Abstract—
The effects the spectrum of incident radiation can have on photobiological processes are studied experimentally and theoretically. The potential effective fluxes of visible light are calculated for energetic and regulatory processes. With these data at hand, we find a correlation between the spectral photoresponse and the energy characteristics of plant photoreceptor and photosynthesis systems. A qualitative model for a photosensitive organic system is formulated as a circuit diagram that includes semiconductor photocells. The thermodynamic and electrical parameters of the modeled system are described. We draw parallels between the functioning of phototransistors and photoreceptor complexes PHOT, CRY, Ph(660), and Ph(730). The control parameters of the energy-distribution process in a living system are estimated using mathematical modeling and an experimental model based on photocells.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Studies on the effects light exerts on living organisms have not lost their relevance today, and presently these are determinants in furthering development in various applied areas, e.g., the use of light-emitting diodes for lighting in greenhouses [1–3]. Not only economic problems can be overcome using these technologies, but we can move forward in controlling the process of interaction between living matter and radiation [4, 5].
It is well documented [6] that in higher plants leaves constitute a key system that is sensitive to light and transforms the energy of light quanta; essentially, they can be called the organic photocells of plants. Radiation in the wavelength region spanning 400−700 nm, which is used by plants, is conventionally called photosynthetically active radiation [7]. For convenience, the whole range of effects associated with the impact of radiation from the visible range are divided into two categories: energetic and regulatory effects. Energetic effects are related to the conversion of the absorbed energy of light quanta into heat and the energy of chemical bonds in organic molecules synthesized by the plant. Regulatory effects that arise due to the absorption of light energy lead to the activation, regulation, and deactivation of various physiological processes in plants. In contrast to energetic effects, photoregulatory effects require from tens to hundreds of times less energy input [6], but so far the exact number has not been determined.
The aim of this study is to build a model that describes the interaction between the organic surface of a plant leaf and radiation and that can be used for identifying, by means of mathematical modeling, the set of process controlling parameters.
THEORETICAL ANALYSIS
Study Objects
Our study is based on the known concept that photosynthesis is a key energy transformation process that enables a plant’s biological functioning and development. The photosynthetic reaction was established to be a source of fluxes of chemical energy (ATP, NADP, µH+, С6Н12О6) necessary for synthesizing organic molecules and for anabolic processes in plant cells [8]. Chlorophyll molecules are responsible for the absorption of visible light. A chlorophyll molecule is excited to the first excited singlet state by absorbing a red photon, while the energy of a blue photon can excite the molecule to the second excited singlet state. The lifetime of the second singlet state is only 10−12 s; therefore, it is too unstable for the excitation energy to be used in chemical reactions. The excited molecule then loses part of this energy as heat; i.e., it is spent on rotational and oscillatory motions in the molecule.
Plant photoreceptors perform assessment of the energy component of visible light and have control over plant regulatory processes. Chemically, these are pigment−protein complexes that transform the energy of absorbed light photons into biochemical signals [8]. Depending on the particular photoreceptor type, two possibilities are distinguished for an associated photochemical reaction. Red-light photoreceptors—Ph660 and Ph730 phytochromes—function by inducing isomerization of a molecule of light sensitive pigment, a change in the protein conformation, and by triggering phosphorylation of a particular domain in the protein complex. By absorbing a photon, a molecule of the blue-light photoreceptors—CRY1 and CRY2 cryptochromes and PHOT1 and PHOT2 phototropins—changes its redox potential and passes the light signal through a system of secondary messengers.
The general features of the effect of visible-region radiation on biological plant systems at the beginning of plant development were published in earlier works [9, 10]. The red- and blue-light photoreceptors CRY1, CRY2, Ph660, and Ph730 are known to control photomorphogenesis. These receptors in particular control the efficiency of photosynthesis by changing the stem length thereby altering the spatial orientation of the plant so that the area of leaves, the plant’s organic photocells, exposed to the radiation flux is increased. The blue-light photoreceptors PHOT1 and PHOT2 are responsible for phototropism, which is the bending of the plant due to nonuniform illumination of its leaves. These receptors thus control the photosynthesis efficiency and adjust the plant orientation in space by altering the leaf surface area exposed to optical irradiation. Chlorophyll synthesis is known to be regulated by photoreceptors [11], but the underlying mechanism has not been fully understood yet.
