Abstract
Almost all wheat species have an unbranched spike. Tetraploid rivet wheat Triticum turgidum L., branched forms of which are widespread and have been known for about 2000 years, is an exception. As for other wheat species, supernumerary spikelet forms are rare, and supernumerary spikelet/branched spike belongs to nonstandard morphotypes. As one of the examples illustrating the law of homologous series in variation, N.I. Vavilov presented the trait “spike branching” peculiar “not only to many wheat and rye species but also to many other genera with spike inflorescence or panicle.” The studies of genetic factors underlying the formation of “supernumerary spikelet/spike branching” trait and the study of peculiarities of the development of inflorescences of nonstandard branched wheat forms made it possible to demonstrate a genetic nature of hereditary variation of this trait. At the same time, the group of supernumerary spikelet/branched-lines is heterogeneous, and different genetic mechanisms can underlie the formation of branched spike. This review presents a retrospective of scientific studies devoted to the creation of supernumerary spikelet wheat forms and to the study of genetics of “supernumerary spikelet/spike branching” trait and demonstrates the results of modern studies of genetic regulation of morphogenesis of cereal inflorescences using supernumerary spikelet lines as genetic models.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Studying a vast diversity of cultivated plants and their wild relatives, Nikolai Ivanovich Vavilov for the first time drew attention to genetically determined similarity and relationship of the traits within the species, as well as larger taxa (plant genera and families). Identification of patterns in manifestation of polymorphism and establishment of polymorphism classes by analogy with homological series in organic chemistry led to the discovery of the law of homologous series in variation. Nikolai Ivanovich formulated the law and for the first time reported it at the Third Congress of Breeders in Saratov in 1920. The law was repeatedly published: initially as a small report in the proceedings of the Third Congress of Breeders, then in 1922, the article was published in the Journal of Genetics, and it was published in expanded version in Theoretical Foundations of Breeding in 1935 [1].
In the century since the discovery of the law of homologous series in variation, its main statements were confirmed by many examples. The universality of the law relative to all living organisms, including the members of the plant and animal kingdoms, was demonstrated. Presenting a formula of accurate facts based on evolutionary theory (according to Vavilov), the law became an integral part of modern phylogenetic concepts and principles of comparative genetics studying the genetic bases of parallelism in hereditary variation and determination of traits and properties. The ideas of Vavilov were developed in various fields of modern breeding.
With the development of molecular biology methods, the focus of research began to shift toward the study of molecular bases of parallel hereditary variation and identification of genetic factors determining homologous traits in genetically related taxa. The approach based on the use of natural diversity according to a certain trait and/or experimental mutagenesis underlies the positional cloning of genes. Another approach involves the isolation of genes by homology and uses the methods of comparative genetics and genomics: based on the structural organization of the genes well studied in model plant species and by its homology, the genes are isolated in other, often less studied, species. Thus, the law of homologous series in variation is directly related to the instruments of modern comparative genetics and genomics.
As examples illustrating this law, Vavilov gave a varietal (racial) variability of the members of Poaceae family, among which hereditarily varying morphological traits of the inflorescence and grain (caryopsis). The spike branching is one of such traits, which “as a race trait is peculiar not only to many wheat and rye species but also to many other genera with spike inflorescence or panicle inflorescence” [1]. The studies of this trait have a long history, and in the last decade, branched spike/supernumerary spikelet lines became widely used models for studying the genetics of cereal inflorescence development.
This review presents a retrospective of scientific studies devoted to the creation of supernumerary spikelet wheat forms and to the study of genetics of “supernumerary spikelet/spike branching” trait; special attention is paid to the studies of genetic regulation of morphogenesis of cereal inflorescences using supernumerary spikelet lines as genetic models.
WHEAT SUPERNUMERARY SPIKELET FORMS
Almost all wheat species have an unbranched spike. Tetraploid rivet wheat T. turgidum L. (BBAA) is an exception. Branched forms of T. turgidum have been known for about 2000 years; Plinius Maior mentioned them under the name ramosum and centigranum (23–79 AD) [2]. For a long history of existence, branched forms of rivet wheat received many different names: Miracle, Mummy, Egyptian, Seven-headed, etc. [2, 3]. In the 18th century, Carl Linnaeus singled out the branched wheat into a separate species T. compositum L. [2]. Subsequently, taxonomists considered it as a special group of the T. turgidum species or its branched varieties. At present, branched forms are allocated to the T. turgidum convar. compositum (L.f) A. Filat. group, which includes about twenty varieties: ramosolusitanicum Flaksb., nachitschevanicum Kulesh., alibekliense Thurn., columbinum (Alef.) Koern., cubinum Dorof., celvinum (Alef.) Koern., etc. [2]. All these branched forms of T. turgidum have a common spike phenotype: additional spikelets are developed on lateral axes (“branches”) of the spikelet rachilla and directly at spike rachis nodes. This type of a spike e was called turgidum type.
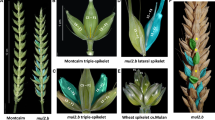
J. Percival [4] gives a detailed description of the turgidum type spike in a monograph The Wheat Plant: the upper part is normal, with a single spikelet at each notch of the rachis, a pair of spikelets placed side by side and arranged at right angles to each other are produced at a few of the lower notches. “Branches” or “secondary spikes” vary in length, the longest reach 3–4 cm, and contain 10–14 spikelets, whose grains often protrude from between the short glumes. The basal secondary spikes are often rudimentary, possessing diminutive spikelets (Fig. 1).
Wheat spikes of different morphotypes. (a) T. аestivum standard type spike; (b) T. turgidum branched spike (genuine branching (GB), ramified spike (RS)); (c) T. aestivum multirow spike (MRS); (d) T. аestivum GB (RS) spike; (e) T. аestivum spike with additional horizontal spikelets (four-rowed spike (FRS); synonym tetrastichon); (f) T. аestivum spike with additional vertical spikelets; (g) T. turgidum spike with false–true ramification (f-tR); (h) T. jakubzineri spike with sham ramification (SHR). Additional “horizontal” spikelet is designated by an arrow; additional “vertical” spikelet is marked by an asterisk.
