Abstract
In order to study the possibility of creating new plant forms resistant to phytopathogens, a collection of transgenic plants of model tobacco culture with new different plant protective genes was obtained by the agrobacterial transformation method. First an addition of a collection with serine proteinase inhibitor BWI-1a (ISP) from buckwheat with fragments of a spidroin gene as putable enhancer by vector constructions different designs was done. Secondly, transgenic plants with an antimicrobial peptide from sinthetic wheat Triticum kiharae and with defensine from Stellaria media. Comparative study of physiological characteristics of transgenic plants in biotests in vivo (with isolated leaves) and in vitro (with well biotests) was carried out. Regardless of the design of the vector construction, the target genes were expressed to a different extent in the tissues of all transgenic plants and their seed and vegetative progenies and gave their tissues antibacterial activity, indicating the synthesis of the functional protein. The introduction to the tobacco tissues of the heterologic plant protective genes of different nature that plants use in different defense mechanisms led to a similar increase in antibacterial activity of the transgenic tobacco tissues.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Identification of gene functions is known to be based on the analysis of phenotypic differences between mutant and wild-type phenotypes. Hence, the collections of insertion mutants may serve the basis for the “direct” and “reverse” genetics. Various proteins are believed to be an essentialpart of the protective mechanisms of plants against pests and diseases. They include defensines, as well as some enzymes, for example, 1,3-gluconases, chitinases, proteases, protease inhibitors, etc. The protective genes of plants provide a wide spectrum of antimicrobial effects and may be inserted directly into the genomes of pathogen-sensitive plants by methods of genetic engineering. A number of studies aimed at increasing the resistance to phytopathogens and pests via introduction of different plant genes to other plant species have been published. These studies demonstrated the expression of such proteins in cells of other species and revealed their protective effect [1–4]. A positive feature of introduction of a protective heterologous transgene is that its influence on the qualitative composition of the recipient plant may either be not observed or be even smaller than that in case of common breeding [5, 6]. At the same time, several studies carried out with transgenic tobacco plants with the nptII marker gene as an example showed that heterologous genes when put into a new plant genome environment may differ in the expression level. Moreover, their expression may change in the case of self-breeding, resulting in deviation from Mendelian segregation [7, 8]. Therefore, in spite of certain achievements, the potential of protective plant genes is far from being fully studied. The development of transgenic plant collections with a variety of introduced genes leads to the necessity of studying the expression and inheritance of transgenes in progeny, as well as revealing the connection between the site of the insertion and the function of the corresponding target gene.
Previous studies carried out at the Belozersky Institute of Physicochemical Biology revealed the full amino acid sequence of the serine protease inhibitor gene of buckwheat, on the basis of which the ISP gene was synthesized and introduced into the vector [9]. The transformed plants of three species (tobacco, potato, and Arabidopsis) which were formerly obtained with this vector and carried the ISP gene. These model plants demonstrated increased phytopathogenic activity of their tissues in compared to the nontransformed plants or the plants transformed with the vector without the target gene. However, interspecies differences in the spectrum of physiological activity and by in the number of morphological mutations were revealed [10–13]. The aforesaid implies that collections of insertion mutants with different heterologous protective plant genes may be considered as a valuable resource in order to study the gene functions and regulation [14].
It has been believed to be interesting to increase phytopathogenic activity of the ISP gene introduced into the tissues of transgenic plants, as well as to compare it with phytopathogenic activity of the another protective plant genes. According to the preliminarily data obtained by K.V. Sidoruk in his study with yeasts, some gene motifs could have served as enhancers for the synthesis of the ISP target gene. It was previously shown that the spidroin 1 synthetic gene does not require modifications to be expressed in plants. Plants were shown to be able to sustain synthetic cobweb protein genes in their genomes, as well as to perform synthesis of the corresponding proteins. The efficiency of expression of these genes, as well as the level of accumulation of their products, depended on the promoter efficacy, number of transgene repeats, organ specificity, and plant species [15, 16].
