Abstract
Oxytocin represents one of the key element regulating social activity and aggressive behavior via its binding to oxytocin receptor (OXTR). Considering the multifactorial nature of developing aggression, the present study aimed to assess the main effects of the OXTR (rs53576, rs237911, rs7632287, rs2254298, rs2228485, rs13316193) gene polymorphisms together with haplotypic and G × E effects on individual differences in aggression level in 623 mentally healthy individuals with sex and ethnicity as covariates. The association of rs2228485*G allele (PFDR = 0.046) and rs237911*G allele (PFDR = 0.046) and decreased aggression level was observed in Tatars. Haplotypic analysis revealed an association of the OXTR G*G*G haplotype (rs53576, rs2228485, and rs237911) and diminished aggression level (Pperm = 0.020) in Tatars. As a result of multiple regression analysis, we observed the modulating effect of smoking and paternal overprotection significantly affecting association of the OXTR rs2228485 and aggression level (P = 0.029 and P = 0.014, respectively) in the total sample.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
The study of aggressive behavior (AB) in humans as an integral part of mental health is becoming more relevant nowadays, which is due to its serious destructive consequences for society and a wide range of individual manifestations of aggression. AB is a complex trait, which implies actions aimed at causing moral, physical, or other damage to another object or to oneself (autoaggression) [1]. According to Rosstat, about 1.5 million individuals repeatedly were subjected to various types of criminal attacks in 2018 in Russia (including 40 000 people subjected to serious harm to their health, while 26 000 individuals died) [2] and a similar pattern is extrapolated to the majority of countries worldwide [3]. Together with the existence of severe forms of AB, an excessive aggression varying within the norm of reaction in population may represent aggressive attacks in everyday life.
A multifactorial nature of the etiology and pathogenesis of AB suggests the involvement of both genetic and environmental components. According to twin studies, the coefficient of inheritance of aggression is about 50% [4]. Together with the role of monoaminergic system genes, the families of neurotrophic factors, and neuronal apoptosis [5], the regulation of AB is caused by the hypothalamic-pituitary-adrenal axis (HPA). An important role in the regulation of this system belongs to oxytocin—sex hormone and neurotransmitter synthesized in magnocellular neurons of the paraventricular and supraoptic nuclei of the hypothalamus and in multiple neurons of the central nervous system (CNS). Oxytocin is involved in control of various functions of the organism directed to species maintenance, including prosocial behavior and stress response [6, 7]. Impaired functioning of the oxytocinergic system was demonstrated on model objects with excessive aggressive behavior [7], while a reduced oxytocin level in the CNS predicted aggressive behavior in humans [6]. Moreover, a decrease in hyperaggressive behavior in mice was detected as a response to injection of oxytocin and oxytocin receptor (OXTR) antagonist [8], which determines the level of oxytocin activity and primarily depends on its binding to corresponding receptor encoded by the OXTR gene.
Association studies also indicated the relation between antisocial and aggressive behavior and the OXTR gene polymorphisms (3p25.3) [9, 10]. One of the most examined loci in the OXTR gene is rs53576 located in intron 3, which was associated with various forms of social behavior [11] and autism spectrum disorders (ASD) [11], which are characterized by a deficit of social activity and aggressive behavior. Previously the association of rs2254298 in intron 3 and rs7632287 in the 3'-untranslated region with social behavior was reported [11], while rs237911 in the promoter region and rs2228485 (p.Asn57) in exon 3 of the OXTR gene were associated with antisocial behavior [9, 10]. Earlier studies examining AB in men who conducted antisocial actions included the association analysis of several SNPs in the OXTR gene in severe forms of AB [10]; however, evaluation of the OXTR gene variants in the individual differences in aggression level in mentally healthy individuals considering social factors was not conducted previously.