Hypothesis Underlying the Model
In developing the model, we made the key assumption that depending on the regulatory signals from each system, an integral signal containing information on the energy efficiency of physiologically important spectral regions of incident radiation—blue (320−500 nm) and red (600−800 nm) regions—is formed. In this way, cell growth in a particular part of the plant is regulated, which is known as photomorphogenesis and phototropism.
The functioning of photoreceptors can be compared to that of valves that control the existing fluxes of the produced and absorbed energy of chemical compounds depending on the spectral region of incident radiation thereby ensuring productive photosynthesis. The fluxes of chemical energy do not pass through the photoreceptors directly, instead pumping into the plant cell is brought about indirectly. Regulatory effects of the photoreceptors lead to the activation of ion pumps and molecular mechanisms localized in the cell membrane; the latter are capable of material transport at the expense of energy released due to ATP hydrolysis or from other sources.
Qualitative Model of the Process
The proposed qualitative model gives a schematic representation of the dependence of the photosynthesis efficiency and of subsequent transformation of the accumulated chemical energy on fluxes of macro- and micronutrients and the spectral region of incident radiation. A physical model of the biological system of a plant is illustrated in Fig. 1. Initially, a major part of the area of the solar cells (panels) 1 and 2, which imitate the leaf surface, is screened by an opaque surface 3. The arrows indicate the horizontal directions the panels can move as a result of the absorption of light quanta. The other arrow indicates the direction of the vertical movement of parts 1−3 by telescopic support pole 4 to which parts 1−3 are attached. This physical model imitates the ability of the plant photosensitive organic system to increase the active surface area as a result of the absorption of the energy of light quanta.
In the circuit diagram shown in Fig. 2, the solar cells G1 and G2 convert photon energy to electrical energy and thus represent the plant’s chlorophyll photosystems that convert the energy of photons to the energy of the chemical bonds in glucose (C6H12O6), amino acids, nucleotides, glycerin, and fatty acids. The electrons produced due to the absorption of light are accumulated in a capacitor C1, which represents the ability of the living plant cell to store the energy of synthesized chemical compounds. Mineral macro- and micronutrients are known to be crucial to plant photosynthesis and metabolism [12]. Nitrogen, phosphorus, and sulfur are constituent elements of organic compounds such as amino acids, nucleotides, phospholipids, peptides, and proteins. Other mineral elements in the plant exist as free ions or are bound in metalloprotein complexes with organic molecules, thus determining the conformation and stability of macromolecules and the membrane potential, among other things. The battery cell C2 is charged by a power source E1 that provides a variable energy supply, which is analogous to the absorption of nutrients by a plant root system. The optical relay VU1 imitates the dependence of photosynthesis efficiency on the supply of mineral nutrients. The capacitor C1 is not charged, if the optical relay VU1 is not open; i.e., there is no voltage in the power source E1. The optical transistors VT1, VT2, VT3, and VT4 imitate the functioning of photoreceptors such as cryptochromes, phototropins, and phytochromes. In phototransistors, the potential barrier of the emitter−base junction drops as a result of light absorption, and a net output signal is formed at the collector. The light-activated flow of electrons through the p−n junctions of the transistors is similar to the phosphorylation of a domain in a photoreceptor’s protein complex and switch-on of the pumping of ions into the cell. Different photoreceptors have different spectral sensitivity. For instance, CRY1 and CRY2 cryptochromes are sensitive to both high and low intensity radiation from the blue spectral region, from 320 to 500 nm. Ph(A)660 and Ph(A)720 phytochromes are sensitive to both red light (λ = 660 nm) of ultralow intensity and far red light (λ = 730 nm) of high intensity, and are not capable of photoconversion. Ph(B)660 and Ph(B)730 phytochromes are interconvertible and are responsible for the absorption of low-intensity radiation. The mechanism of the functioning of phototropins is least studied; however, experimental studies showed that it exists in two forms sensitive to blue light of both low and high intensity [8]. In our circuit diagram, the same transistors are shown as one circuit element. The optical transistors VT1 and VT2 (Fig. 2) correspond to PHOT1 and PHOT2. The phototropins PHOT1 and PHOT2 correspond to solar cells 1 and 2 of Fig. 1. The cryptochromes CRY1 and CRY2 are represented by the optical transistor VT3 (CRY). The phytochromes Ph(A)660, Ph(A)730, Ph(B)660, and Ph(B)730 are represented by the optical transistor VT4 (Ph).
