Abstract
Results of the study of pollen emission from 32 anemophilous plant species into the atmosphere are presented. The percentage of clusters of two or more pollen grains in the total number of pollen particles entering the atmosphere is estimated. It is shown that despite the presence of morphological features that prevent the formation of clusters in anemophilous plants, similar clusters were formed in significant quantities in all series of the experiments. The percentage of pollen clusters ranged from 6.8 to 66.1% of the total number of pollen particles; the percentage of pollen grains in the composition of the clusters varies from 15.3 to 94.8%.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Transport of pollen of anemophilous seed plants in the atmosphere is the necessary condition of their propagation by seeds and genetic exchange between individuals and remote populations. Anemophily is peculiar to gymnosperms and representatives of 60 families of angiosperms (∼16% of the total families richness [1]). Ranking below entomophilous plants in the number of species (in Novosibirsk region, 29.5% [2]), anemophilous plants predominate in the number of individuals in the vegetation cover of extratropical regions (53% of the land surface without Antarctica) and are main biomass producents.
Anemophilous plants feature high pollen production. For example, production of radiata pine in the forests of New Zealand reaches 1–3 t of pollen per one hectare [3]. An enormous number of pollen grains (PGs) entering the atmosphere turns pollen into one of the most massive and ubiquitous components of the coarsely dispersed fraction (d > 1 µm) of bioaerosol [4]. During the blossom season of white birch, the contribution of its pollen to the total mass concentration of atmospheric aerosol (AA) amounts to 16.4% on the average and reaches 57–66% in individual samples [5]. Anemophilous plant pollen plays an important part in the transport of chemical elements in biocoenoses [6], has an effect on optical properties of the atmosphere [7], and causes allergic diseases in 30% of the population [8, 9].
To predict the pollen content in the atmosphere, networks for monitoring the AA pollen component were deployed. As of 2016, there were 879 active stations collecting samples of pollen aerosol in the world; among them, 9 in Africa, 151 in America, 182 in Asia (143 in Japan), 525 in Europe, and 12 in Oceania [8] (https://www.zaum-online.de/pollen-map.html). In the Russian Federation, there are nine stations (in Moscow, St. Petersburg, Yekaterinburg, Krasnodar, Perm, Rostov-on-Don, Ryazan, Stavropol, and Tyumen) (https://allergotop.com).
At 70% of stations, pollen is collected according to Hirst (Hirst, Burkard, and Lanzoni traps). At the remaining 30% of stations, automatic and Rotorod samplers are used. Large size of PGs of anemophilous plants (the average diameter is 20–40 µm) and complex morphology of pollen particles (PPs) [10, 11] are the causes of significant difficulties when collecting AA samples. Sampling by the devices used is nonisokinetic [12, 13], it is accompanied by destruction of clusters [14], and the sampler’s ability to grade particles into sizes is insufficient [8]. Moreover, even in some European regions, there are few observation points, which makes the quality of the pollen transport prognosis questionable [8].
In the Russian Federation, the large area of the country and low population density call into question the possibility to deploy a sufficient number of stations for AA pollen monitoring. It seems more promising to construct pollen transport models based on taking into account the pollen production of plants and physical processes of dissemination, atmospheric transport, and sedimentation of PPs. The distance of PP transport in the atmosphere is determined by the source height, wind velocity, turbulence, vertical coefficient of diffusion, and PP sedimentation rate. The latter depends on the size and density of PGs and cluster composition of PPs entering the atmosphere.
In this work, pollen emission into the atmosphere is studied for 32 species of anemophilous plants displayed in the Central Siberian Botanical Garden, Siberian Branch, Russian Academy of Sciences.
MATERIALS AND METHODS
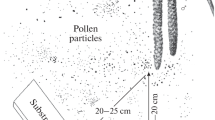
Pollen was blown by wind from inflorescences to slides covered with glycerin gelatin with addition of Coomassie blue (Fig. 1). The distance from the substrates was 20–25 cm, which made it possible to avoid the contact with the inflorescences. Pollen samples of each species were collected fivefold with short intervals.
The exposure lasted several seconds. At the same time, using the Center 311 device, the temperature and relative air humidity were measured. The pollen particles (individual PGs and their clusters) were counted on ten transects at 10–40-fold magnification of the microscope lens (Fig. 2).
Pollen grains of anemophilous plants have a set of features impeding their coalescence and formation of clusters. One can assume that individual PGs enter the atmosphere during the emission from anthers, and the clusters are formed on the slide.
The number of possible clusters of at least two PGs on the substrate was estimated under the following assumptions: (i) sedimentation of PGs onto the substrate does not depend on sedimentation of other PGs and (ii) PGs position themselves in a cluster in one layer. With an increase in the number of PGs in clusters, their number decreases. If the mathematical expectation of the number of clusters of N PGs is less than unity, then particles of at least N PGs are not formed on the specimen at a given number of PG per unit area.