Several environmental factors may trigger the manifestation of excessive aggression via mechanisms of epigenetic regulation. Twin studies provide evidence that the development of a phenotype related to impaired types of behavior varied depending on the place of residence (rural/urban), child–parental relationships, the presence of chronic disorders and addiction, ethnicity, and sex, which might be caused by differences in functioning of the nervous and endocrine systems together with transcription profiles between men and women [12]. The data on the influence of maternal psychiatric disorder, deviant behavior in close relatives [13], and addictive behavior during prenatal development on an individual’s epigenetic state and, hence, on the expression of the OXTR gene and manifestation of deviant behavior in childhood and AB in adult life [14] are of interest. For the complete coverage of the OXTR gene and considering a better prognostic strength of haplotypic analysis, the present study was based on genotyping of six SNPs in the OXTR gene. Accordingly, the present study aimed to estimate the main effect of the OXTR gene polymorphisms (rs53576, rs237911, rs7632287, rs2254298, rs2228485, rs1331619) together with a haplotypic effect and gene × environment interactions (G × E) in developing AB in healthy individuals aged 17–25 years considering sociodemographic parameters.
MATERIALS AND METHODS
The study comprised 623 mentally healthy individuals (81.11% women)—students at universities of the Republic of Bashkortostan and Udmurt Republic (mean age 19.53 ± 1.75 years) consisting of Russians (N = 225), Udmurts (N = 218), Tatars (N = 141), and individuals of mixed ethnicity (N = 39). All enrolled individuals were not registered in the Psychiatric Registry and did not have familial history of mental illness. The study participants underwent psychological testing, and sociodemographic data on ethnic origin (up to three generations) together with specificity of child–parent relationships (parenting style, episodes of childhood abuse, rearing in a full/incomplete family), socioeconomic status, maternal and paternal age at birth, place of residence (urban/rural), sibship size and birth order, chronic diseases, and smoking were obtained.
The Russian version of the Buss-Perry Aggression Questionnaire (BPAQ-29) consisting of 29 issues was used for the assessment of aggressiveness. The material for the study included DNA samples isolated from the peripheral blood leukocytes via the standard phenol-chloroform technique. The genotyping of the OXTR gene SNPs (rs53576, rs237911, rs7632287, rs2254298, rs2228485, rs13316193) was conducted via real-time PCR with fluorescent detection (FLASH/RTAS, State Research Institute of Genetics and Selection of Industrial Microorganisms of the National Research Center Kurchatov Institute, Moscow) using a CFX96 PCR machine (BioRad, United States) with the possibility of endpoint genotyping.
The resemblance of quantitative data to the normal distribution (Gaussian distribution) was performed using the Shapiro–Wilk W test (P > 0.05). Nonparametric tests (Mann–Whitney test) were used to identify the relationship between environmental parameters and the level of aggressiveness. The estimate of the main effect of SNPs and haplotypes in individual variance in aggression was carried out with linear regression analysis using various statistical models (additive, dominant, recessive) in PLINK v.1.07. The genotypes and environmental factors served as independent factors in G × E analysis, while aggression level was the dependent variable. Haplotypic blocks were constructed on the basis of the method of confidence intervals. The assessment of linkage disequilibrium was performed via Haploview 4.2 software. A detection of a statistically significant model of G × E interaction was followed by multiple regression analysis including sex and ethnicity as covariates (STATA 11.0). The correction for multiple testing was conducted by the FDR procedure (False Discovery Rate) or permutation test (10 000 permutations) during haplotypic analysis (PLINK v.1.07).
RESULTS
As a result of the present study, we conducted the association analysis of the OXTR gene polymorphisms (rs53576, rs237911, rs7632287, rs2254298, rs2228485, rs13316193) and estimated their involvement in aggression level in individuals without mental disorders considering several sociodemographic parameters together with sex and ethnicity. The results of the distribution of allele and genotype frequencies of the OXTR gene corresponded to the Hardy–Weinberg equilibrium (P = 1.000 for rs53576, P = 0.559 for rs237911, P = 1.000 for rs7632287, P = 0.299 for rs2254298, and P = 0.147 for rs2228485), except for rs13316193 (P < 0.005). The aggression score in the examined sample was congruent to the Gaussian normal distribution (P = 0.200). Owing to the absence of statistically significant differences in the distribution of allele and genotype frequencies of the studied loci between men and women and individuals of different ethnicities (P > 0.05), the association analysis was conducted in the total sample, as well as in groups stratified by sex and ethnicity.
