Abstract
Electrical and dielectric properties of ternary glasses in the Sb2O3–PbCl2–AgCl system were investigated across a broad temperature and frequency range. The studied glass system is interesting since it possesses a high ionic conductivity. The (Sb2O3)x–(PbCl2)100 –y – x–(AgCl)y glasses were prepared by melt-quenching method from high purity components. Different batches of these glasses were investigated with varying molar content of both Sb2O3 (45 ≤ x ≤ 70 mol %) and AgCl (5 ≤ y ≤ 25 mol %). The colour of the prepared chloro-antimonite glasses varies between yellow and brown. The glass transition temperature (Tg) decreases with increasing AgCl concentrations. DC and AC electrical conductivities and complex electrical modulus were measured across a temperature range from room temperature up to 200°C and across a frequency range between 0.2 and 105 Hz. The dependence of DC conductivity on temperature follows the so-called Arrhenius-like equation. The DC conductivity at constant temperature significantly increases with increasing AgCl or PbCl2 content. It was found that the activation energy of conduction process decreases with the substitution of PbCl2 by AgCl from 1 eV down to 0.56 eV for (Sb2O3)50-(PbCl2)45–(AgCl)5 and (Sb2O3)50–(PbCl2)25–(AgCl)25, respectively. The influence of the composition on the AC conductivity of the investigated glasses is also discussed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Antimony oxide-based glasses, as a major family of heavy metal oxide glasses, show promising potential for applications in nonlinear optical devices such as ultrafast optical switches and/or power limiters [1–4]. They also have potential for application in broadband optical amplifiers operating around at 1.5 μm, and silicate glasses containing antimony were tested for optical amplification in communications in the C-band (1530–1560 nm) [5].
Stable binary glasses are formed in the (Sb2O3)1 – x–(PbCl2)x system [6, 7], which enables additions of other oxide compounds such as MoO3 or TeO2 and/ormetal halides (CuI, LiCl, ZnCl2), as related in other works [7–15].
The Sb2O3–PbCl2–AgCl glass system is particularly interesting due to its high ionic conductivity due to the presence of Ag. In addition to electrical conductivity, these glasses are transparent across a wide optical range (400 nm–6.5 µm) and they thus have a potential for applications in optoelectronics [16].
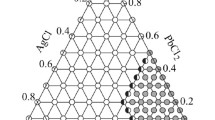
In this paper, the compositions of the investigated glasses belong to a wide range of compositions for which [Sb2O3] ∈ (45; 70), [PbCl2] ∈ (5; 40) and [AgCl] ∈ (5; 25). The glass transition temperatures of the prepared glasses are given. Moreover, the electrical and dielectric properties of the Sb2O3–PbCl2–AgCl glasses across a broad range of temperatures and frequencies are presented. This allows the temperature dependence of DC and AC conductivity as well as the dielectric properties of the glass samples to be investigated.
EXPERIMENTAL
The glasses were prepared using the standard processing steps of melting homogenised mixtures of the starting compounds, fining, cooling, melt casting, and annealing of glass bulks. Starting materials—99.9% Sb2O3 (Acros organics), 99% PbCl2 (Hichem), and 99.9% AgCl (Alfa Aesar)—were thoroughly mixed in an agate mortar and placed into a silica tube. Silica is not an ideal material for preparing this glass as it gradually dilutes in the glass melt. However, it is a better choice than platinum or gold, which both can be attacked by metallic particles resulting from oxidation-reduction reactions during heating of the batch and the glass melting process [16]. The contamination of the melt by SiO2 can be kept under the limit of detection by EDS semi quantitative chemical analysis if the melting time is kept as short as possible just to obtain a homogeneous glass melt [7]. During the melting process, the tube fills up with vapours from the glass melt which prevent the contact of the melt with ambient air and/or with combustion products from the flame used for heating.
After fining at about 850°C the melt was rapidly cooled down to approximately 600°C and poured on a brass plate preheated to 250°C (near the glass transition temperature Tg). After solidification, the sample was placed into an oven heated at Tg to remove thermally induced stresses. After a few hours at Tg, the temperature of the oven is slowly decreased down to room temperature [17].