The optical relay VU2 controls the flow of electrons to the emitter of the optical transistor VT5 and imitates the dependence of glucose, amino acid, and nucleotide transport into the plant cell on the concentrations of ions and mineral substances transported from the root system. The transistor VT5 integrates the energy fluxes, with the integral signal from the system of optical transistors VT3 and VT4 being the base input current.
The transistor VT6 controls the current that flows to the electromotor M1 and light-sensitive panel 1, with the signal from the optical transistor VT1 (PHOT1) being the input base current. The transistor VT7 controls the current to the electromotor M2 and light-sensitive panel 2, with the signal from the optical transistor VT2 (PHOT2) being the input base current. These circuit elements imitate the phototropin’s ability to block the transport of both auxin, the growth hormone, and nutrients to the cells of leaves receiving less light.
As a consequence, the electromotors M1, M2, and M3 move solar cell G1, depending on the spectrum of incident radiation. The electromotors M1 and M2 allow movement of solar panels 1 and 2 in the horizontal plane and this corresponds to changing the active surface area and an increase in biomass production in leaves 1 and 2. The electromotor M3 allows movement of the photosensitive elements in the vertical direction, which imitates stem growth.
The accumulated energy is then distributed during the operation of the photoreceptors and activated signaling cascades. The analysis of the spectral parameters of photobiological processes enabled us to calculate the photon-energy flux of an artificial light source. This energy can potentially be used for photoreceptor activation and the phototropic reaction, i.e., photomorphogenesis. Thus, the spectral sensitivity of photoreceptors can be determined similarly to that of optical transistors.
THEORY OF OUR APPROACH
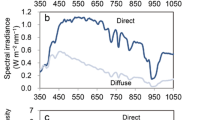
The energy produced and stored in the form of carbohydrates Cx(H2O)y can be estimated from theoretical considerations. Figure 3 shows the action spectra of plant photobiological reactions: photosynthesis, Kph(λ); dissipation of energy as heat, Kh(λ); phototropic reaction, Kphtrop(λ); chlorophyll synthesis, Kchl(λ); photomorphogenesis mediated by phytochrome 660, KP660(λ); and photomorphogenesis mediated by phytochrome 730, KP730(λ).
The energy produced and stored in the form of carbohydrates as a result of the absorption of visible light quanta can be estimated from the spectral efficiency of photosynthesis Kph(λ) in combination with the absorption spectra of leaves.
The photon flux density, at wavelength λ, of photosynthetically active radiation can be calculated using the formula
where D(λ) is the photon flux density of the light source and Kn(λ) is the absorption coefficient of an n-th leaf (Fig. 4). Figure 4 shows the absorption spectra of cotyledons 1 (C1) and 2 (C2) and mature leaves 1 (L1) and 2 (L2); each of these spectra was obtained by averaging ten measurements.
To find out the number of carbohydrate molecules produced due to the absorption of incoherent radiation, we must know the energy capacity of each spectral region. The energy transferred by one mole of photons with the wavelength λ is calculated as below:
where Na is Avogadro’s number.