The characteristics of the studied sample considering differences in aggression level depending on each of the examined sociodemographic parameters are shown in Table 1. As a result of analysis, we observed statistically significant differences in the mean aggression score depending on ethnic origin (P < 0.001), birth order (P = 0.029), sibship size (number of children in a family) (P = 0.031), childhood maltreatment (P = 0.001), smoking (P = 0.022), maternal care (P = 0.001), and protection (P < 0.001). To estimate the effect of individual age, weight at birth (1500–4950 g), and maternal age at delivery (16–44 years) on differences in aggression level, a linear regression analysis was carried out with inclusion of sociodemographic factors as independent variables and aggression level as a dependent variable. No statistically significant models were detected as a result of this analysis considering age (19.45 ± 1.48 years; β = –0.353; P = 0.462), weight at birth (3381 ± 533 g; P = 0.932), and maternal age at delivery (25.61 ± 5.43 years; β = ‒0.191; P = 0.183).
As a result of linear regression analysis after FDR correction, the association of minor alleles of rs2228485 (β = –8.412; P = 0.014; PFDR = 0.046) and rs237911 (β = –8.771; P = 0.015; PFDR = 0.046) with a decreased aggressiveness was observed in Tatars under the dominant statistical model (G/G + G/A vs. A/A) (Table 2). The analysis of additive (G/G vs. G/A vs. A/A) and recessive (G/G vs. G/A + A/A) effects of the minor rs2228485*G allele demonstrated the association with a reduced aggression level in both the total sample (β = –4.538; P = 0.011; PFDR = 0.065 and β = –8.569; P = 0.015; PFDR = 0.091, respectively), and women (β = –4.322; P = 0.040; PFDR = 0.240 and β = –8.228; P = 0.049; PFDR = 0.243, respectively); however, these associations did not achieve the level of statistical significance after FDR correction (PFDR > 0.05).
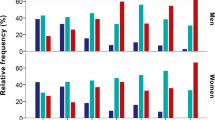
Considering sexual dimorphism with respect to oxytocin receptor level and the necessity to include sex as a covariate together with examined independent variables, we performed the analysis of G × E interactions, which estimates both the main effect of the OXTR gene polymorphisms and 21 sociodemographic parameters. It was observed that smoking (β = –10.38; r2 = 0.020; Р = 0.029) and paternal protection (β = 7.985; r2 = 0.020; Р = 0.014) modulated association of rs2228485 with aggression level (Fig. 1). A subsequent one-way ANOVA revealed that smoking carriers of major rs2228485*A/A genotype were characterized by higher aggression level compared to nonsmoking individuals (Р = 0.021). Moreover, carriers of rs2228485*G allele, which reported paternal overprotection as the rearing style, demonstrated a statistically significant increase in aggression level compared to those with paternal low protection rearing style (Р = 0.020) (Fig. 1).
Multiple linear regression analysis demonstrating the modulating effect of G × E interactions—rs2228485* “smoking” (a), rs2228485* “paternal protection” (b)—on individual differences in aggression level including sex and ethnicity as covariates. The signs “–” and “+” stand for the absence (–) or presence (+) of smoking (a), low (–) or high (+) level of paternal protection (b). The mean values of aggression score depending on the OXTR rs2558485 genotype and standard deviations of the means are shown. The arcs mark the comparison groups in one-way ANOVA. The variables included in the regression model are reported. β—regression coefficient; P—level of significance (Р-value). * P < 0.05.
As a result of analysis, we observed a linkage disequilibrium between rs53576, rs2228485, and rs237911 in all examined groups (D' > 0.720), which constructed a single haplotype block (Fig. 2). Haplotype frequencies are reported in Fig. 2. As a result of linear regression analysis of haplotypes, we detected the association of *G*G*G haplotype (based on rs53576, rs2228485, rs237911, respectively) with a decreased aggressiveness in Tatars (β = –9.761; Р = 0.003; Pperm = 0.020). Moreover, a trend for enhanced aggression in men bearing the *G*A*A haplotype was demonstrated (β = 4.964; Р = 0.027; Pperm = 0.160).