Despite a wide prevalence, branched wheat T. turgidum is not cultivated on a large scale. The growing areas and ecological characteristics of the T. turgidum convar. compositum and T. turgidum convar. turgidum groups coincide in general; however, branched forms are absent among the Tibetan ecotype [2].
Among the members of T. durum Desf. (BBAA), branched forms are less common [2, 5]. In 1924, F.A. Coffman [6] reported the emergence of spontaneous supernumerary spikelet mutants in the crops of T. durum wheat of the Mindum cultivar. The spike phenotype of detected mutants differed from the turgidum type in the fact that two (less often three) additional sessile spikelets developed directly at a rachis node. In 1952, M.M. Jakubziner found branched forms of durum wheat in the Akmolinka 5 and Gordeiforme 10 cultivars and were assigned to new varieties ramosohordeiforme Jakubz. and ramosoapulicum Jakubz. [5]. During an expedition across Transcaucasia in 1961–1964, V.F. Dorofeev revealed the original forms of durum wheat with a branched spike (var. ramosohordeiforme and var. ramosoapulicum) under natural conditions of wheat growing, where many varieties of durum, rivet, and bread wheat were found [5].
Branched forms and forms with paired of spikelets were found in the T. dicoccum (Schrank) Schuebl. (BBAA) tetraploid wheat; a hybrid origin of some branched accessions of this species was noted. Branched forms were occasionally found among the samples of the tetraploid T. polonicum L. (BBAA) species [4].
Diploid wheat species (AA) have an unbranched spike; no branched forms of these wheat species were described [2, 7]. The induced mutants with branched spike of T. monococcum L. (AmAm) were obtained by Dr. Yamashita in the second half of the last century [8].
The formation of branched spike is not typical of the bread wheat T. aestivum L. (BBAADD), or of hexaploid wheat in general. According to F.A. Coffman [6], the bread wheat forms with additional spikelets at spike rachis nodes were described by Meunssier in 1918. According to the spike phenotype, these forms resembled spontaneous mutants of the durum wheat Mindum cultivar. J. Percival described the formation of additional (supernumerary) spikelets in the T. aestivum bread wheat, paying attention to the fact that they can develop (1) at right angle to the normally placed spikelet at one rachis node or (2) arranged in parallel to the normal spikelete [4]. Such additional spikelets (of second type) are often rudimentary and consist only of minute of misshapen glumes, but occasionally one to two grains are developed in them. Most often, additional spikelets of such type are found in Chinese bread wheat cultivars on latest tillers, while the spikes which developed first looks normal. The trait is manifested every season and can occur repeatedly [4].
Branched bread wheat forms can emerge as a result of wide hybridization [9, 10] and of the effect of mutagens [11–14]. Thus, M.S. Swaminathan et al. [14] reported obtaining the bread wheat N.P. 797 mutant with a turgidum type of spike as a result of the effect of the S35 isotope on the bread wheat seeds. The bread wheat lines with additional/supernumerary spikelets at a rachis node were isolated by V.M. Mel’nik [11] during the treatment of the seeds of Saratovskaya 29 cultivar with a chemical mutagen nitrosomethylurea.
Branched forms of bread wheat rarely appear spontaneously. S. Koric [15, 16] reported obtaining the branched form of bread wheat based on a spontaneous mutant (branched teratological plant found among the offspring from crossing the bread wheat cultivars with a normal spike). The spike phenotype of this form was similar to the turgidum spike type of T. turgidum convar. compositum. The trait was stably inherited and was transferred to different bread wheat cultivars, which it was proposed to assign to a separate group T. aestivum ramifera S.K. Tibetan triplespikelet wheat T. aestivum L. conv. tripletum is one more example of the spontaneous formation of supernumerary spikelet forms of bread wheat [17].
DIFFERENT MORPHOTYPES OF SUPERNUMERARY SPIKELET WHEAT FORMS
In the second half of the last century, it was proposed to use one general term “supernumerary spikelets (SS)” to describe branched spikes and spikes with additional sessile spikelets [18]. The term became widespread and is used at present to describe all supernumerary spikelet wheat forms.
The group of supernumerary spikelet wheat forms (SS forms) is heterogeneous. Already in early studies, S. Koric [15, 16], A.L. Pennell and G.M. Halloran [18] distinguished two types. The first type was called ramified spike, RS (a synonym, genuine branching, GB), in which the formation of lateral axes/ “branches” is observed, and additional spikelets are developed both at the rachis nodes and on the “branches.” This type includes a turgidum branching typeof T. turgidum. In the second type of supernumerary spikelet forms, the additional spikelets are sessile and are formed directly at a rachis node, most frequently two spikelets per the node. Such type was called four-rowed spike, FRS (a synonym, tetrastichon); it is found both in tetraploid T. durum and T. turgidum species [6, 19] and in hexaploid T. aestivum wheat [20].
Depending on the mutual location of additional sessile spikelets at rachis node in wheat, P. Martinek and J. Bednar [20, 21] proposed to distinguish several nonstandard spike morphotypes (Fig. 1):
(1) multirow spike (MRS)—clusters of supernumerary spikelets (up to ten spikelets) are located at a rachis node [20, 21];
(2) horizontal spikelets (HS) (synonym, tetrastichon sessile spikelets)—two or three spikelets are located in horizontal position at a rachis node; this morphotype includes the above-described FRS [20, 21];
(3) vertical sessile spikelets (VSS)—a pair of spikelets located at a rachis node one above the other in parallel plains; this phenotype was also called “banana spikelets” or “tween spikelets” [20–22];
(4) genuine branching (GB) morphotype completely corresponds to the previously described phenotype RS/turgidum type of spike branching [15, 16, 18].
