Abstract
Satellite DNA, whose monomers form long arrays of tandem repeat ranging from hundreds or thousands of copies to several million base pairs, makes up at least 10% of the human genome. Application of new methods of sequencing and bioinformatics analysis opens the way to investigate the organization and functioning of human satellite DNA and contributes to the revision of the long-standing view about this part of the genome as “junk DNA.” One of the important features of satellite DNA is its participation in structural rearrangements in the human karyotype. This review examines the mechanisms of participation of satellite DNA in the formation of structural rearrangements, as well as the nature of transcription of tandem repeats in structural rearrangements in the karyotype of normal and tumor cells.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Repeated DNA sequences constitute a significant part of the eukaryotic genome, causing the C-value paradox phenomenon: when the number of transcribed sequences of structural and regulatory genes does not match the amount of DNA in the haploid set [1]. In the human genome, the share of duplicate elements accounts for more than two-thirds of the total DNA [2]. One example of this type of DNA is satellite DNA (satDNA), whose monomers form long arrays of tandem repeats ranging from hundreds or thousands of copies to several million base pairs (bp) in the genomes. The share of satDNA in the human genome is at least 10% [3].
Human satDNA is represented by various classes of tandem repeats that differ both in the length of monomers and in their enrichment in AT and CG base pairs. For example, α-satDNA and satDNA I are the AT-rich fraction of the genome, while β-satDNA is predominantly CG-rich [4]. Classical satellites II and III include the AT and CG pairs of DNA bases [5]. SatDNAs I, II, and III have a repeating sequence length of 5–20 bp, while α-, β-, and γ-satDNAs type are 171, 68, and 220 bp, respectively [5, 6].
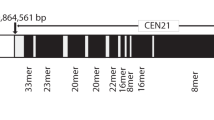
SatDNA has a specific location in the human karyotype. For example, centromeric regions consist of α-satDNA tandem repeats with a monomer length of about 171 bp, which are grouped into higher order repeats (HORs) [5, 6]. Separate monomers of satDNA in the composition of a HORs of nonhomologous chromosomes may differ, but within the centromere of a single chromosome, they have a very high level of homology, which is 95–98% [7]. They form extended areas in regions of constitutive heterochromatin. The length of high order repeats of α-satDNA can vary from 0.5 to 5 million bp, and the size of the centromeric unit of satDNA of individual chromosomes of the set may differ in various individuals by an order of magnitude [5].
The pericentromeric regions of chromosomes flanking the centromeres are characterized by lower levels of homogeneity of high order repeating elements. Their α-satDNA monomers are interspersed with other sequences and repeating elements [8]. In addition to the mobile elements of the genome from the LINE and SINE families, the pericentromeric regions of individual chromosomes are characterized by the presence of classical satellites in them, as well as β- and γ-satDNA [1, 6, 9].
Chromatin of the pericentromeric regions of most cell types has typical features of constitutive heterochromatin with a high degree of DNA methylation and trimethylation of histone H3 for lysine in position 9 [10], as well as interaction with nonhistone protein HP-1 (heterochromatin protein 1), a marker of repressed chromatin [11].
Centromeric chromatin has a unique epigenetic status. In addition to the presence of proteins characteristic of kinetochore formation, such as CENP-A, the DNA of the centromere region itself is hypomethy-lated [12]. In nucleosomes containing CENP-A, histone H4 is monomethylated for lysine in position 4 [13], and in nucleosomes carrying histone H3, it is additionally dimethylated for lysines in positions 9 and 27 [14]. In addition, in the human karyotype, α‑satDNA of centromeres of all chromosomes except for chromosome Y contains the CENP-B box: a motif of 17 base pairs that binds the CENP-B protein and participates in nonrandom phasing of nucleosomes in centromere regions [9, 15].
SatDNA is involved in the occurrence of structural rearrangements both in meiosis during the formation of hereditary information of gametes and in the transfer among a number of somatic cells of the body [5, 16]. Structural adjustments in the human karyotype can be divided into categories: balanced and unbalanced. In balanced rearrangements, in contrast to unbalanced ones, no loss or acquisition of genetic material is observed [17]. Although satDNA consists of tandem repeats and does not contain genes whose structure could be changed as a result of structural rearrangements, it has important structural and functional characteristics, the disturbance of which may have a phenotypic manifestation.