The glass transition temperature Tg was measured by heating at a linear slope of 10°C/min using the TA Instruments DSC Q20 differential scanning calorimeter.
Samples for measurements of electrical and dielectric properties were cut and polished, and contact surfaces were coated with a conductive graphite layer. The DC conductivity was determined by measuring the electric current passing through the sample at a constant voltage of 10 V by Novocontrol Concept 90, across a temperature of 20–200°C. The current was measured by the Keithley 6517B picoammeter and the temperature was controlled by a Pt/PtRh thermocouple with an accuracy of ±1°C. Temperature dependence of the DC conductivity was measured during the phase when the temperature increased at a rate of 5°C/min [16].
The AC measurements (from 20 up to 150°C) were performed using the LCR Hi-tester Hioki 3522-50 at a frequency range 100 Hz–100 kHz. The measurements were performed in steps of 10°C after the temperature remained constant for 20 min [9, 18, 19]. Typically, as the frequency increases, the influence of electron transport processes increases, so at higher frequencies it is possible to see the influence of chemical elements for which different valence states are possible (Sb3+, Sb5+, W6+, W5+).
RESULTS
The acronyms of the respective glass compositions and their corresponding compositions expressed in molar percentages are shown in Table 1. The table summarizes also the electric and dielectric properties described in following text. The dependencies of the transition temperature Tg on the AgCl content in the studied Sb2O3–PbCl2–AgCl glasses are shown in Fig. 1. The Tg values decrease with increasing AgCl content in glasses with both 50 and 70 mol % Sb2O3. The temperature dependence of DC conductivity, σdc, of the investigated glasses is presented in Fig. 2. At temperature sranging between room temperature and 200°C the observed dependences follow Arrhenius-like equation
where σ0 is the pre-exponential factor, Edc is the conduction activation energy, k is the Boltzmann constant, and T is the thermodynamic temperature. The determined parameters of Eq. (1) for the linear parts of the recorded temperature dependences of DC conductivity are summarized in Table 1.
The AC conductivity σac of Sb2O3–PbCl2–AgCl glasses increases with increasing temperature and frequency. In Fig. 3, frequency dependencies of AC conductivity measured at 150°C are presented. AC conductivity frequency dependency can be described using the following formula
where f is the frequency, and A and n are the parameters. The value of σ01 corresponds to DC conductivity, and its values for the temperature of 150°C are shown in Table 1. The value of σ01 is subject to an error due to the fact that it is an extrapolated value and also that the contribution of other carriers of electric charge (for example electrons) is more pronounced at higher frequencies in AC measurements than in DC measurements (mainly ionic conductivity in the investigated glasses). Therefore in some cases the σdc and σ01values may differ significantly from each other. The parameter A increases and the exponent n slightly decreases with increasing PbCl2 and AgCl contents.
The dielectric response was studied using modular spectroscopy. Complex electrical modulus M* was introduced [20–22] as the reciprocal value of the complex permittivity ε* by the equation
The measured modular spectra, i.e. the dependencies of complex electrical modulus in complex plane, are shown in Fig. 4. At lower AgCl content, the shapes of modular spectra can be characterized as semicircles with a centre just below the real axis with linear tails appearing at high frequencies. In these cases, dielectric relaxation can be characterized by a relatively narrow range of relaxation times. At higher AgCl content, the centres of the semicircles shift more under real axis. Consequently, the range of relaxation times expands.
The relaxation times as a function of temperature τ = τ(T) were calculated as reciprocal value of angular frequency 1/(2πfm), where fm is the frequency of maxima M " obtained from frequency dependency of the imaginary part of complex electric modulus. As an example, Fig. 5 shows these dependencies of M " for glass E30-15 at various temperatures. For glasses where relaxation time values could be determined, the temperature dependencies of relaxation times follows the Arrhenius equation (see Fig. 6).