Genes of antimicrobial peptides (AMPs), the defensine Sm of chickweed (Stellaria media), and the WAMP peptide from Triticum kiharaе, which were previously obtained and characterized in the Laboratory of Molecular Genetic Principles of Plant Immunity of the Institute of General Genetics of the Russian Academy of Sciences, were used in order to compare the effect of introduction of other protective plant genes and to transform tobacco plants [17–20].
MATERIALS AND METHODS
Plant Material
The objects of the study were previously obtained transgenic tobacco plants (Nicotiana tabacum L.), cultivar Samsun, which carried the serine protease inhibitor gene (BWI-1a) from buckwheat seeds (ISP) [9–11]. To perform new transformation cycles, the constructions newly designed on the basis of the previous one, which contained the ISP target gene, were used. These new constructions contained the additional number of spidroin genes: the 2s motif of the spidroin 1 gene, variation E, was repeated twice; the 4s motif of the spidroin 1 gene, variation E, was repeated four times. Both transformation and obtainment of transgenic plants were performed as described in [11, 12].
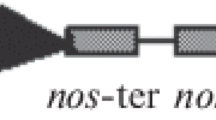
Moreover, new vectors which carried the defensine Sm gene of chickweed (the Sm line) and the WAMP antimicrobial peptide gene of Triticum kiharae were constructed in two modifications: prepropeptide (W) and the combination of the signaling and mature peptide (the SHP lines). The vectors were constructed on the basis of the pORE04 plasmid and contained neomycin phosphotransferase marker gene (nptII), as well as the protective target genes under the strongly constitutive 35S promoter of the cauliflower mosaic virus (Fig. 1).
Schematic diagrams of the vector constructions carrying the Sm defensine gene of chickweed and the WAMP gene of Triticum kiharae (рORE-SmD, рORE-W, рORE-SHP). SP—signaling peptide encoding motif; CD—C-terminal pro-domain encoding motif; Sm-D1 and WAMP1—the mature peptide encoding motives. PENTCUP2—the cryptic constitutive tobacco promoter. Р35SCaMV—the 35S promoter of the cauliflower mosaic virus (CaMV). TNos—nopalin synthase gene terminator. nptII—the kanamycin resistance gene. RB and LB—the insertion sites in the plant genome. N—the NotI restriction site. K—the KpnI restriction site.
These genes were previously obtained and characterized in the Laboratory of Molecular Genetic Principles of Plant Immunity of the Institute of General Genetics of the Russian Academy of Sciences [17–20].
Monitoring of the Presence of the nptII and Target Genes in Transgenic Plants
Monitoring of the presence of the transgenic insertion of the buckwheat protease inhibitor gene (trypsin inhibitor) and the AMP genes in the transgenic tobacco plants was performed by multiplex PCR, in which more than one pair of oligonucleotide primers were used simultaneously, resulting in coamplification of several DNA templates. One pair of primers provides the amplification of the target gene, while the other provides the amplification of a housekeeping gene fragment, which was used as the inner control of the reaction in a certain sample in order to exclude a false positive PCR result. The tobacco β-actin gene was used as the housekeeping gene. DNA was isolated from the top leaves of transgenic plants of the same line. The DNA isolated was used for the PCR as the template with primers specific to the ISP target gene (ISP-Dir 5'-GAGAACGAAGATGTGCGCG-3' and ISP-Rev 5'-ACATCACCACTGGGGTGTC-3'; 127 bp PCR product is synthesized) and to the tobacco actin gene (TOB-Act-For 5'-CTGGCATTGCAGATCGTATGA-3' and TOB-Act-Rev 5'-GCGCCACCACCTTGATCTT-3'; 75 bp PCR product is synthesized). The presence of the nptII marker gene in transgenic plants was verified with the primers specific to the nptII gene (nptII-Dir 5'-GCCAACGCTATGTCCTGATA-3' and nptII-Rev 5'-TATTCGGCTATGACTGGGCA-3'; 620 bp PCR product is synthesized). PCR was carried out using the ScreenMix kit (Eurogen, Russia) with the Mastercycler amplifier (Eppendorf, Germany). The PCR program was as follows: pre-denaturation at 94°С for 5 min; 38 cycles (94°С for 30 s, 60°С for 20 s, 72°С for 40 s); and additional amplification at 72°С for 3 min. The amplification products were separated in 3% agarose gel (for multiplex PCR) and 1% agarose gel (for the nptII PCR). The accumulation of PCR products was assessed by the intensity of DNA fragment luminescence in transmitted UV light.