Theoretically, to cover the energy expenditures associated with the conversion of 1 mole of carbon to a carbohydrate through the combination of water and carbon dioxide, a reaction which also gives rise to dioxygen, at least 120 kcal/mol (502.080 kJ/mol) of energy is required, which corresponds to three red light quanta (680 nm):
Emerson and Arnold [13], however, established that in nature this process requires more energy, and the quantum requirement measures eight to ten quanta per absorbed carbon dioxide molecule and resulting dioxygen molecule; i.e., with the maximum consumption of ten photons, the required energy is \({{E}_{{{\text{C}}{{{\text{O}}}_{2}}}}}\) = 1760 kJ/mol. Considering this, the minimum quantum requirement at wavelength λ is
Considering that it is thought that a single photon can contribute to an elementary chemical reaction, the minimum quantum requirement γ(λ) should be rounded to the next nearest integer.
Using the data on the effective photon number Dn(λ) and the quantum requirement of photosynthesis γ(λ), the intensity of carbon dioxide conversion to carbohydrates for an n-th leaf can be calculated with the formula (units: \([{{I}_{{{\text{C}}{{{\text{O}}}_{2}}n}}}(\lambda )] = [{{\mu {\text{mol}}} \mathord{\left/ {\vphantom {{\mu {\text{mol}}} {{{{\text{m}}}^{2}}\,\,{\text{s}}}}} \right. \kern-0em} {{{{\text{m}}}^{2}}\,\,{\text{s}}}}]\)):
Let us denote the surface areas of the first and second cotyledon leaves and the first mature leaf by S1c, S2c, and S2m, respectively, and λ1–λ2 is the wavelength range of incoherent radiation. The number of converted carbon-dioxide molecules can then be calculated as below:
where \({{t}_{1}}\) and \({{t}_{2}}\) are the time moments at which the surface irradiation started and ended and n is the leaf number (the first and second cotyledon leaves and the first mature leaf). The total number of converted CO2 molecules is
To find the amount of synthesized carbohydrates from the mass of converted carbon dioxide, we must make use of the correction factor k1 = 0.64, as 1 g of CO2 absorbed by a plant is equivalent to 0.64 g of produced carbohydrates.
The rate of diffusion of carbon dioxide to the cells of plant photosynthetic organs is another limiting factor. Normally, photosynthesis requires a continuous supply of carbon dioxide to the cells. As a leaf absorbs carbon dioxide, a concentration gradient of the gas is established, so that the diffusion of CO2 occurs toward the plant’s photosynthetic organs. According to Fick’s law, the rate of diffusion is directly proportional to the concentration gradient and inversely proportional to the diffusion resistance. Carbon dioxide diffuses from the atmosphere into leaves and then into intercellular space, and from intercellular ducts to cells and then to chloroplasts. The faster carbon dioxide is used during photosynthesis, the lower is its partial pressure inside the intercellular ducts and the higher the CO2 flux toward the ducts [14].
We must also take into account the expenditure of synthesized carbohydrates for plant respiration. With the dependence of the rate of CO2 absorption on the irradiation intensity [15] given in Fig. 5, energy losses and alteration of the carbon-dioxide flux due to respiration can be taken into account by introducing the correction factor k2:
where Vabs and tabs are the rate and duration of CO2 absorption, respectively; Vresp and tresp are the rate and duration of respiration, respectively; and Vabs,max is the maximum possible rate of CO2 absorption.
With all the correction factors considered, the amount of produced carbohydrates Сх(Н2О)у is
where \(M{{r}_{{{\text{C}}{{{\text{O}}}_{2}}}}}\) = 44.01 g/mol is the carbon-dioxide molar mass.
With each synthesized glucose molecule, the Gibbs free energy ΔG increases. Knowing the total number of synthesized glucose molecules and ΔG of the glucose-synthesis reaction per glucose molecule, we can calculate the increase in the system total free energy due to radiation absorption during photosynthesis:
Photosynthesis redox reactions can also be described in terms of the redox reaction potential ΔE, which is related to the reaction Gibbs free energy via the expression [16]
where \(F\) is the Faraday number (the charge of 1 mol of elementary charges, 96.480 (A s)/mol).
The redox potential and the amount of synthesized chemical energy can be used in modeling a plant’s photosensitive system.
EXPERIMENTAL
If the lateral dimensions of a plant leaf, the length L and width W, are known (Fig. 6), we can estimate the leaf surface area S theoretically. We made estimates of the leaf surface area for cotyledon and mature leaves.