Activation energy Eτ for Arrhenius-type dielectric relaxation was determined using the expression
where τ0 is the pre-exponential factor, Eτ is the conduction activation energy, k is the Boltzmann constant, and T is the thermodynamic temperature. The value of Eτ correlates with the value of Edc (Table 1), i.e. the activation energy of the dielectric relaxation is affected by transport of the same type of electric charge.
DISCUSSION
The decrease of Tg values with increasing AgCl content in investigated glasses corresponds to the AgCl roleplays as a modifier in the antimonite glass network. According to [16], the increase in the molar concentration of AgCl leads to decrease of Tg values due to the formation of weaker Ag–Cl chemical bonds instead of stronger Sb–O bonds. The glass can accept only certain amount of modifiers without deterioration of the glassy network stability. As a consequence, the glass stability and density decrease significantly at high AgCl concentrations. As far as DC conductivity is concerned, temperature dependencies can be well described by the Arrhenius law with a single dominant mechanism of electric charge transport. In terms of the glass composition, polaronic conductivity Sb3+–Sb5+ and/or ionic conductivity by means of Ag+, or Cl– ions, respectively, could be considered as probable transport mechanisms. Transition from polaronic conductivity to ion conductivity mediated by Ag+ ions at lower concentrations of AgO (>0.1 mol %) has been reported recently [23]. It could be assumed that Ag+ ions are dominant charge carriers in Sb2O3–PbCl2–AgCl glasses. The DC conductivity values increase and the DC conductivity activation energy decreases with increasing AgCl content. The contribution of Cl– ions to DC conductivity is probably not significant. The DC conductivity values also increase with higher PbCl2 content. It is important, that this compound causes the increase of mobility of ions due to a relaxation of the glass network and an increase of the molar volume [16]. The authors investigated the possibility of electric transport using Cl– ions in detail in a similar glass system of Sb2O3–PbCl2–LiCl [14]. Their analysis showed that the influence of Cl– ions on electrical conductivity is negligible. It means that the DC conductivity increases at higher AgCl and PbCl2 contents due to the expansion of the glass network lead to an increase of the ion mobility. Moreover, in the case of AgCl an increase of the concentration of charge carriers occurs. The glass network relaxation is connected with a decrease of activation energies of DC conductivity in an interval ranging from 1 eV (A45-05) to 0.56 eV (A25-25). The A25-25 glass also reaches the highest value of conductivity at 150°C.
At measured temperatures, AC conductivity increases with increasing angular frequency and changes according to the power-law described by relation (2). The changing values of σ01 and A shown in Table 1 suggest that the dominant underlying mechanism of AC conductivity is the transport of the same type of electric charge as in the case of DC conductivity (σdc (150°C) in Table 1). The shape of the measured modular spectra (Fig. 4) indicates significant influence of ion movement. Also the activation energy of dielectric relaxation corresponds to the activation energy of DC conductivity. This also means that the influence of Ag+ ions transport is significant.
CONCLUSIONS
The results obtained by electrical measurements and the observed dependencies of the parameters of the studied materials show that the conductivity of Sb2O3–PbCl2–AgCl glasses show an Arrhenius-type behaviour. The dominant mechanism of charge transport in the investigated glasses is an ionic mechanism involving Ag+ ions. On the other hand, the contribution of Cl– ions and/or electrons to the transport of electric charge in these materials is less significant.
The values of direct electric conductivity are spread across a range spanning 5 orders of magnitude. The highest value of DC conductivity, around 3.6 × 10−5 S m−1 at 150°C with a corresponding activation energy of 0.56 ± 0.03 eV, was obtained for the (Sb2O3)50–(PbCl2)25–(AgCl)25 glass. Positive influence of the addition PbCl2 on electric mobility of the charge carrier Ag+ has been highlighted. The transport of electric charge by Ag+ ions has also an effect on values of alternate electric conductivity and dielectric relaxation of glasses.