A cluster is formed if the distance between the geometric PG centers does not exceed two radii. The mathematical expectation of the number of clusters of at least two PGs can be represented by the relation
where N≥ 1 is the number of PGs in clusters of at least one PG (in fact, the total number of PGs settled on the substrates);
is the fraction of the surface occupied by PGs, Spg is the average area of PG projection, and ST is the area of the examined specimens.
In the common use center of microscopic analysis of biological objects at the Institute of Cytology and Genetics, Siberian Branch, Russian Academy of Sciences, photographs of ∼200 PGs of all examined plant species were taken. The areas of PG projections were found by processing their image with the MapInfo Professional geoinformation system.
The mathematical expectation of the number of clusters of two PGs can be estimated by the relation
where
is the mathematical expectation of the number of clusters of at least three PGs.
The mathematical expectations of the number of clusters N≥ j and Nj of at least j PGs can be represented by the relations
The mathematical expectation of the number of individual PGs
After sedimentation of pollen, PGs appear on the substrate either individually or as a component of a cluster. Therefore, the problem is reduced to comparison of fractions of individual PGs of the total number of PGs settled on the substrates, i.e., to estimation of the significance of differences between two samples in the frequency of occurrence of this index characterized by an alternative distribution. For this purpose, the Fisher criterion with the φ-transformation (Fisher angular transformation) was used. It is intended for comparison of two samples in the frequency of occurrence of an index examined:
where φ1 and φ2 are the transformed fractions and Na ≥ 1 and Nb ≥ 1 are the sample volumes (in this case, the total numbers of PGs on the substrates). The value obtained was compared with the tabular one at the given level of significance (α) and number of the degrees of freedom df1 = 1 and df2 = Na ≥ 1 + Nb ≥ 2 − 2.
When the sample volumes number in hundreds and thousands, F = 3.8 at α = 0.05; 6.6 at α = 0.01; and 10.8 at α = 0.001. If the calculated values of F exceed the abovementioned levels, the null hypothesis at the given level of significance should be rejected [15].
RESULTS
The cluster composition of the pollen entering the atmosphere from 14 species of woody plants, 11 species of grasses, and 7 species of herbs has been studied.
Clusters of at least two PGs are found in the collected pollen samples of all anemophilous plants (Tables 1–3). For woody plants (Table 1), the percentage of clusters of at least two PGs of the total number of PPs settled on the substrate varies from 6.8 (Alnus glutinosa) to 66.1% (Salix glauca). The cluster composition includes 15.3–94.8% of the total number of PGs settled on the substrates. Such a wide variation range is apparently caused by differences in morphology of pollen shells in species of different families and different flowering phases.
For grasses (Table 2), the percentage of clusters in the total amount of PPs varies from 11.5 (Arrhenatherum bulbosum) to 32.3% (Poa alpina). Their composition included from 27.3 (Panicum virgatum) to 61.9% of the total amount of PGs settled on the substrates. Morphology of pollen shells is monotypic and the differences seem to be caused by different flowering phases.
For other herbaceous plants, herbs (Table 3), the percentage of clusters in the total amount of PPs varies from 25.2 (Plantago lanceolata) to 67.9% (Carex altaica). Their composition included from 52.7 (Carex vesicaria) to 90.8% of the total amount of PGs settled on the substrates. The variation is apparently caused by differences in morphology of pollen shells in species of different families and different flowering phases.
Tables 4–6 present (i) the total number of pollen grains settled on the substrates (N≥ 1); (ii) the percentage of the specimen surface occupied by pollen grains (p, %); (iii) actually observed number of clusters of j PGs on the substrates (nj) and its mathematical expectation (Nj); and (iv) calculated values of the Fisher criterion (F) when comparing fractions of individual PGs.
The total area of the projection of PGs settled on the substrates for woody plants (Table 4) varies from 0.03 to 3.46% of the examined specimen surface. The cluster compositions of pollen sedimentations on the substrates differ markedly from those that would appear with the formation of clusters on slides after sedimentation of individual PGs on them. Values of Fφ vary from 15.4 to 113.9, which considerably exceeds its value at α = 0.001.
The area of the projection of settled PGs for grasses (Table 5) varies from 0.03 to 0.4% of the examined specimen surface. Values of Fφ vary from 16.4 to 45.2, which considerably exceeds its value at α = 0.001.
The area of the projection of settled PGs for herbs (Table 6) varies from 0.05 to 1.07% of the examined specimen surface. Values of Fφ vary from 33 to 64.8, which considerably exceeds its value at α = 0.001.