Positions of the studied SNPs in the OXTR gene, haplotype frequencies constructed on the basis of rs53576, rs2228485, rs237911, and results of linear regression analysis of haplotypes in the examined groups. (a) Schematic structure of the OXTR gene, location of the studied SNPs, and distance between them (kb). (*) Excluded SNP due to its deviation from the Hardy–Weinberg equilibrium. UTR—untranslated region. (b) Haplotype frequencies in each of the studied groups (in total sample, among women, men, individuals of Russian, Tatar, Udmurt ethnic origin). (1) rs53576, (2) rs2228485, (3) rs237911. Freq—haplotype frequency. P (Pperm)—P-value before and after permutation test (P-value for haplotypes associated with aggression level is shown). Dashes indicate the haplotype frequencies under 0.01. Statistically significant differences (before and after 10 000 permutation test) are marked in bold. (1)–(7) are haplotype numbers. Constructed haplotype blocks (marked in triangles) in the examined groups based on Lewontin’s statistics (D') were calculated in Haploview v.4.2.
DISCUSSION
The present study revealed an ethnicity-specific pattern of association of rs2228485 and rs237911 in the OXTR gene with aggression level in mentally healthy individuals. The functional studies of the OXTR gene in knockout mice indicate their increased aggression [15]. Molecular genetic studies devoted to the analysis of the OXTR gene in humans also point to its involvement in the regulation of neurobiological processes underlying AB. The rs2228485 (c.171C>T, p.Asn57) and rs237911 (с.-135C>T) examined in the present study do not cause amino acid substitution, and their functional significance remains unclear. To date only one study based on the analysis of these loci in manifestation of AB was published. Namely, rs2228485*G/G genotype was associated with a reduced risk, while rs237911*A allele was associated with an enhanced risk for developing AB in men with mental illness of Tatar ethnic origin [10]. These findings are congruent with the detected character of association between minor rs2228485*G and rs237911*G alleles demonstrated in the present study with a reduced aggression level in healthy individuals of Tatar ethnicity and in the total group and women at a trend level (P < 0.05; PFDR > 0.05). On the basis of these findings, it may be suggested that rs2228485*G and rs237911*G alleles appear to be markers of lower risk of manifesting aggression independent of the presence of psychiatric illness; however, several sociodemographic parameters are known to modulate the association of genetic main effect and aggression level. They comprise smoking and the level of paternal protection, which modified the aggression level. In the first case, interindividual differences in aggressiveness depending on the presence of smoking were observed only among carriers of rs2228485*A/A genotype, which were characterized by an increased aggression level compared to carriers of other genotypes. Therefore, it can be assumed that genetically determined high aggression level (in individuals with rs2228485*A/A genotype) will be significantly higher in smokers compared to nonsmoking individuals. According to published findings, smoking caused individual changes such as increased aggression even in the case of passive smoking [16], in particular, owing to the effect of nicotine on the epigenetic profile of individuals [17]. In the second case, we detected differences in aggression level only among carriers of minor rs2228485*G allele, and a higher aggression score was observed in individuals reporting paternal overprotection in childhood compared to paternally low-protected responders. According to the published data, the level of paternal protection also positively correlated with social aggression [18], which is congruent to our findings.
Although no studies reported the functional significance of rs2228485 (c.171C>T, p.Asn57), its location in one of the CpG islands (according to Methyl Primer Express Software v. 1.0, Applied Biosystems) in the promoter gene region may point to its potential involvement in a precise regulation of gene expression via allele-specific differential methylation. Moreover, multiple genes overlap with other genes and noncoding RNAs, which are oppositely transcribed. In particular, previously we examined Taq1A (rs1800497) polymorphism located in the dopamine D2 receptor gene (DRD2), which actually resides in the ANKK1 gene overlapping it, and demonstrated the involvement of this SNP in the development of individual differences in emotional instability [19]. The rs2228485 in the OXTR gene examined in the present study is simultaneously located in the CAV3 gene encoding caveolin-3 and noncoding RNA (ncRNA) and involved in the transport of another sex hormone (estrogen, ER) to the cellular membrane [20]. Caveolin-3 belongs to auxiliary proteins regulating the functioning of polarization-activated cyclic nucleotide ion channels. The latter are responsible for the fine tuning of neuronal excitation, while its impaired functioning may be related to mental illness [21].