Along with a true spike branching of the bread wheat, the spike of T. vavilovii (Thum.) Jakubz. (BBAADD) is also frequently called branched. However, the spike phenotype significantly differs from the spike with a turgidum type of branching, since additional spikelets at a rachis node of this wheat are not developed, but there is an elongation of the spikelet rachilla, on which many flowers are formed [2]. Such type of branching is called vavilovii, while the morphotype is called “sham ramification” (SHR) [20]. Tetraploid wheat T. jakubzineri Udacz. et Schachm. (BBAA) has a similar phenotype, false-branched spike [23]. It was demonstrated that the forms with a vavilovii type of spike branching can emerge as a result of wide hybridization [10].
Along with a true and false spike branching, a false–true ramification (f-tR) or false–true ramified spike (f-tRS) is distinguished [24]. In this morphotype, an elongated spikelet rachilla on which both supernumerary spikelets in the distal part and a pair of normal grains in the basal part are developed (Fig. 1). This morphotype was described in the accessions of T. turgidum tetraplod wheat [24].
Thus, supernumerary spikelet/branched wheat varieties are a heterogeneous group; the trait varies widely in its manifestation; and in order to distinguish homologous variability from similar variability, detailed studies of the spike using microscopy and genetic analysis were required.
PECULIARITIES OF DEVELOPMENT OF INFLORESCENCES IN SUPERNUMERARY SPIKELET WHEAT FORMS
Normally, the wheat inflorescence is determined. The apical meristem of the inflorescence consistently gives rise to lateral meristems that developed into lateral spikelets until a terminal spikelet is formed. Each primary axial (spikelet) meristem develops into a single lateral spikelet consisting of many florets [25].
Detailed analysis of developing inflorescences of supernumerary spikelet lines using a light and electron microscopy demonstrated that changes in the development of inflorescences do not affect the apical meristem, which is developed in all lines along the standard pathway for wheat, or to the floret meristems initiating the floret organs as in wild type inflorescences [25, 26]. Anomalies in the development occur only with spikelet meristems and lead to the formation of inflorescences with a changed morphology (different supernumerary spikelet phenotypes) [27–29].
The detected regularities in the formation of additional spikelets made it possible to divide supernumerary spikelet wheat forms into groups with similar changes in morphogenesis. The first group of supernumerary spikelet forms includes morphotypes with a multirow spike (MRS), genuine branching (GB), horizontal additional spikelets (HS/FRS), and triple spikelets of T. aestivum, T. durum, T. turgidum, and T. monococcum wheat species; the second is represented by a morphotype “false–true ramification (f-tR) of spike” of T. turgidum; and the third group includes the forms with vertical sessile spikelets (VSS) of T. aestivum (Fig. 1).
The peculiarities of the development of inflorescence in the members of the first group consist in changes in spikelet development associated with disruption of the identity of spikelet meristems in the transition to establishing the identity of floret meristems of either the basal part or all of the spikelet that are accompanied by a change in phyllotaxis [27, 28]. Despite the morphological heterogeneity of this group, which includes different morphotypes, differences in the development of inflorescences between the members of morphotypes are quantitative and are associated with different number of floret meristems replaced with ectopic spike meristems and/or with different degree of elongation of the spikelet rachilla. This leads to the formation of a different number of additional spikelets or to the development of branchlike structure at a rachis node. Both the clusters of sessile supernumerary spikelets and “branches” with supernumerary spikelets at a rachis node of this group are modified spikelets. These changes in the development of inflorescence [27, 28] are similar to previously found peculiarities of the development of rice Oryza sativa L. fzp [30], maize Zea mays L. bd1 [31], and Brachypodium distachyon (L.) P. Beauv. mos1 [32] mutants. Despite different types of inflorescences in rice, maize, B. distachyon, and wheat mutants (panicle, ear, spike), general patterns in the development of their inflorescences are traced associated with disruption of establishing the identity of floret meristems, where ectopic spikelets are developed, as a result of which extremely branched panicles [30, 31] or ectopic “branches” in the spike or ear [28–32] are formed. Thus, a parallel hereditary variation of the trait “spike branching” registered by Vavilov in different species of the members of Poaceae family [1] can be a consequence of similar violations of the program of development of inflorescences of these species [27, 28, 30–33].
Changes associated with the disruption of establishing the identity of the floret meristem of the distal part of the spike, accompanied by a change in phyllotaxis and a change in determinacy of the spikelet meristem, are observed in the development of the second group spike (“false–true ramification of spike” morphotype). Violations in the program of development of the inflorescence of this group come later than in the first group lines and fzp/bd1/mos1 cereal mutants, and the first two florets in the basal part of the spikelet are developed according to a standard scheme. The development of subsequent florets is violated and occurs in a similar way to the previous group: ectopic spikelets are developed in place of florets; their development is accompanied by a change in phyllotaxis [29]. The peculiarities of the development of this morphotype include the formation of an ectopic terminal spikelet, which is not typical of the members of the above-described first group. The mutants of other cereal species with similar changes in development were not described in the literature.
Unlike the first two groups, the development of additional spikelets in the VSS morphotype occurs outside the primary spikelet. The additional spikelet is developed later than the primary one and is located lower at a rachis node o, and its formation is not accompanied by a change in phyllotaxis and thus is not associated with a change in identity/determinacy of the spikelet meristem [34].
Thus, supernumerary spikelet wheat forms representing different morphotypes can be united into groups on the basis of the similarity of disruption of morphogenesis. The study of genetic control of the trait “supernumerary spikelet/spike branching” in the members of these groups demonstrated that similar changes in genetic programs of inflorescence development underlie the formation of their phenotypes [28, 33, 34].