On the basis of the foregoing, one should discard the original hypothesis about the formation of pollen clusters directly on the substrate for the studied 14 species of woody plants, 11 species of grasses, and 7 species of herbs. Their clusters are formed directly in anthers as the pollen enters the atmosphere.
CONCLUSIONS
The cluster composition of pollen entering the atmosphere has been studied for ∼1/12 of the total number (417) of anemophilous plants of Novosibirsk region. The anemophilous plant pollen entering the atmosphere is not monodispersed. In addition to individual PGs, clusters containing two and more PGs enter the atmosphere. The percentage of such clusters of the total number of forming PPs varies over a wide range and can be markedly different in different plant species. Such variation is apparently caused by differences in morphology of PG shells and different flowering phases of the anemophilous plants.
Morphological features of the structure of pollen grains of anemophilous plants do not prevent the formation of clusters in the process of pollen emission into the atmosphere.
Anemophilous plant pollen entering the atmosphere is not monodispersed but represented both by individual PGs and by their clusters of two or more PGs.
The fraction of clusters of the total number of particles formed and the percentage of PGs in their composition vary over a wide range.
REFERENCES
J. D. Ackerman, “Abiotic pollen and pollination: Ecological, functional, and evolutionary perspectives,” Plant Syst. Evol. 222, 167–185 (2000).
I. M. Krasnoborov, M. N. Lomonosova, D. N. Shaulo et al., Classification of Plants of Novosibirsk Region (Nauka, Sibirskoe predpriyatie RAN, Novosibirsk, 2000) [in Russian].
G. Erdtman, Handbook of Palynolody (Munksgaard, 1969).
V. R. Despre, A. J. Huffman, S. M. Burrows, C. Hoose, A. S. Safatov, G. Buryak, J. Frohlich-Nowoisky, W. Elbert, M. O. Andreae, U. Posch, and R. Jaenicke, “Primary biological aerosol particles in the atmosphere: A review,” Tellus B 64, 1–58 (2012).
V. V. Golovko, K. P. Kutsenogii, and V. L. Istomin, “Number and mass concentrations of the pollen component of atmospheric aerosol measured near Novosibirsk during blossoming of arboreal plants,” Opt. Atmos. Okeana 28 (6), 529–533 (2015).
L. G. Greenfield, “Weight loss and release of mineral nitrogen from decomposing pollen,” Soil Biol. Biochem. 31 (3), 351–353 (1999).
S. Bohlmann, X. Shang, E. Giannakaki, M. Filioglou, S. Romakkaniemi, M. Komppula, and A. Saarto, “Detection and characterization of birch pollen in the atmosphere using a multiwavelength raman polarization lidar and hirst-type pollen sampler in Finland,” Atmos. Chem. Phys. 19 (23), 14559–14569 (2019).
J. T. M. Buters, C. Antunes, A. Galveias, K. C. Bergmann, M. Thibaudon, C. Galan, C. Schmidt-Weber, and J. Oteros, “Pollen and spore monitoring in the world,” Clin. Transl. Allergy 8, 9 (2018).
P. J. Beggs, “Allergen aerosol from pollen-nucleated precipitation: A novel thunderstorm asthma trigger,” Atmos. Environ. 152, 455–457 (2017).
L. A. Kupriyanova and L. A. Aleshina, Pollen and Spores of Dicotyledons in Flora of European Russia (Nauka, Leningra, 1978), vol. 1 [in Russian].
B. Crook, “Inertial samplers: Biological perspectives,” in Bioaerosols Handbook, Ed. by C.S. Cox and C.M. Wathes (Lewis Publishers, Boca Raton, Florida, 1995).
B. Crook, “Non-inertial samplers: Biological perspectives, in Bioaerosols Handbook, Ed. by C.S. Cox and C.M. Wathes (Lewis Publishers, Boca Raton, Florida, 1995).
N. A. Fuks, Mechanics of Aerosols (Publishing House of the Academy of Sciences of USSR, 1955) [in Russian].
V. V. Golovko, A. P. Belanova, and G. A. Zueva, “Study of the cluster composition of pollen particles entering the atmosphere during the bloom of anemophilic plants,” Opt. Atmos. Okeana 32 (6), 476–481 (2019). https://doi.org/10.15372/AOO20190610
E. V. Ivanter and A. V. Korosov, Elementary Biometry (Publishing House of Petrozavodskg State Univ., Petrozavodsk, 2010) [in Russian].
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
The authors declare that they have no conflicts of interest.
Additional information
Translated by A. Nikol’skii
Rights and permissions
About this article
Cite this article
Golovko, V.V., Zueva, G.A. & Kiseleva, T.I. Anemophilous Plant Pollen Grains Entering the Atmosphere: Cluster Composition. Atmos Ocean Opt 34, 483–490 (2021). https://doi.org/10.1134/S1024856021050092
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1024856021050092