To date, rs53576 is the most examined SNP in the OXTR gene despite its intronic location (Fig. 2). Nevertheless, rs53576*G allele is related to the increased transcription of the OXTR gene and, hence, to enhanced receptor sensitivity to oxytocin [22]. Previously it was studied in several psychopathologies (schizophrenia, bipolar disorder, depression, and suicidal behavior) and specific social behavior [11, 22]. Considering a negative correlation between oxytocin level in cerebrospinal fluid and a history of suicidal attempts and AB in patients reported in one study [23], an increased expression of the OXTR gene associated with rs53576*G allele may represent a marker of decreased risk of developing AB owing to compensation of a low oxytocin level [22]. Although we failed to detect association of rs53576 with individual differences in aggressiveness, this locus comprising a specific haplotype (*G*G*G haplotype based on rs53576, rs2228485, rs237911) was associated with a reduced aggression in Tatars, which is at odds with previous studies to some extent [10, 22, 23]. One of the possible mechanisms regulating expression of the OXTR gene may be attributed to allele-dependent methylation of the OXTR gene, whose differential levels are related to rs53576 variants. In particular, it was detected that homozygous rs53576*G/G genotype was characterized by a significant decrease in the methylation level at the second exon of the OXTR gene compared to carriers of rs53576*A allele [24], which corresponds to a negative correlation between methylation level and gene expression.
It is suggested that rs7632287 examined in the present study is functional, since it is located within binding sites of transcription factors COUP-TF (related to the presence of rs7632287*A allele) and N-MYC, ARNT, and USF (related to the presence of rs7632287*G allele); however, the question on the mechanism of action on the OXTR gene expression remains unclear [25]. Despite previous findings on the association of the rs7632287*A allele with AB in adolescent boys [9] and men independent of the presence of mental illness [10], the present study failed to detect association of this locus with variance in aggression level. Similar results were reported by Johansson et al. [26], who also did not observe association of rs7632287 with developing aggression in men.
The data published to date indicate the involvement of rs2254298 located in intron 3 in the regulation of processes related to social behavior [9, 11]. Previously it was demonstrated that rs2254298*A allele was associated with severe social deficit in ASD and less severe social impairments in ADHD [11], while carriers of rs2254298*G allele with psychopathologies were characterized by higher risk of developing AB [10]. In the present study, we failed to detect association of rs2254298 with aggression level, which may be caused by the differences between the examined samples, since our sample did not comprise clinical forms of aggression.
As a result of the present study, for the first time, we established an ethnicity-specific role of the OXTR gene (based on rs53576, rs2228485, rs237911) in individual differences in aggression level in mentally healthy individuals, which is congruent to previously published ethnicity-specific association of candidate genes with individual personality traits [27]. Moreover, a modulating effect of smoking and paternal protection on the association of rs2228485 in the OXTR gene with examined personality construct was reported.
Although the present study has several advantages (sample homogeneity by age and education level, analysis of several SNPs in the same gene, the analysis of a significant number of sociodemographic parameters which may directly or indirectly affect regulation of gene expression, correction for multiple comparison), we did not include other significant components of the HPA axis (in particular, oxytocin gene (OXT), arginine-vasopressin (AVP), and corresponding receptors (AVPR1A, AVPR1B)) responsible for the regulation of hormonal and neurotransmitter functioning as a whole. Therefore, future research in this field has to consider interactions of the hypothalamic-pituitary-adrenal axis and other systems and include large-scale samples, which will make it possible to overcome limitations existing in the present study (average sample size and the absence of replication sample).
REFERENCES
Baron, R.A. and Richardson, D.R., Human Aggression, New York: Plenum, 1994.
http://www.gks.ru/free_doc/new_site/population/pravo/10-05.doc.
United Nations Office on Drugs and Crime, Reports on world crime trends, 2017. https://www.unodc.org/unodc/en/data-and-analysis/statistics/reports-on-world-crime-trends.html.