GENETIC REGULATION OF “SUPERNUMERARY SPIKELET/SPIKE BRANCHING” TRAIT IN WHEAT
The genetics of wheat spike branching/supernumerary spikelet began to be studied more than 100 years ago. In 1910, E. von Tschermak reported that the trait of T. turgidum spike branching is genetically determined and is under a monogenic recessive control. The gene was designated as bh from Latin brachitus [7]. The recessive nature of the trait inheritance in tetraploid and hexaploid wheat was later confirmed by the results of many studies [18–21, 35].
It was found that environmental factors, including temperature and photoperiod, affect the stability of expression of the spike branching trait. At the same time, a stable expression of the trait was found in individual lines, which indicates the effect of the genotypic environment [18].
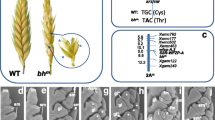
B.C. Sharman [13] reported a monogenic type of inheritance of the spike branching in tetraploid wheat. According to the results of D.L. Klindworth et al. [19], the phenotype of branched line of tetraploid wheat T. turgidum is determined by one major gene, the expression of which is affected by genes with minor effects. Using a series of disomic substituted lines, the main bh gene was localized on chromosome 2A [36]; then its localization in the short arm 2AS was clarified using the 2A-telocentric line [37].
The study of the forms of hexaploid bread wheat with branched spike or many sessile spikelets at a rachis node began later. First of all, this was associated with the fact that most of such forms arose as a result of mutagenesis [13, 14], being a consequence of wide hybridization [9] or aneuploidy [22], while branched forms of the T. turgidum wheat are widespread and have been known for about two thousand years [2, 3].
According to the results of studies [15, 16] performed on the offspring of a spontaneous branched mutant (T. aestivum ramifera), hexaploid wheat varieties carry two complementary interacting genes Rm (ramifera) and Ts (tetrastichon), as well as a dominant inhibitor, Nr (Normalizator).
A.L. Pennell and G.M. Halloran [18] reported the presence of two recessive genes determining supernumerary spikelet in bread wheat and, in addition to them, the presence of branching suppressor. It was demonstrated that commercial wheat cultivars with a standard spike carry the spike branching genes that are not manifested because of the presence of a suppressor gene [18]. D.L. Klindworth et al. [36] confirmed the presence of a spike branching suppressor gene in the bread wheat genome and localized it in chromosome 2D.
Using a monosomic analysis, Z.S. Peng et al. [35] found that chromosomes 2D, 4A, 5A, and 4B are involved in the genetic control of a turgidum spike type in the bread wheat line “Yupi Branching,” thus demonstrating a polygenic nature of the trait inheritance. The effect of genetic factor(s) of the chromosome 2D on the formation of supernumerary spikelets in induced bread wheat mutant MC1611 was also found by L.I. Laikova et al. [38] using a monosomic analysis.
D.F. Sun [39] demonstrated that the SS phenotype of line 51885 obtained as a result of crossing of octoploid triticale and bread wheat is under the control of two dominant genes and several genes with minor effects.
In the mid-1950s, E.R. Sears described the emergence of spikes with “spikelet reduplication” in nullisomic plants by chromosomes of 2A and 2D of bread wheat [22]. Later, M. Muramatsu [40] demonstrated that the effect of nullisomy can be completely compensated by an increase in the number of homeologous chromosomes, and the spikes of nuli-tetrasomic Tetra-2A Nulli-2D line (2n = 42, 19" + 1"") have a wild phenotype, and one spikelet develops at a rachis node. Chromosomal rearrangements (including deletions) and the absence of a whole chromosome were also found in other bread wheat lines with sessile additional spikelets and branching of the spike rachis [14, 41]. By means of the methods of cytogenetics and genotyping using DNA markers, it was found that the chromosome 2D substitutions and deletions in the short arm of the bread wheat chromosome 2D can affect the spike morphology, causing the development of additional sessile spikelets and ectopic “branches” on the spike rachis branches. The results of molecular genetic mapping demonstrated that the presence of deletions of the chromosome 2DS can determine up to ~50% of the variability of the studied trait (the presence of additional spikelets on the ledges) [28, 42].
Despite some inconsistency of data relative to the number and nature of inheritance of the genes determining the wheat SS phenotype (which can be explained in part by a genetic heterogeneity of the studied lines of different origin), the results of studies demonstrated the involvement of genetic factors of the chromosome of wheat homeologous group 2 in the control of SS phenotype [22, 35, 36, 38, 42, 43]. Later, these results were confirmed using the methods of molecular genetic mapping. Thus, the genetic locus determining the phenotype of the Tibetan three-spike form of bread wheat was mapped in chromosome 2AS [17]; the genes bh and bhm determining the spike branching of tetraploid and diploid wheats were mapped in chromosomes 2AS and 2AmS, respectively [8, 43].
Studying the genetic control of supernumerary spikelet in the bread wheat lines of different origin with similar changes in morphogenesis, the Multirow spike 1 (Mrs1) gene and qSS-2D and qSS-2A quantitative trait loci co-localized on 2DS or located in the regions of conserved synteny of homeologous chromosomes 2DS and 2AS were identified [27, 28, 44]. Thus, it was demonstrated that the formation of the branched spike (GB morphotype) and the spike with additional sessile spikelets (MRS, HS morphotypes) is caused by similar violations in the development and is under the same genetic control. Along with the main contribution of the genetic locus of the chromosome 2D, the influence of QTL with less effects was demonstrated [45]. This confirmed the result of previous works on the presence of minor genes affecting the trait “spike branching” and the results demonstrating the effect of genotypic environment in manifestations of the mutant gene of the chromosome 2D [27]. On the basis of hybrids from crossing branched T. turgidum accession and lines with a standard spike, R.Q. Zhang et al. [46] managed to obtain near isogenic lines with branched phenotype and four-row spike with sessile spikelets and to confirm the involvement of the common genetic factor in the genetic control of these different morphotypes.