The shape of a cotyledon leaf is ellipsoidal (Fig. 6a) [17]; therefore, the leaf surface area is calculated using the formula
A mature leaf has a more intricate shape, as illustrated in Fig. 6b. To estimate the leaf surface area via known lateral dimensions, different regression models can be applied [18]. The leaf surface area of the first mature leaf can be determined from the regression equation
In this way, we can compare the theoretical estimations of the amount of photosynthesis products produced and stored in the plant to the experimental data upon weighing dried tissue of the plants that were exposed to radiation sources with different spectral compositions.
The dynamics of the biological development of plants and the distribution of the chemical energy, as produced by photosynthesis during plant development, can be obtained from daily measurements of a leaf’s lateral dimensions. We studied the effect of two different light sources—a DNaT 250 W tubular sodium arc lamp and a SID 46 W light-emitting diode lamp (their emission spectra are shown in Fig. 7 [10])—upon plant growth. The data on the rate of carbohydrate synthesis are shown in Fig. 8; we note that the measurement day corresponds to the synthesis rate during the following 24 hours. Listed in Table 1 are the theoretical estimates of the total amount of synthesized carbohydrates, \({{M}_{{{{{\text{C}}}_{x}}{{{({{{\text{H}}}_{{\text{2}}}}{\text{O}})}}_{y}}}}},\) and average weight of dried specimens (experimental data).
With the available data on the variation of the leaf absorption spectrum at different stages of development as well as spectra of the efficiency of photobiological reactions [6], the effect the spectrum of incident visible radiation has on the biological systems of plants at different stages of development can be assessed. The potential spectrum efficiency Deff for photobiological reactions can then be obtained via the expression
where Keff(λ) is the efficiency of a photobiological reaction (Fig. 3). The effect of the radiation spectrum of different light sources on the efficiency of photobiological reactions was estimated at four development stages of vegetative growth of the cucumber hybrid Cucumis sativus L. “Kuraj”; namely, the first and second cotyledons and the first and second mature leaf were studied.
Energetic effects in plants are known to require from tens to hundreds times more energy input than photoregulatory ones, but so far the exact number has not been established. In studies on modeling the efficiency of photobiological processes in plants, the following expression was proposed for the efficiency of regulatory processes:
Suppose that Dph, Dh, Dphtrop, Dchl, DP660, and DP730 are the effective radiation fluxes of corresponding photobiological reactions. The photon flux absorbed by a leaf at a particular stage of development is defined by the expression
where Dabs is the photon flux density of absorbed photons for a particular light source.
Due to the extreme complexity of both individual system elements and the whole system, we will simplify matters by assuming that the coefficient Kusg takes the same value for all considered regulatory processes. That said, the total flux of absorbed radiation having a particular spectrum can be transformed to give the expression
where Kusg is the coefficient of photon energy usage in regulatory processes controlled by photoreceptors. The photon usage coefficient is calculated according to the expression
At a low photon flux density of photosynthetically active photons, the heat losses due to the absorption and conversion of incident radiation are negligible. Formula (16) can then be adjusted for a SID 46 W lamp to give the expression
On the basis of the results of modeling, we obtained estimates for Dph, Dh, Dphtrop, Dchl, DP660, DP730, and the values of Kusg for each spectrum (see Table 2).
RESULTS AND DISCUSSION
By the time the first measurement of the leaf lateral dimensions was taken, the plant tissues and root tissues had formed; i.e., the weight of synthesized carbohydrates must be lower than the weight of dried plant tissue. The theoretically calculated weight of synthesized carbohydrates and its experimental value agreed to within an order of magnitude. The differences can be rationalized by recognizing that the correction factor k2 does not take into account the spectral features of the used light sources and that it indirectly points to the existence of a dependence of CO2 diffusion on the gas-exchange rate. The factor k1 was statistically averaged and all features of the natural (real) process were not considered either. We thus see that our model for the estimation of photosynthesis dynamics in relation to the spectrum of incoherent incident radiation can be employed in numerical modeling of the effects of different light sources. It can also be used in the development of a qualitative model of a plant. We showed that the energy of the produced carbohydrates is redistributed during plant morphogenesis. With our measurements of the dry weight of different plant organs taken during vegetation and theoretical estimations of the effects of the incident radiation spectrum on the dynamics of carbohydrate synthesis, it is possible to obtain estimates for chemical-energy fluxes that are controlled by different plant photoreceptor systems, and this may be used in designing a solid state model of the biological system of a plant.