To prove the expression of the АМР and ISP target genes and the expression of the nptII gene in the seed progeny obtained from individual plants, RNA was isolated with the ExtractRNA kit (Eurogen, Russia), cDNA was synthesized with the Mint kit (Eurogen, Russia), and PCR was performed with primers specific to a certain target gene or to the nptII gene.
Bacterial Culture
Tests for the resistance to bacterial infection were performed in well biotests using several phytopathogen bacterial strains (Pseudomonas syringae pv. tomatoe, Erwinia carotowora, and Clavibacter michiganensis pv. michiganensis), which were kindly provided by the Department of Plant Protection of the Russian State Agrarian University—Moscow Timiryazev Agricultural Academy.
Assessment of Antibacterial Activity of Plants
To prepare the lawn, 18 h bacterial cultures, which were grown on the liquid LB medium were used. Eight to ten wells with diameter of 0.5 cm were made in Petri dishes, which contained the agar LB medium and the lawn of the corresponding bacteria sown ex tempore, using a sterile screw for resin stoppers. The wells were filled with either tissue homogenates of aseptic plants of similar weight or equal volumes of cell-free extracts. Zones of bacterial growth inhibition around the wells were registered in 24 and/or 48 h.
To perform the biotests, isolated leaves were put in a humid chamber, injury was made with a sterile scalpel, and one drop of the night-old culture of Erwinia carotovora was introduced into the injury. The reaction results were registered in 18–20 h.
RESULTS AND DISCUSSION
The previously obtained collection of transgenic tobacco plants which carried the buckwheat trypsin inhibitor gene revealed the possibility to increase resistance to phytopathogens via introduction of one of the heterologous protective plant genes [10, 11]. The further work was also aimed at studying the possibility to increase the expression of the target antimicrobial peptide in transgenic plants. The ISP gene was introduced into the vectors which carried the nptII kanamycin resistance gene as the marker. In contrast to the original construction, the newly developed vectors contained additional elements (spodroin gene fragments). According to the aforesaid, data preliminarily obtained for yeasts showed that these fragments could have worked as enhancers and upregulated the target gene. As a result of agrobacterial transformation, each variation of the vector construction allowed us to obtain new series of transgenic plants carrying the BWI-1a buckwheat protease inhibitor gene (ISP). Plants containing the corresponding insertions were selected using the selective media with 150 mg/L kanamycin with subsequent verification of the DNA transformants by PCR.
It also appeared interesting to compare the phytopathogenic activity of transgenic plant tissues after the introduction of heterologous protective plant genes of different nature. To reach this goal, the chickweed defensine gene (Sm) and the protective wamp gene of Triticum kiharae were used to transform the tobacco plants. New vector constructions, some of which were used to perform agrobacterial transformation, were developed in order to study the protective effect of the AMP genes. Besides the nptII marker gene, they contained the following AMP genes: the chickweed defensine gene (Sm), the WAMP propeptide gene of Triticum kiharae (W1), and the signaling + mature WAMP peptide gene (SHP) (Fig. 1). As in the case of the buckwheat ISP protease inhibitor gene, the selection of regenerants was performed on the selective medium which contained 150 mg/L kanamycin. Designations of the transgenic lines, which carried the AMP genes were as follows: Sm—lines containing defensine from Stellaria media; SHP—lines containing both signaling and mature WAMP peptides from Triricum kiharae; and W—lines containing the WAMP prepropeptide.