Porsch, R.M., Middeldorp, C.M., Cherny, S.S., et al., Longitudinal heritability of childhood aggression, Am. J. Med. Genet.,Part B, 2016, vol. 171, no. 5, pp. 697—707. https://doi.org/10.1002/ajmg.b.32420
Davydova, Yu.D., Litvinov, S.S., Enikeeva, R.F., et al., Recent advances in genetics of aggressive behavior, Vavilovskii Zh. Genet. Sel., 2018, vol. 22, no. 6, pp. 716—725. https://doi.org/10.18699/VJ18.415
Lee, R., Ferris, C., Van de Kar, L.D., and Coccaro, E.F., Cerebrospinal fluid oxytocin, life history of aggression, and personality disorder, Psychoneuroendocrinology, 2009, vol. 34, no. 10, pp. 1567—1573. https://doi.org/10.1016/j.psyneuen.2009.06.002
Lucht, M.J., Barnow, S., Sonnenfeld, C., et al., Associations between the oxytocin receptor gene (OXTR) and affect, loneliness and intelligence in normal subjects, Prog. Neuropsychopharmacol. Biol. Psychiatry, 2009, vol. 33, no. 5, pp. 860—866. https://doi.org/10.1016/j.pnpbp.2009.04.004
Tan, O., Musullulu, H., Raymond, J.S., et al., Oxytocin and vasopressin inhibit hyper-aggressive behaviour in socially isolated mice, Neuropharmacology, 2019, vol. 156, article 107573. https://doi.org/10.1016/j.neuropharm.2019.03.016
Hovey, D., Lindstedt, M., Zettergren, A., et al., Antisocial behavior and polymorphisms in the oxytocin receptor gene: findings in two independent samples, Mol. Psychiatry, 2016, vol. 21, no. 7, pp. 983—988. https://doi.org/10.1038/mp.2015.144
Valiullina, A.R., Studying the role of genes of neuropeptides and neurotransmitter systems in the development of aggressive human behavior, Cand. Sci. (Biol.) Dissertation, Ufa: Institute of Biochemistry and Genetics of Ural Scientific Centre, Russian Academy of Sciences, 2013, p. 159.
Baribeau, D.A., Dupuis, A., Paton, T.A., et al., Oxytocin receptor polymorphisms are differentially associated with social abilities across neurodevelopmental disorders, Sci. Rep., 2017, vol. 7, no. 1, p. 11618. https://doi.org/10.1038/s41598-017-10821-0
Mustafin, R.N., Kazantseva, A.V., Enikeeva, R.F., et al., Epigenetics of aggressive behavior, Russ. J. Genet., 2019, vol. 55, no. 9, pp. 1051—1060. https://doi.org/10.1134/S1022795419090096
Fragkaki, I., Cima, M., Verhagen, M., et al., Oxytocin receptor gene (OXTR) and deviant peer affiliation: a gene—environment interaction in adolescent antisocial behavior, J. Youth Adolesc., 2019, vol. 48, no. 1, pp. 86—101. https://doi.org/10.1007/s10964-018-0939-x
Cecil, C.A., Lysenko, L.J., Jaffee, S.R., et al., Environmental risk, oxytocin receptor gene (OXTR) methylation and youth callous-unemotional traits: a 13-year longitudinal study, Mol. Psychiatry, 2014, vol. 19, no. 10, pp. 1071—1077. https://doi.org/10.1038/mp.2014.95
Caldwell, H.K., Aulino, E.A., Freeman, A.R., et al., Oxytocin and behavior: lessons from knockout mice, Dev. Neurobiol., 2017, vol. 77, no. 2, pp. 190—201. https://doi.org/10.1002/dneu.22431
Khorasanchi, Z., Bahrami, A., Avan, A., et al., Passive smoking is associated with cognitive and emotional impairment in adolescent girls, J. Gen. Psychol., 2019, vol. 146, no. 1, pp. 68—78. https://doi.org/10.1080/00221309.2018.1535485
Sikdar, S., Joehanes, R., Joubert, B.R., et al., Comparison of smoking-related DNA methylation between newborns from prenatal exposure and adults from personal smoking, Epigenomics, 2019. https://doi.org/10.2217/epi-2019-0066
Tisak, J., Tisak, M.S., Baker, E.R., et al., The association among parental bonding, depression, social aggression, and criminal assault: are there gender differences between male and female youth offenders?, J. Interpers. Violence, 2017. https://doi.org/10.1177/0886260517744192