Along with the main genes and quantitative trait loci of wheat spike branching/supernumerary spikelet, the presence of the genes determining supernumerary spikelet phenotypes was demonstrated in S. cereale L. cultivated rye (monstrosum 1, mo1) and barley (branched 1, brc1 syn. compositum 2, com2) [33, 48, 49]; these genes are located in the regions of conserved synteny of the chromosomes of the homeologous group 2, which assumed the presence of orthologous series of genes in the genomes of the members of Triticeae tribe.
Studying the genetic control of the “false–true ramification of spike” morphotype in the lines of T. turgidum tetraploid wheat, Y. Amagai et al. [23] established that the trait is under a monogenic recessive control. The gene determining the mutant phenotype was designated as sham ramification 2 (shr2), since at this stage of the study the authors found no differences between the phenotypes of the studied lines and lines with true (vavilovii) type of branching of the spike with the sham ramification (SHR) morphotype. Later, detailed analysis of the line spike phenotype made it possible to distinguish a separate morphotype “false–true ramification” [24]; however, the gene itself is still called shr2. The shr2 gene was localized in the long arm of chromosome 2A [23]. This morphotype was studied to a lesser extent, and no reports on the presence of a series of orthologous genes were found to date.
STRUCTURAL AND FUNCTIONAL ORGANIZATION OF GENES CONTROLLING SUPERNUMERARY SPIKELET/SPIKE BRANCHING
The determination of nucleotide sequences of the genes whose mutations cause the formation of nonstandard supernumerary spikelet spike phenotypes became an important stage in the study of supernumerary spikelet/spike branching forms of wheat and genetically related species.
Using a positional cloning, the sequence of the Mrs1 gene controlling the formation of supernumerary spikelets in the bread wheat of “multirow spike” (or MRS) morphotype was determined. It turned out to be the ortholog of the FRIZZY PANICLE rice gene (wheat fzp, wfzp) encoding the transcription factor with the functional APETALA 2/ERF domain [28, 44]. According to the results of resequencing of supernumerary spikelet wheat lines united in a single group taking into account the peculiarities of the development of inflorescences, it was demonstrated that their phenotypes are caused by mutations in the functional domain AP2 of the WFZP-A and WFZP-D homeolog genes. For all bread wheat wfzp mutants, similar changes in the development of inflorescence are typical (the formation of ectopic spikelet meristems at the place of floret meristems in the basal part of the spikelet or in the whole spikelet). On the basis of the analysis of the mutant phenotype, conclusions were made about the functional role of the WFZP gene in the development of the bread wheat spike (the genetic control of transition to establishing the identity of inflorescence floret meristems). The gene mutations cause violations in establishing the identity of flowering meristems and development of ectopic secondary axial meristems (ectopic spikelet meristems) instead of floret meristems.
Similar results were obtained regarding the role of WFZP-A gene in the development of inflorescences of tetraploid (T. turgidum and T. durum) and diploid (T. monococcum) wheat species [29, 33]. N. Poursarebani et al. [33] isolated the bh1/WFZP-A gene in the genome of T. turgidum tetraploid wheat and demonstrated that the same TtBH-A1 mutation led to the formation of the spike of turgidum type of branching in 30 branched T. turgidum accessions of different origin; this suggests a monophyletic origin of this allele. The area and ecological characteristics of the accessions with a normal phenotype of T. turgidum convar. turgidum spike and branched T. turgidum convar. compositum samples generally coincide; the only exception is the Tibetan ecotype, among the samples of which no branched plants were found [2]. Consequently, the TtBH-A1 mutation originated at early stages of the evolutionary history of the T. turgidum species. The mutation could have been transmitted to other species (for example, T. durum) with spontaneous hybridization [29].
R.Q. Zhang et al. [46] demonstrated that, along with the wfzp-A/TtBH-A1 gene, there are other genetic factors involved in the control of the turgidum type of spike branching. These factors determine the development of a spikelet with an extended spikelet rachilla (ramified spike, RS) and the formation of the branched spike of a typical turgidum type.
In general, it was demonstrated that the structural organization of genetic loci and functions of cereal FZP ortholog genes are highly conserved. Thus, the formation of ectopic axial meristems and replacement of florets with ectopic branches were observed in rice fzp mutants; this led to repeated cycles of inflorescence branching, on the basis of which it was concluded that the function of the FZP gene consists in the suppression of the development of the axial meristems and in the control of the transition to establishing the identity of floret meristems of the rice inflorescence [30]. The results of the studies of N.N. Rao et al. [50] confirmed the fact that FZP suppresses the formation of axial meristems of the inflorescence. The maize BD1 gene is an ortholog of the fzp rice gene, bd1 and fzp mutants have a similar inflorescence phenotype, and the functions of the BD1 and FZP genes are conserved [31].
P. Derbyshire and M.E. Byrne [32] obtained the induced more spikelets1 (mos1) mutant of the B. distachyon species with violations in development and phenotypic peculiarities of the inflorescence morphology similar to bd1 and fzp mutants. When determining the structural organization of the gene homologous to FZP, insertion in the promoter region associated with a small (20%) but statistically significant decrease in the expression was found in the mos1 mutant. A screening of TILLING population of the B. distachyon species detected two new alleles with mutations in the functional domain AP2/ERF of the ortholog FZP gene associated with the mutant phenotype (formation of additional spikelets in the inflorescence) [28].
The ortholog of the FZP gene (COMPOSITUM 2 (COM2)) was isolated in the barley genome [33]. The com2 mutations cause the formation of a phenotype similar to branched wheat forms. The functional role of the gene in the development of inflorescence does not differ from FZP orthologs of other cereals. It is interesting that the Com1 gene, recessive mutations in which induce similar defects in the development of inflorescence, is present in barley. The nucleotide sequence of the gene is unknown [51].