Using these constructions, agrobacterial transformation of tobacco plants was carried out and new series of individual Km-resistant transformants were obtained. Screening of the lines for the presence of the nptII genes was performed along with removal of agrobacteria. Plants which carried the insertions were selected on the kanamycin-containing selective media, and the DNA transformants were further verified by PCR (Fig. 2). In experiments with vector constructions containing the ISP gene with different number of cobweb gene fragments, the regeneration rate and survival of transformants depended on the size of the vector. As a result, 17 new Km-positive transgenic plants with normal morphological traits carrying two spidroin genes (the IP 2s line) were selected via the analysis of the ISP genes, as well as five transformants carrying four spidroin genes (the IP 4s line).
The analysis of DNA-transformed plants for the presence of the nptII gene. Plants carrying the Sm defensine gene of chickweed (top row) and the plants carrying the antimicrobial peptide WAMP gene from Triricum kiharae of the SHP line (bottom row). M—the molecular weight marker; K0—nontransformed control plant; K+—plasmid DNA containing the vector construction; K—reaction purity control.
Use of the vector carrying the antimicrobial prepropeptide gene of the T. kiharae (the W plant line) also affected the regeneration rate and purity of the transformants and allowed selection of only three kanamycin-resistant plants with normal morphological traits. At the same time, more than 50 regenerants were selected for a shorter period of time using the vector constructions carrying the defensine gene from Stellaria media (the Sm line), as well as the genes encoding the mature antimicrobial signal peptide of Triticum kiharae (SHP lines).
Therefore, it was only an increase in the complexity of the vector construction that led to impediment of the transformation process, decrease in the percentage of transformed plants, and modifications of their phenotype regardless of the target gene nature. These plants were often characterized by growth inhibition, dwarfism, vitrification, and often extinction during the selection process as compared with plants which underwent transformation with simpler vector constructions. The selection of the AMP-carrying transgenic plants resulted in obtainment of 15 Sm tobacco plants carrying the defensine gene from chickweed, 25 SHP plants carrying the gene of the mature antimicrobial signal peptide of Triticum kiharae, and only one W1 plant carrying the propeptide gene of T. kiharae. All the plants obtained were sustained on the aseptic medium by the method of cutting. In some of the plants, elimination of a part of the vector or distortion of its integrity occurred during the selection process (Table 1). It is noteworthy that periodic verification of the collection of IP transgenic tobacco plants carrying the protease inhibitor gene, which were previously obtained by transformation with the construction without any additional genes (47 lines), did not reveal significant morphological modifications either in primary transformants or in the seed progeny of the first and second generations.
Biotests
We also performed comparative well biotests based on the suppression of the Erwinia lawn growth with tissues and sap of the new series of transgenic tobacco plants carrying genes of the aforesaid groups of protective proteins. They also revealed the presence of functional proteins in all the variations studied (Fig. 3). The comparative analysis of the ISP line collection in well biotests with the transformants carrying the new constructions showed that the plants of all experimental variations exceeded the control plants in the level of antimicrobial activity on average by 1.5–3 times with respect to both gram-positive and gram-negative bacteria. However, no statistically significant difference between them was observed (Table 2, Fig. 3).
The dish biotest for suppression of the Erwinia lawn by sap of transgenic tobacco plants carrying the protective target genes in a variety of vector constructions. K0—the control nontransgenic tobacco plant; K1—the control plant subjected to transformation with “empty” vector construction without target genes. (a) Wells 1–4—plant tissues transformed with the constructions with two spidroin genes; wells 5–7—the constructions with four spidroin genes; (b) wells 8–10—plants transformed with constructions carrying the Sm genes; wells 11–13—plants transformed with constructions carrying the wamp-1 gene of the W1 line; wells 14–16—plants transformed with constructions carrying the wamp-1 gene of the SHP line; (c) С11, С18—lines carrying the single ISP gene; C12—line losing the inserted gene.