Thus, satDNA participates in the formation of kinetochore and proper segregation of chromosomes [9], encodes regulatory molecules [11, 18], determines the topology of the nucleus [19], secures fixed positioning of centromeres on chromosomes [20], and participates in the evolution of karyotypes [21, 22]. In addition, the movement of a chromosomal locus as a result of rearrangements into the satDNA region can lead to a change in the activity of genes located in that locus because of the “position effect” [23]. Disturbed topology at the chromatin stacking level in the nucleus can also affect the differential activity of genes by changing the position relative to regulatory elements [24]. In addition, owing to the homology of the nucleotide sequences of satDNA of a number of chromosomes, synapsis and segregation of nonhomologous chromosomes may become disturbed, and also nonreciprocal exchanges in meiotic division may arise [25].
MECHANISMS CAUSING STRUCTURAL CHROMOSOME REARRANGEMENTS WITH PARTICIPATION OF SATELLITE DNA
At present, it is known that physical chromosome breaks often occur precisely in satDNA of centromeric and pericentromeric chromosome regions [15]. This is partly due to the nature of its forming units: repeating DNA sequences help slow down or stop the replication fork, which can cause the formation of double-strand breaks in these areas [26]. It was shown that normally up to 40 double-strand breaks occur in cells during replication [27], which are restored by homologous recombination during synthetic and postsynthetic periods of the cell cycle [28]. Such recombination may jeopardize the stability of the genome, since it “allows” genetic exchange between homologous repetitive sequences distributed across the genome, which will provoke structural chromosomal rearrangements [29].
For example, one of the most common unbalanced translocations without phenotypic manifestation (cytogenetically visible copy number variations) is a rearrangement between satDNA III of the distal heterochromatin band of Y chromosome and short arms of acrocentric chromosomes, mainly chromosomes 15 and 22 [6, 30]. Owing to the homology of the satDNA III sequence constituting these regions, the sexual bivalent is associated with the short arms of acrocentric chromosomes and moves from the periphery to the central part of the nucleus at the pachytene stage of the prophase of the first division of meiosis, which disturbs the synapsis and further chromosome segregation [6, 25, 31]. Such an association may result in double-strand breaks of satDNA with the subsequent formation of a derived autosome containing the satDNA material of Y chromosome.
Another example of rearrangements involving satDNA are balanced Robertsonian translocations, which involve long arms of nonhomologous or (less commonly) homologous acrocentric chromosomes, with double-strand breaks and their recovery in centromeric or pericentromeric regions [21, 29, 32, 33].
Double-strand breaks, leading to abnormal (transverse) centromere separation, can simultaneously cause reduplication of one of the chromosome arms and the formation of an isochromosome [29, 32]. Recoupling of double-stranded breaks in the pericentromeric regions of sister chromatids (isochromatid break) by the U-type can lead to the formation of a dicentric isochromosome [29, 32].
At the same time, it was found that the very blocks of highly repetitive satDNA regions arise owing to recombination and repair of double-strand breaks [34]. Moreover, the features of the organization of pericentromeric regions of different chromosomes indicate that the formation of heterochromatic blocks is predominated by interchromosomal exchanges [35]. Slowing the replication fork can also lead to slipping of the polymerase and rereplication of individual DNA sections [36]. Normally, there are several parallel systems in cells that prevent polymerase slipping; however, such events may happen in the case of spontaneous disruptions of the cell cycle [37]. This leads to an increase in the copy number of satDNA monomers, which may not have a phenotypic manifestation and not be subjected to elimination.
An increase in the copy number of the repeating α‑satDNA elements contributes to stronger binding of the centromere to the spindle apparatus during meiosis [38]. An important role in maintaining the stability of α-satDNA belongs to the CENP-A protein, which not only is responsible for the proper formation of the kinetochore and the attachment of spindle apparatus microtubules [39] but also suppresses recombination in centromere regions of satDNA in human proliferating cells [20]. This makes it possible to distance hot recombination points from centromeres and prevent unwanted exchanges during cell divisions [20, 40].
At the same time, one of the factors that increase instability in the centromere regions of chromosomes is the peculiarity of the spatial organization of DNA of these regions, which is characterized by the formation of complex secondary and tertiary packing and the presence of regions with noncanonical DNA helix and hairpins [26, 41]. In addition to higher torsion stress, which provokes active introduction of double-strand breaks followed by the need to repair them, melting the DNA duplex in such areas requires a large amount of energy, which hinders the passage of the replication fork and causes it to stop and accumulate replication stress factors in centromere regions [26, 42, 43]. Thus, on one hand, repetitive satDNA blocks in the pericentromeric chromosome regions stabilize centromeres and, on the other hand, increase genome instability and contribute to the evolution of karyotypes [21, 22].