Kazantseva, A., Gaysina, D., Malykh, S., and Khusnutdinova, E., The role of dopamine transporter (SLC6A3) and dopamine D2 receptor/ankyrin repeat and kinase domain containing 1 (DRD2/ANKK1) gene polymorphisms in personality traits, Prog. Neuropsychopharmacol. Biol. Psychiatry, 2011, vol. 35, no. 4, pp. 1033—1040. https://doi.org/10.1016/j.pnpbp.2011.02.013
Wong, A.M., Scott, A.K., Johnson, C.S., et al., ERαΔ4, an ERα splice variant missing exon4, interacts with caveolin-3 and mGluR2/3, J. Neuroendocrinol., 2019, vol. 31, no. 6. e12725. https://doi.org/10.1111/jne.12725
DiFrancesco, J.C., Castellotti, B., Milanesi, R., et al., HCN ion channels and accessory proteins in epilepsy: genetic analysis of a large cohort of patients and review of the literature, Epilepsy Res., 2019, vol. 153, pp. 49—58. https://doi.org/10.1016/j.eplepsyres.2019.04.004
Parris, M.S., Grunebaum, M.F., Galfalvy, H.C., et al., Attempted suicide and oxytocin-related gene polymorphisms, J. Affective Disord., 2018, vol. 238, pp. 62—68. https://doi.org/10.1016/j.jad.2018.05.022
Jokinen, J., Chatzittofis, A., Hellström, C., et al., Low CSF oxytocin reflects high intent in suicide attempters, Psychoneuroendocrinology, 2012, vol. 37, no. 4, pp. 482—490. https://doi.org/10.1016/j.psyneuen.2011.07.016
Reiner, I., Van IJzendoorn, M.H., Bakermans-Kranenburg, M.J., et al., Methylation of the oxytocin receptor gene in clinically depressed patients compared to controls: the role of OXTR rs53576 genotype, J. Psychiatr. Res., 2015, vol. 65, pp. 9—15. https://doi.org/10.1016/j.jpsychires.2015.03.012
Campbell, D.B., Datta, D., Jones, S.T., et al., Association of oxytocin receptor (OXTR) gene variants with multiple phenotype domains of autism spectrum disorder, J. Neurodev. Disord., 2011, vol. 3, no. 2, pp. 101—112. https://doi.org/10.1007/s11689-010-9071-2
Johansson, A., Bergman, H., Corander, J., et al., Alcohol and aggressive behavior in men-moderating effects of oxytocin receptor gene (OXTR) polymorphisms, Genes Brain Behav., 2012, vol. 11, no. 2, pp. 214—221. https://doi.org/10.1111/j.1601-183X.2011.00744.x
Kazantseva, A.V., Kutlumbetova, Y.Y., Malykh, S.B., et al., Arginine—vasopressin receptor gene (AVPR1A, AVPR1B) polymorphisms and their relation to personality traits, Russ. J. Genet., 2014, vol. 50, no. 3, pp. 298—307. https://doi.org/10.1134/S1022795414030041
Funding
The present study was supported within the framework of the state task of the Ministry of Education and Science of the Russian Federation (project no. AAAA-A16-116020350032-1) and partially supported by a grant from the Russian Foundation for Basic Research (project no. 17-29-02195_ofi_m). DNA samples were obtained from the Shared Access Center Collection of Human Biological Materials at the Institute of Biochemistry and Genetics, Ufa Federal Research Centre, Russian Academy of Sciences, supported by the Program of Collections of Biological Resources of FASO of Russia (project no. 007-030164/2).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest. The authors declare no conflict of interest.
Statement of compliance with standards of research involving humans as subjects. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants involved in the study.
Additional information
Translated by A. Kazantseva
Rights and permissions
About this article
Cite this article
Davydova, Y.D., Kazantseva, A.V., Enikeeva, R.F. et al. The Role of Oxytocin Receptor (OXTR) Gene Polymorphisms in the Development of Aggressive Behavior in Healthy Individuals. Russ J Genet 56, 1129–1138 (2020). https://doi.org/10.1134/S1022795420090057
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1022795420090057