Data of biotests were confirmed by molecular methods, which revealed the expression of the trypsin inhibitor target protein in tissues of all transgenic plants studied, including formerly obtained model tobacco, potato, and Arabidopsis plants [10, 22]. In a limited number of plants carrying the ISP gene, the expression of the gene introduced was carried out in order to confirm the presence of the functional target protein (Fig. 3).
(a) Verification of the presence of the transgenic insertion of the ISP gene of protease inhibitor of buckwheat (trypsin inhibitor) and β-actin control gene in transgenic tobacco plants of the T1 generation. Lanes 1–3—the seed progeny of the line C1; lanes 4, 5—the C7 line; lanes 6, 7—the C10 line; lanes 8–13—the C18 line; lanes 14–16—the C22 line. K0—nontransformed control plant. K+—plasmid DNA containing the ISP target gene. (b) Analysis of the ISP and npt II gene expression in transgenic tobacco plants of the T2 generation. Lane 4—plant 9 of the line C10; lanes 8, 9—plants 1 and 2 of the line C18 losing the Km resistance and phytopathogenic activity in vitro.
However, in spite of the general increase in phytopathogenic properties of tissues, some of the new transformants carrying the ISP gene of protease inhibitor together with additional parts of the spidroin gene demonstrated a tendency to decrease in their toxic effect in comparison with plants transformed with the ISP gene construction the only (Table 2). This was also confirmed in biotests with infection of isolated leaves of transgenic plants from different lines with Erwinia (Table 3). In biotests with isolated leaves, a difference between the transformants carrying only the ISP gene (С10, С18) and the plants transformed with more complex constructions was observed more clearly. In plants carrying additional genes, the bacterial growth was suppressed less effectively. These differences may most likely be explained by the fact that the most effective variations from the collection of transgenic plants carrying only the ISP gene were chosen as a result of long-term selection. Therefore, data of molecular genetic analysis and biological tests showed that tissues of all transgenic plants with the trypsin inhibitor target gene (ISP) suppressed the bacterial lawn growth and decreased the lysis zone in experiments with isolated plants more effectively than the control ones, regardless of the construction and the number of additional sidroin genes.
At the same time, no clear difference was observed between the suppression efficiency and the number of suggested additional enhancer spidroin genes in the vector construction. Apparently, in plant tissues, in contrast to yeasts, the spidroin genes did not produce a stimulatory effect on the target gene expression, though the increase in the size of the used vector led to the inhibition of morphogenesis.
Similar experiments were carried out for a series of new transformants carrying other protector genes. To compare the functional activity of different target genes expressed in the transgenic plants (ISP, Sm, and wamp), experiments with isolated leaves from the new collection were also performed (Table 4).
The data represented in the table show that all plants differed from one another by the level of resistance within each series, whereas between plants carrying different protector genes, no significant difference in the level of protection against phytopathogens was observed. Close to the control plants, clones were found among the series of transgenic plants carrying both the ISP trypsin inhibitor gene (the IP2s-2 line) and the wamp-1 gene (the SHP2 and SHP8 lines).
The most homogenous was the group of lines carrying the defensine gene of chickweed. In all other groups, the dispersion was observed even toward the increase in lysis (the SHP8 plant). However, the expected significant increase in the resistance to the pathogen was not observed. Most likely, as we expected previously, the site of an alien gene insertion and the vector construction affected the level of protection against a pathogen. However, the genetically determined reaction norm of an organism, apparently, limits the increase in the new peptide synthesis in addition to already available proteins.