REFERENCES
Dubois, B., Aomi, H., Videau, J.J., Portier, J., and Hagenmuller, P., New oxyhalide glasses involving Sb2O3, Mat. Res. Bull., 1984, vol. 19, no. 10, p. 1317.
Zavadil, J., Ivanova, Z.G., Kostka, P., Hamzaoui, M., and Soltani, M.T., Photoluminescence study of Er-doped zinc-sodium-antimonite glasses, J. Alloy. Compd., 2014, vol. 611, p. 111.
Soltani, M.T., Hamzaoui, M., Houhou, S., Touiri, H., Bediar, L., Ghemri, A.M., and Petkova, P., Physical characterization of Sb2O3–M2O–MoO3 (M = Li, K) new glasses, Acta Phys. Pol. A, 2013, vol. 123, p. 227.
Hamzaoui, M., Azri, S., Soltani, M.T., Lebullenger, R., and Poulain, M., Thermal and elastic characterization of Sb2O3–Na2O–ZnO glasses, Phys. Scr., 2013, vol. 157, p. 014029. https://doi.org/10.1088/0031-8949/2013/T157/014029
Minelly, J. and Ellison, A., Applications of antimony-silicate glasses for fiber optic amplifiers, Opt. Fiber Technol., 2002, vol. 8, p. 123.
Dubois, B., Videau, J.J., Couzi, M., and Portier, J., Structural approach of the (xPbCl2-(1-x)Sb2O3) glass system, J. Non-Cryst. Solids, 1986, vol. 88, p. 355.
Bošák, O., Kostka, P., Minárik, S., Trnovcová, V., Podolinčiaková, J., and Zavadil, J., Influence of composition and preparation conditions on some physical properties of TeO2–Sb2O3–PbCl2 glasses, J. Non-Cryst. Solids, 2013, vol. 377, p. 74.
Goumeidane, F., Legouera, M., Iezid, M., Poulain, M., Nazabal, V., and Lebullenger, R., Synthesis and physical properties of glasses in the Sb2O3–PbCl2–MoO3 system, J. Non-Cryst. Solids, 2011, vol. 357, p. 3572.
Labaš, V., Poulain, M., Kubliha, M., Trnovcová, V., and Goumeidane, F., Electrical, dielectric and optical properties of Sb2O3–PbCl2–MoO3 glasses, J. Non-Cryst. Solids, 2013, vol. 377, p. 66.
Macháček, J., Kostka, P., Liška, M., Zavadil, J., and Gedeon, O., Calculation and analysis of vibrational spectra of PbCl2–Sb2O3–TeO2 glass from first principles, J. Non-Cryst. Solids, 2011, vol. 357, p. 2562.
Gedikoglu, N., Ersundu, M.C., Kostka, P., Basinova, N., and Ersundu, A.E., Investigating the influence of transition metal oxides on temperature dependent optical properties of PbCl2–TeO2 glasses for their evaluation as transparent large band gap semiconductors, J. Alloys Compd., 2018, vol. 748, p. 687.
Poirier, G., Poulain, M., and Poulain, M., Copper and lead halogeno-antimoniate glasses, J. Non-Cryst. Solids, 2001, vol. 284, p. 117.
Cozic, S., Bréhault, A., Usuki, T., and Le Coq, D., GeS2–Ga2S3–LiCl glass system: electrical conductivity and structural considerations, Int. J. Appl. Glass Sci., 2016, vol. 7, no. 4, p. 513.
Castro, A., Bréhault, A., Carcreff, J., Bošák, O., Kubliha, M., Trnovcová, V., Dománková, M., Šiljegović, M., Calvez, L., Labaš, V., and Le Coq, D., Lithium and lead chloride antimonate glasses, J. Non-Cryst. Solids, 2018, vol. 499, p. 66.
Sahar, M.R., Ahmed, M.M., and Holland, D., The crystallisation of Sb2O3–PbCl2–ZnCl2 glasses, Phys. Chem. Glasses, 1990, vol. 31, no. 3, p. 126.