At present, wfzp mutants are the most studied among supernumerary spikelet wheat forms, while the morphotype “false–true ramification of spike” under the control of the sham ramification 2 (shr2) gene is studied to a lesser extent [23, 24, 29]. The position of shr2 in the long arm of chromosome 2A was determined [23]; the structural organization of the gene is unknown at the present time. The functions of the Shr2 gene in the spike development, established on the basis of the analysis of the peculiarities of the development of the mutant phenotype, consist in the genetic control of establishing the identity of floret meristems and determinacy of the spikelet meristem [29].
The results of classical genetic analysis demonstrated that, despite some similarities in the development of mutant phenotypes wfzp and shr2, the genes that determine them are inherited independently [29]. Thus, establishing the identity of floral meristems of basal and distal parts of multi-floret wheat spikelet occurs under the control of the WFZP and Shr2 genes acting independently at different stages of spikelet development and belonging to different genetic pathways of regulation of the inflorescence development. At the same time, it was demonstrated that SHR2 interacts with the ramified spike (RS) gene (genes) presumably determining the formation of an elongated spikelet rachilla in branched forms of the T. turgidum species [29]. It should be noted that the conserved WFZP gene functions in the basal part of the spikelet in both multi-floret and single-floret spikelets of the members of the Poaceae family, while the SHR2 gene acts in the distal part of the multi-floret spikelet. There is no mention in the literature about the mutants with phenotypes similar to shr2 in rice or in other cereals. It is possible that this gene belongs to a specific pathway of regulating the development of multi-floret wheat spikelet.
The formation of paired vertical spikelets at the nodesl of wheat spike occurs owing to some changing in the identity of spikelet meristems. Molecular genetic analysis detected 18 quantitative trait loci responsible for the formation of this trait; the gene controlling the response to photoperiod (PHOTOPERIOD-1, Ppd-1) makes the most significant contribution [34]. It was found that Ppd-1 affects the spikelet development, changing the expression of the FLOWERING LOCUS T1 (FT1) gene, an integrator of genetic pathways that regulate flowering. The formation of paired spikelets is also affected by some alleles of the TEOSINTE BRANCHED1 (TB1) gene [52]. It is assumed that TB1 coordinates the formation of axial meristems during the transition from the vegetative to generative stage of development.
Thus, at present, the structural and functional organization of the WFZP and Ppd-1 genes, mutations in which cause changes in spike morphogenesis and the formation of additional spikelets in two different groups of supernumerary spikelet wheat, was studied in detail. The shr2 gene determining the “false–true ramification of spike” wheat morphotype was less studied; the localization of this gene in chromosome 2A and functions based on the analysis of a mutant phenotype are known. The RS genes that cause the elongation of the spikelet rachilla of the T. turgidum and T. aestivum “true spike branching” morphotype remain the least studied; to date, their localization in the genome and structural organization has not been clarified to date.
CONCLUSIONS
Changes in homologous genes with a common origin (ortholog genes) underlie homologous hereditary variation of supernumerary spikelet/branched wheat forms. The formation of a similar mutant phenotype (the emergence of additional sessile spikelets and branches in wheat wfzp, barley com2, rye mo1, rice fzp, maize bd1, and B. distachyon moc1 mutants as a result of mutations in FZP ortholog genes) is the best example. The presence of series of mutants made it possible to characterize in detail the structural and functional organization of FZP ortholog genes and to determine the role of functional domain. A high level of conservation in all studied members of cereals is typical of FZP.
The studies of the peculiarities of the development of supernumerary spikelet wheat lines and identification of common features and patterns of development made it possible to divide them into three main groups, each of which unites the mutants for a particular major gene (wfzp, shr2, Ppd-1). Thus, the collections of supernumerary spikelet lines are at present genetic models for studying the processes of development of inflorescence. In general, wheat forms with violations of morphogenesis leading to changes in the spike morphology (architecture) have a large potential in the study of the genetic bases of development of inflorescence and formation of economically valuable traits.
REFERENCES
Vavilov, N.I., Zakon gomologicheskikh ryadov v nasledstvennoi izmenchivosti (Law of Homological Series in Hereditary Variation), Leningrad: Nauka, 1987.
Dorofeev, V.F., Filatenko, A.A., Migusheva, E.F., et al., Kul’turnaya flora SSSR: pshenitsa (Cultural Flora of the USSR: Wheat), Leningrad.: Kolos, 1979, vol. 1.
Dahlgren, B.E., Wheat, Chicago: Field Museum of Natural History, 1922.
Percival, J., The Wheat Plant, London: Duckworth, 1921.
Dorofeev, V.F., Spontaneous mutations as a factor in the wheat formation, Vestn. S.-kh.Nauki, 1968, vol. 7, pp. 16—26.
Coffman, F.A., Supernumerary spikelets in Mindum wheat, J. Hered., 1924, vol. 5, pp. 187—192.
Goncharov, N.P., Sravnitel’naya genetika pshenits i ikh sorodichei (Comparative Genetics of Wheat and Their Relatives), Novosibirsk: Geo, 2012.
Amagai, Y., Martinek, P., Watanabe, N., et al., Microsatellite mapping of genes for branched spike and soft glumes in Triticum monococcum L., Genet. Resour. Crop Evol., 2014, vol. 61, pp. 465—471. https://doi.org/10.1007/s10722-013-0050-9
Tsitsin, N.V., Branched winter rye, in Otdalennaya gibridizatsiya (Remote Hybridization), Moscow: Sel’khozgiz, 1954, pp. 313—322.
Alieva, A.J. and Aminov, N.K., Influence of D genome of wheat on expression of novel type spike branching in hybrid populations of 171ACS line, Russ. J. Genet., 2013, vol. 49, no. 11, pp. 1119—1126. https://doi.org/10.1134/S1022795413110021
Mel’nik, V.M., Kozlovskaya, V.F., Pastukhov, G.P., et al., Study of allelic relationships in induced spring wheat mutants, Aktual’nye voprosy genetiki i selektsii rastenii (Current Issues of Genetics and Plant Breeding) (Proc. Sib. Region Conf.), Shumnii, V.K. and Kalinina, I.P., Eds., Novosibirsk, 1980.