As an additional test, germination of spores of Aspergillus nidulans was assessed in the extracts of leaves of both control and transgenic plants carrying the protease inhibitor gene. Plants from different generations carrying the original construction and plants transformed with the vector construction and carrying two and four spidroin genes apart from the target gene were tested (Table 5). It was shown that all plants obtained as a result of the agrobacterial transformation with vector constructions carrying heterologous protector genes of different nature acquired increased resistance to bacterial and fungal pathogens.
The seed progeny of the Т0 and Т1 generations of several individual ISP plants from different transgenic and control lines were analyzed. These included nontransformed plants (K0) and plants transformed with the “empty” vector without target gene (Ktr0). The quality of seeds was assessed, and their resistance to the selective agent (kanamycin) was selectively estimated. Progeny of the control lines demonstrated total absence of resistance to kanamycin. These plants were characterized by total loss of color and death of their seedlings in three-week growth on the selective medium, though the germination capacity of their seeds on the kanamycin-containing medium demonstrated little difference from that of the transgenic line seeds. The number of defective seeds in the first generation progeny of the transgenic lines carrying a single target gene (С10-2, С11-5, and С22-6) slightly exceeded the number of such seeds in the progeny of nontransformed plants (Table 6). The analysis of some transgenic lines carrying the AMP genes provided similar results.
It was shown that all plants transformed with more complex vector constructions demonstrated an increased number of defective seeds (sometimes up to 50%). Two plants carrying an additional spidroin gene (2s-3, 2s-8) were characterized by extremely low seed viability, in contrast to the seed progeny of other transgenic lines. At the same time, the Т1 seed progeny of the lines carrying the single ISP gene (С10-2, С11-5, and С22-6) demonstrated high quality of seeds and resistance to kanamycin. Apparently, introduction of new fragments of heterologous DNA often induces the distortion of microsporogenesis in transgenic plants, which is expressed in the appearance of an increased number of defective and unviable seeds, as well as in disturbance segregation by the trait of kanamycin resistance (the nptII marker gene) in comparison with the monogenic one in the first seed generation after self-pollination. Similar results were obtained in the study of the long-term subcultured collection of the transgenic tobacco lines carrying the single ISP gene [22]. These deviations may be considered to be the evidence of multiple transgene insertions [8]. It appears that longer selection of the effective variations may help select more stable and viable transgenic clones, which would demonstrate both a protective effect and normal phenotype even in further generations.
We observed these disorders in the analysis of insertion mutants of Arabidopsis transformed by the same construction with the ISP gene. The analysis revealed differences in the spectrum of insertion mutations obtained by transformation of Arabidopsis and tobacco plants with the same construction. Arabidopsis was characterized by a significantly higher number of morphological and phenological deviations from the normal type. This may be due to the significant differences in the genome structures of these two objects. The genome of Arabidopsis consists of preferably unique sequences, and insertion of any alien DNA may cause a mutation in it. Moreover, its mutation spectrum also depended on the vector complexity [12].
Therefore, the results obtained demonstrate the possibility to increase the resistance to phytopathogens in different plant species via introduction of additional heterologous plant genes (for example, the trypsin inhibitor gene). The comparative analysis of transgenic plants of different species transformed with the same gene revealed significant species-specific differences. The transgenic tissues of potato clones demonstrated slight phytopathogenic activity [10]. However, the prolonged selection made it possible to obtain more effective and stable clones of all plant species under study carrying the target gene of the inhibitor and demonstrating significant phytopathogenic activity. The investigaton of plants carrying the vector construction with additional spidroin genes showed that it was ineffective to use these genes as enhancers in higher plant tissues. On the whole, the efficiency of the target gene expression in transgenic plant tissues is affected by several factors, such as the transgene insertion site, the complexity of the vector construction, the number of copies inserted, and the efficiency of selection of transformants.
REFERENCES
Charity, J.A., Hughes, P., Anderson, M.A., et al., Pest and disease protection conferred by expression of barley β-hordothionin and Nicotiana alata proteinase inhibitor genes in transgenic tobacco, Funct. Plant Biol., 2005, vol. 32, pp. 35—44.