Yezli, D., Legouera, M., El Abdi, R., Poulain, M., and Burgaud, V., Mechanical, thermal, and optical properties of new chloroantimonite glasses in the Sb2O3–PbCl2–AgCl system, Mat. Sci., 2016, vol. 52, no. 1, p. 33.
Kubliha, M., Investigating Structural Changes and Defects of Non-Metallic Materials via Electrical Methods, 1 st ed., Dresden: Forschungszentrum Dresden-Rossendorf, 2009.
Kalužný, J., Kubliha, M., Labaš, V., Poulain, M., and Taibi, Y., Electrical and dielectrical properties of Sb2O3–V2O5–K2O glasses, J. Non-Cryst. Solids, 2009, vol. 355, nos. 37–42, p. 2031.
Kubliha, M., Soltani, M.T., Trnovcová, V., Legouera, M., Labaš, V., Kostka, P., Le Coq, D., and Hamzaoui, M., Electrical, dielectric, and optical properties of Sb2O3–Li2O–MoO3 glasses, J. Non-Cryst. Solids, 2015, vol. 428, p. 42.
Moynihan, C.T., Boesch, L.P., and Laberge, N.L. Decay function for electric-field relaxation in-vitreous ionic conductors, Phys. Chem. Glasses, 1973, vol. 14, p. 122.
Molak, A., Paluch, M., Pawlus, S., Klimontko, J., Ujma, Z., and Gruszka, I., Electric modulus approach to the analysis of electric relaxation in highly conducting (Na0.75Bi0.25)(Mn0.25Nb0.75)O3 ceramics, J. Phys. D: Appl. Phys., 2005, vol. 38, p. 1450.
Davidson, D.W. and Cole, R.H., Dielectric relaxation in glycerine, J. Chem. Phys., 1950, vol. 18, p. 1417.
Ashok, J., Kostrzewa, M., Ingram, A., Venkatramaiah, N., Srinivasa Reddy, M., Ravi Kumar, V., Piasecki, M., and Veeraiah, N., Structural and dielectric features of silver doped sodium antimonate glass ceramics, J. Alloy. Compd., 2019, vol. 791, p. 278.
Funding
This work was supported by the Slovak Science Foundations, projects VEGA 1/0235/18, VEGA 1/0144/20, APVV SK-FR-19-0007, and APVV DS-FR-19-0036, P. Kostka acknowledges the Czech Science Foundation—project no. 19-07456S and the Ministry of Education, Youth and Sports of the Czech Republic—project no. 8X20053.This publication is partially supported by the European Union through the European Regional Development Fund (ERDF), the Ministry of Higher Education and Research, the French region of Brittany and Rennes Métropole.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
CONFLICT OF INTERESTS
The authors declare that they have no conflict of interest.
INFORMATION OF PERSONAL CONTRIBUTIONS OF AUTHORS
D. Le Coq prepared samples of glasses for experiments and performed DSC experiments. O. Bošák and M. Kubliha performed measurements of direct electrical conductivity and modular spectra. S. Minarik analysed modular spectra. O. Bošák, M. Kubliha, M. Domankova, and P. Kostka wrote the first draft of the manuscript. All authors edited the manuscript and approved the final version.
ADDITIONAL INFORMATION
Authors ORCID ID. O. Bošák (0000-0001-6467-5398), M. Kubliha (0000-0003-4987-6233), P. Kostka (0000-0003-2868-1322), S. Minarik (0000-0002-6851-0053), M. Domankova(0000-0002-0595-1943), D. Le Coq (0000-0001-7898-3463).
Additional information
Based on the materials of the report at the 15th International Meeting “Fundamental Problems of Solid State Ionics,” Chernogolovka, 30.11.–07.12.2020.
Rights and permissions
About this article
Cite this article
Bošák, O., Kubliha, M., Kostka, P. et al. Electrical and Dielectric Properties of Sb2O3–PbCl2–AgCl Glass System. Russ J Electrochem 57, 681–687 (2021). https://doi.org/10.1134/S1023193521070041
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1023193521070041