M. et al. The Effect of Calcination Temperature on the Morphology, Size and the Crystalline Phase of the As-Synthesized Products by Direct Calcination of FeSO4 at the Presence of Benzoic Acid. J. Surf. Investig. 14, 1191–1194 (2020). https://doi.org/10.1134/S1027451020060051
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1027451020060051