Lee, S.C. and Hwang, B.K., CASAR82A, a pathogen-induced pepper SAR8.2, exhibits an antifungal activity and its overexpression enhances disease resistance and stress tolerance, Plant Mol. Biol., 2006, vol. 61, pp. 95—109. https://doi.org/10.1007/s11103-005-6102-6
Ntui, V.O., Azadi, P., Thirukkumaran, G., et al., Increased resistance to Fusarium wilt in transgenic tobacco lines co-expressing chitinase and wasabi defensin genes, Plant Pathol., 2011, vol. 60, pp. 221—231. https://doi.org/10.1111/j.1365-3059.2010.02352.x
Scotton, D.C., Da, Silva., Azevedo, M., et al., Expression of the Theobroma cacao Bax-inhibitor-1 gene in tomato reduces infection by the hemibiotrophic pathogen Moniliophthora perniciosa,Mol. Plant Pathol., 2017, vol. 18, no. 7, pp. 1101—1112. https://doi.org/10.1111/mpp.12463
Khalf, M., Goulet, C., Vorster, J., et al., Tubers from potato lines expressing a tomato Kunitz protease inhibitor are substantially equivalent to parental and transgenic controls, Plant Biotechnol. J., 2010, vol. 8, pp. 155—169. https://doi.org/10.1111/j.1467-7652.2009.00471.x
Herman, R.A., Fast, B., Scherer, P.N., et al., Stacking transgenic event DAS-Ø15Ø7-1 alters maize composition less than traditional breeding, Plant Biotechnol. J., 2017, vol. 15, no. 10, pp. 1264—1272. https://doi.org/10.1111/pbi.12713
Deineko, E.V., Novoselya, T.V., Zagorskaya, A.A., et al., Inactivation of alien genes in transgenic tobacco plants (review), in Izuchenie genoma i geneticheskaya transformatsiya rastenii (Study of the Genome and the Genetic Transformation of Plants), Novosibirsk: Nauka, 2001, pp. 132—142.
Deineko, E.V., Study of the expression of heterologous and own genes in transgenic plants (on the example of Nicotiana tabacum L.), Doctoral (Biol.) Dissertation, Moscow: N. I. Vavilov Inst. Gen. Genet. Russ. Acad. Sci., 2004.
Belozersky, M.A., Dunaevsky, Y.E., Musolyamov, A.X., and Egorov, T.A., Complete amino acid sequence of the protease inhibitor from buckwheat seeds, FEBS Lett., 1995, vol. 371, pp. 264—266.
Dunaevsky, Y.E., Khadeeva, N.V., Belyakova, G.A., and Belozersky, M.A., Properties, physiological role and possible use in biotechnology of proteinase inhibitor from buckwheat seeds, Eur. J. Plant Sci. Biotechnol., 2009, vol. 3, spec. issue 1, pp. 39—44.
Khadeeva, N.V., Kochieva, E.Z., Cherednichenko, M.Yu., et al., Use of buckwheat seed protease inhibitor gene for improvement of tobacco and potato plant resistance to biotic stress, Biochemistry (Moscow), 2009, vol. 74, no. 3, pp. 260—267. https://doi.org/10.1134/S000629-7909030031
Khadeeva, N.V. and Yakovleva, E.Yu., Inheritance of marker and target genes in seed and vegetative progenies of transgenic tobacco plants carrying the buckwheat serine protease inhibitor gene, Russ. J. Genet., 2010, vol. 46, no. 1, pp. 50—56. https://doi.org/10.1134/S1022795410010084
Khadeeva, N.V., Yakovleva, E.Yu., Dunaevskii, Ya.E., and Belozerskii, M.A., Comparative analysis of tobacco and Arabidopsis insertional mutants, transformed with equal vector constructions, Russ. J. Genet., 2012, vol. 48, no. 2, pp. 170—178. https://doi.org/10.1134/S1022795412010097
Perez-Martin, F., Yuste-Lisbona, F.J., Pineda, B., et al., A collection of enhancer trap insertional mutants for functional genomics in tomato, Plant Biotechnol. J., 2017, vol. 15, pp. 1439—1452. https://doi.org/10.1111/pbi.12728
Abdeeva, I.A., Musiichuk, K.A., Abdeev, R.M., et al., Construction of transgenic tobacco plants expressing synthetic genes encoding proteins—analogues of proteins of the Nephila clavipes spider silk carcass, Kletochnye yadra i plastidy rastenii: biokhimiya i biotekhnologiya (Cell Nuclei and Plastids of Plants: Biochemistry and Biotechnology) (Proc. Int. Conf.), Minsk, 2004, pp. 135—140.