Mel’nik, V.M. and Pastukhov, G.P., Geneticheskie issledovaniya indutsirovannykh mutantov yarovoi pshenitsy: khimicheskii mutagenez v povyshenii produktivnosti sel’skokhozyaistvennykh rastenii: (Genetic Studies of Induced Mutants of Spring Wheat: Chemical Mutagenesis to Increase the Productivity of Crops), Moscow: Nauka, 1984.
Sharman, B.C., Branched head in wheat and wheat hybrids, Nature, 1944, vol. 153, pp. 497—498.
Swaminathan, M.S., Chopra, V.L., and Sastry, G.R.K., Expression and stability of an induced mutation for ear branching in bread wheat, Curr. Sci., 1966, vol. 35, pp. 91—92.
Koric, S., Branching genes in Triticum aestivum, Proceedings of 4th International Wheat Genetics Symposium, Sears, E.R. and Sears, L.M.S., Eds., Columbia, MO, USA, 1973, pp. 283—288.
Koric, S., Study of branched gene complex of T. aestivum ssp. vulgare and its significance for wheat breeding, J. Sci. Agric. Res., 1980, vol. 142, pp. 271—282.
Li, J., Wang, Q., Wei, H., et al., SSR mapping for locus conferring on the triple spikelet trait of the Tibetan triple-spikelet wheat (Triticum aestivum L. concv. tripletum), Triticeae Genomics Genet., 2011, vol. 2, pp. 1—6. https://doi.org/10.5376/tgg.2011.02.0001
Pennell, A.L. and Halloran, G.M., Inheritance of supernumerary spikelets in wheat, Euphytica, 1983, vol. 32, pp. 767—776.
Klindworth, D.L., Williams, N.D., and Joppa, L.R., Inheritance of supernumerary spikelets in a tetraploid wheat cross, Genome, 1990, vol. 33, pp. 509—514.
Martinek, P., Gene resources with non-standard spike morphology in wheat, in Proceedings of 9th International Wheat Genetics Symposium, Saskatoon, Saskatchewan, Canada, 1998, vol. 2, pp. 286—288.
Martinek, P. and Bednar, J., Changes of spike morphology (multirow spike—MRS, longglumes—LG) in wheat (Triticum aestivum L.) and their importance for breeding, Genetic Collections, Isogenic and Alloplasmic Lines (Proc. Int. Conf. Novosibirsk, Russia), 2001, pp. 192—194.
Sears, E.R., The Aneuploids of Common Wheat, Columbia, MO: Univ. Missouri, 1954, pp. 3—58.
Amagai, Y., Aliyeva, A.J., Aminov, N.Kh., et al., Microsatellite mapping of the genes for sham ramification and extra glume in spikelets of tetraploid wheat, Genet. Resour. Crop Evol., 2014, vol. 61, pp. 491—498. https://doi.org/10.1007/s10722-013-0052-7
Amagai, Y., Aliyeva, A.J., Aminov, N.Kh., et al., The third glume phenotype is associated with rachilla branching in the spikes of tetraploid wheat (Triticum L.), Genet. Resour. Crop Evol., 2017, vol. 64, pp. 835—842. https://doi.org/10.1007/s10722-017-0503-7
Bonnet, O.T., The development of the wheat spike, J. Agr. Res., 1936, vol. 53, pp. 445—451.
Shitsukawa, N., Kinjo, H., Takumi, S., et al., Heterochronic development of the floret meristem determines grain number per spikelet in diploid, tetraploid and hexaploid wheats, Ann. Bot., 2009, vol. 104, pp. 243—251. https://doi.org/10.1093/aob/mcp129
Dobrovolskaya, O.B., Badaeva, E.D., Adonina, I.G., et al., Investigation of morphogenesis of inflorescence and determination of the nature of inheritance of “supernumerary spikelets” trait of bread wheat (Triticum aestivum L.) mutant line, Russ. J. Dev. Biol., 2014, vol. 45, pp. 361—366. https://doi.org/10.1134/S1062360414060034
Dobrovolskaya, O., Pont, C., Sibout, R., et al., FRIZZY PANICLE drives supernumerary spikelets in bread wheat (T. aestivum L.), Plant Physiol., 2015, vol. 167, pp. 189—199. https://doi.org/10.1104/pp.114.250043
Dobrovolskaya, O.B., Amagai, Y., Popova, K.I., et al., Genes WHEAT FRIZZY PANICLE and SHAMRAMIFICATION 2 independently regulate differentiation of floral meristems in wheat, BMC Plant Biol., 2017, vol. 17, suppl. 2, article 252. https://doi.org/10.1186/s12870-017-1191-3
Komatsu, M., Chujo, A., Nagato, Y., et al., FRIZZY PANICLE is required to prevent the formation of axillary meristems and to establish floral meristem identity in rice spikelets, Development, 2003, vol. 130, pp. 3841—3850. https://doi.org/10.1242/dev.00564
Chuck, G., Muszynski, M., Kellogg, E., et al., The control of spikelet meristem identity by the branched silkless1 gene in maize, Science, 2002, vol. 298, pp. 1238—1241. https://doi.org/10.1126/science.1076920
Derbyshire, P. and Byrne, M.E., MORE SPIKELETS1 is required for spikelet fate in the inflorescence of Brachypodium distachyon,Plant Physiol., 2013, vol. 161, pp. 1291—1302. https://doi.org/10.1104/PP.112.212340
Poursarebani, N., Seidensticker, T., Koppolu, R., et al., The genetic basis of composite spike form in barley and “Miracle-Wheat”, Genetics, 2015, vol. 201, pp. 155—165. https://doi.org/10.1534/genetics.115.176628
Boden, S.A., Cavanagh, C., Cullis, B.R., et al., Ppd-1 is a key regulator of inflorescence architecture and paired spikelet development in wheat, Nat. Plants, 2015, vol. 1, article 14016. https://doi.org/10.1038/nplants.2014.16
Peng, Z.S., Yen, C., and Yang, J.L., Chromosomal location of genes for supernumerary spikelet in bread wheat, Euphytica, 1998, vol. 103, pp. 109—114. https://doi.org/10.1023/A:1018323310621
Klindworth, D.L., Williams, N.D., and Joppa, L.R., Inheritance of supernumerary spikelets in a tetraploid wheat cross, Genome, 1990, vol. 33, pp. 509—514. https://doi.org/10.1139/g90-076
Klindworth, D.L., Klindworth, M.M., and Williams, N.D., Telosomic mapping of four genetic markers in durum wheat, J. Hered., 1997, vol. 88, pp. 229—232. https://doi.org/10.1093/oxfordjournals
Laikova, L.I., Arbuzova, V.S., Popova, O.M., et al., Study of spike branching in mutant lines of bread wheat variety Saratov 29, in Aktual’nye zadachi selektsii i semenovodstva sel’skokhozyaistvennykh rastenii na sovremennom etape: doklady i soobshcheniya IX genetiko-selektsionnoi shkoly. (5—9 aprelya 2004 g.) (Relevant Problems of Breeding and Seed Production of Agricultural Crops at the Present Stage: Abstracts and Reports of the IX Genetic-Breeding School (April 5—9, 2004)), Novosibirsk, 2005, pp. 388—393.