CONCLUSIONS
The qualitative model proposed in this study creates the conditions for theoretical calculations of the energy consumption in a complex system by using numerical methods and for projecting the spectral efficiency of light sources in photoregulated and energetic processes via feedback with a plant. The approach proposed here for modeling the effects of the light spectrum on processes that take place in plants can be adapted for modeling a variety of biological systems, e.g., the use of low-intensity irradiation in medicine and animal husbandry as well as in modern hybrid high-technology systems for the conversion of radiant energy, to name a few. We carried out a theoretical study of the effects visible-light radiation can have on the physical properties of condensed matter by the example of the plant biological system. In addition, we developed and investigated the physical technological principles for improving, by using engineering approaches, equipment for the irradiation of living systems.
REFERENCES
B. Bhushan, Philos. Trans. R. Soc., A 367, 1445 (2009).
J. K. Patra and S. Gouda, J. Endocrinol. Thyroid Res. 5 (5), 104 (2013).
C. Liu, B. C. Colon, M. Ziesack, et al., Science 352 (6290), 1210 (2016).
X. Y. Wang, X. M. Xu, and J. Cui, Photosynthetica 53 (2), 213 (2015).
D. Singh, C. Basu, M. Meinhardt-Wollweber, and B. Roth, Renewable Sustainable Energy Rev. 49, 139 (2015).
V. N. Volkov, I. I. Sventitskii, P. I. Storozhev, and L. A. Tsareva, Artificial Radiation Processing of Plants (Institute of Agricultural Chemistry and Soil Sciences Russ. Acad. Sci., Pushchino, 1982) [in Russian].
K. J. McCree, in Physiological Plant Ecology I (Springer, 1981), p. 41.
N. D. Alekhina, Yu. V. Balnokin, V. F. Gavrilenko, et al., Physiology of Plants (Akademiya, Moscow, 2005) [in Russian].
S. I. Supel’nyak, V. G. Kosushkin, and S. A. Adarchin, Elektromagn. Volny Elektron. Sist. 22 (8), 25 (2017).
S. I. Supel’nyak, V. G. Kosushkin, and S. A. Adarchin, Elektromagn. Volny Elektron. Sist. 22 (7), 21 (2017).
H. Kasemir, Cell Biol. Int. Rep. 3 (3), 197 (1979).
M. A. Grusak, M. R. Broadley, and P. J. White, Plant Macro- and Micronutrient Minerals, in Encyclopedia of Life Sciences (Wiley, 2016). https://doi.org/10.1002/9780470015902.a0001306.pub2
R. Emerson, Annu. Rev. Plant Physiol. 9 (1), 1 (1958).
N. I. Yakushkina and E. Yu. Bakhtenko, Physiology of Plants (VLADOS, Moscow, 2004) [in Russian].
M. A. Alam, MT Thesis (Norwegian Univ. of Life Sciences, 2016).
H.-W. Heldt, Pflanzenbiochemie (Spektrum Akademischer Verlag, Heidelberg, 2003).
F. G. Gregory, Ann. Bot., No. 1, 93 (1921).
F. F. Blanco and M. V. Folegatti, Hortic. Bras. 21 (4), 666 (2003).
ACKNOWLEDGMENTS
This work was supported by the Federal Agency for Scientific Organizations (agreement no. 007-GZ/Ch3363/26).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated by A. Kukharuk
Rights and permissions
About this article
Cite this article
Supelnyak, S.I., Kosushkin, V.G. Spectral Photoresponse of Biological Systems. J. Surf. Investig. 13, 525–532 (2019). https://doi.org/10.1134/S1027451019010348
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1027451019010348