Abdeeva, I.A., The study of the expression of synthetic spidroin genes and the stability of their products in plants, Cand. Sci. (Biol.) Dissertation, Moscow: N.I. Vavilov Inst. Gen. Genet. Russ. Acad. Sci., 2004.
Rogozhin, E.A., Slavokhotova, A.A., Grishin, E.V., et al., A novel antifungal peptide from leaves of the weed Stellaria media L., Biochimie, 2015, vol. 116, pp. 125—132.
Istomina, E.A., Korostyleva, T.V., Rozhnova, N.A., et al., Genes encoding hevein-like antimicrobial peptides WAMPs: expression in response to phytohormones and environmental factors, Russ. J. Genet., 2016, vol. 52, no. 11, pp. 1176—1185. https://doi.org/10.1134/S1022795416110053
Odintsova, T.I., Korostyleva, T.V., Utkina, L.L., et al., Wheat antimicrobial peptides, Russ. J. Genet.: Appl. Res., 2013, vol. 3, no. 1, pp. 40—46. https://doi.org/10.1134/S2079059713010103
Odintsova, T.I., Slavokhotova, A.A., Istomina, E.A., et al., Molecular genetic basis and the role of antimicrobial peptides in plant resistance to pathogens, Science Review: Proceedings of International Scientific Conference, Czech Republic, Karlovy Vary–Russia, Moscow, 2015, Karlovy Vary: Skleněný Můstek—Kirov, MCNIP, 2015, pp. 49—51.
Korostyleva, T.V., Istomina, E.A., Konopkin, A.A., et al., Generation of vector constructs and transgenic tobacco plants carrying AMP genes, Russ. J. Genet., 2018, vol. 54, no. 13, suppl. C, pp. S41—S45. https://doi.org/10.1134/S0016675818130088
Khadeeva, N.V., Yakovleva, E.Y., Sydoruk, K.V., et al., Molecular genetic analysis of collection of transgenic tobacco plants with buckwheat serine proteases inhibitor gene during long-term subculture, Russ. J. Genet., 2017, vol. 53, no. 11, pp. 1—12. https://doi.org/10.1134/S1022795417110047
Funding
This work was carried out within the scope of governmental program no. 0112-2019-0002, “The Genetic Technologies in Biology, Medicine, and Agricultural and Nature Management.” The study was partially supported by the Russian Foundation for Basic Research (RFBR) and RFBR-Belarus, project nos. 19-016-00069 and 18-54-00008 respectively, as well as by the Russian Science Foundation, project no. 16-16-00032 (the designing of constructions to express the AMP genes of wheat T. kiharae).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflict of interest. This article does not contain any studies involving animals or human participants performed by any of the authors.
Additional information
Translated by M. Bibov
Rights and permissions
About this article
Cite this article
Khadeeva, N.V., Yakovleva, E.Y., Korostyleva, T.V. et al. Comparative Analysis of Transgenic Tobacco Plants with Different Heterologic Plant Defensive Genes. Russ J Genet 56, 307–316 (2020). https://doi.org/10.1134/S1022795420030084
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1022795420030084