Sun, D.F., Inheritance of genes controlling supernumerary spikelet in wheat line 51885, Euphytica, 2009, vol. 167, pp. 173—179. https://doi.org/10.1007/s10681-008-9854-7
Muramatsu, M., A presumed genetic system determining the number of spikelets per rachis node in the tribe Triticeae, Breed. Sci., 2009, vol. 59, pp. 617—620. https://doi.org/10.1270/jsbbs.59.617
Košner, J. and Foltyn, J., Chromozomalní poměry pšenice obecné (Triticum aestivum L.) s větevnatým klasem, Sbor. Ú-VTIZ, Genet. Šlecht., 1989, vol. 25, no. 1, pp. 11—17.
Dobrovol’skaya, O.B., Martinek, P., Adonina, I.G., et al., Effect of rearrangements of homoeologous group 2 chromosomes of bread wheat on spike morphology, Vavilovskii Zh. Genet. Sel., 2014, vol. 18, no. 4/1, pp. 672—680. https://doi.org/10.7868/S0475145014060032
Haque, M.A., Martinek, P., Kobayashi, S., et al., Microsatellite mapping of genes for semi-dwarfism and branched spike in Triticum durum Desf. var. ramosoobscurum Jakubz.“Vetvistokoloskaya,” Genet. Resour. Crop Evol., 2012, vol. 59, pp. 831–837. https://doi.org/10.1007/s10722-011-9722-5
Dobrovolskaya, O., Martinek, P., Voylokov, A.V., et al., Microsatellite mapping of genes that determine supernumerary spikelets in wheat (T. aestivum) and rye (S. cereale), Theor. Appl. Genet., 2009, vol. 119, pp. 867–874. https://doi.org/10.1007/s00122-009-1095-1
Echeverry-Solarte, M., Kumar, A., Kianian, S., et al., Genome-wide genetic dissection of supernumerary spikelet and related traits in common wheat, Plant Genet., 2014, vol. 7, pp. 1–16. https://doi.org/10.3835/plantgenome2014.03.0013
Zhang, R.Q., Hou, F., Chen, J., et al., Agronomic characterization and genetic analysis of the supernumerary spikelet in tetraploid wheat (Triticum turgidum L.), J. Integr. Agr., 2017, vol. 16, pp. 1304–1311. https://doi.org/10.1016/S2095-3119(16)61469-7
Benito, C., Zaragoza, C., Gallego, F.J., et al., A map of rye chromosome 2R using isozyme and morphological markers, Theor. Appl. Genet., 1991, vol. 82, pp. 112–116.
Castiglioni, P., Pozzi, C., Heun, M., et al., An AFLP-based procedure for the efficient mapping of mutations and DNA probes in barley, Genetics, 1998, vol. 149, pp. 2039–2056.
Rossini, L., Vecchietti, A., Nicoloso, L., et al., Candidate genes for barley mutants involved in plant architecture: an in silico approach, Theor. Appl. Genet., 2006, vol. 112, pp. 1073–1085. https://doi.org/10.1007/s00122-006-0209-2
Rao, N.N., Prasad, K., and Kumar, P.R., Distinct regulatory role for RFL, the rice LFY homolog, in determining flowering time and plant architecture, Proc. Natl. Acad. Sci. U.S.A., 2008, vol. 105, pp. 3646–3651. https://doi.org/10.1073/pnas.0709059105
Schnurbush, T., Wheat and barley biology: towards new frontiers, J. Integr. Plant Biol., 2019, vol. 62, pp. 198–203. https://doi.org/10.1111/jipb.12782
Dixon, L.E., Cockram, J.R., Mellers, G., et al., TEOSINTE BRANCHED 1 regulates inflorescence architecture and development in bread wheat (Triticum aestivum), Plant Cell, 2018, vol. 30, no. 3, pp. 563–581. https://doi.org/10.1105/tpc.17.00961
Funding
This work was supported by the Russian Foundation for Basic Research (project no. 18-04-00483-a) and by the budgetary project no. AAAA-A16-116061750188-4.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The author declares that she has no conflict of interest. This article does not contain any studies involving animals or human participants performed by the author.
Additional information
Translated by A. Barkhash
Rights and permissions
About this article
Cite this article
Dobrovolskaya, O.B. Supernumerary Spikelet Wheat Forms as Models for Studying Genetic Regulation of Inflorescence Development. Russ J Genet 56, 1298–1307 (2020). https://doi.org/10.1134/S1022795420110034
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1022795420110034