An increase in satDNA arrays requires maintaining their epigenetic status, while its disruption leads to chromosome fragility [44, 45]. Thus, disturbed methylation of the pericentromeric satellite II on human chromosomes 1 and 16 and satellite III of chromosome 9 [46] leads to the fragility of these chromosomes and the development of a ICF syndrome (Immunodeficiency, Centromeric Instability, Facial Anomalies), which is also characterized by severe mental retardation [47]. Cytogenetically, the ICF syndrome is manifested by decondensation of the pericentromeric heterochromatin regions of these chromosomes, associations in the centromeric regions on metaphase chromosomes of lymphocytes with the formation of radial structures, and by deletions with breaks in the pericentromeric satDNA [48]. It has been found that the development of this syndrome can be triggered by mutations in different genes, but all of them are directly or indirectly associated with maintaining the level of DNA methylation [49–51]. The exact mechanism leading to chromosome fragility in the areas of satDNA in the case of disturbed methylation has not been fully studied. However, indirectly, it can be caused by dysregulation of transcription in these regions or activation of mobile elements present in pericentromeric chromatin blocks, which will destabilize the centromeres [52, 53]. In addition, the enrichment in mobile elements with the monomers being located in the opposite direction can also provoke chromosomal rearrangements [54].
TRANSCRIPTIONAL ACTIVITY OF SATELLITE DNA IN THE PRESENCE OF STRUCTURAL REARRANGEMENTS IN KARYOTYPE
On the basis of the fact that the centromeric and pericentromeric regions of chromosomes lack protein-coding genes, it was historically believed that these regions are transcriptionally inert [15]. However, at present, satDNA transcription is shown for many organisms and cell lines [3, 55–57].
A large number of chromosomal rearrangements detected in karyotypes of hematological and solid tumors [16, 58], balanced and unbalanced rearrangements without phenotypic manifestation [6, 17, 59], and chromosome variants [6] involve the satDNA of centromeric and pericentromeric chromosome regions, which may disturb the transcriptional status of satDNA of these chromosomal regions [60].
Thus, the activation of transcription of satDNA II and α-satDNA is observed in a number of tumors, where overexpression of satellite II is specific namely to tumor cells [61]. It should be noted that the sequence of satDNA II of chromosome 1 (region 1q12) is one of the frequent break points in rearrangements in hematological tumors [16]. In addition, such rearrangements can promote the effect of heterochromatinization of the genes that should normally be transcribed or, conversely, promote the transcription of the genes that as a result of the rearrangement are distant from constitutive heterochromatin [58].
In general, it should be noted that aberrant transcription of classical satellites I, II, and III, as well as α-satDNA, is observed in many types of tumors [60, 62, 63] regardless of the involvement of satDNA regions in the rearrangement, which, apparently, is related to global genetic and epigenetic disorders of the genome of these cells [60, 64].
Thus, mutations or knockouts of the tumor suppressor genes KDM2A and BRCA1 lead to disruption of epigenetic modifications of histones in the centromeric and pericentromeric chromosome regions and their interaction with the nonhistone protein HP-1, which contributes to aberrant activation of satDNA transcription and leads to instability of the genome [60, 64].
In addition, it is assumed that the α-satDNA transcription hyperactivation of centromere repeats may lead to a decrease in the content or delocalization of the CENP-A protein [15, 20], which, in turn, may contribute to an increase in the number of chromosomal rearrangements and aneuploidy because of disturbed attachment of the spindle apparatus.
Hypomethylation of satDNA and especially the satellite II is also one of the characteristic epigenetic features of centromeric and pericentromeric chromosome regions in tumor cells [63]. However, it is known that satDNA transcription occurs regardless of the methylation status [65]. For example, unlike most tumors, no aberrant transcription of satDNA II was found in lymphocytes from patients with the ICF syndrome [66], in which this satellite is also hypomethylated.
A special place among chromosomal rearrangements belongs to the above-mentioned translocations between satDNA III of Y chromosome and autosomes, which have the population frequency of 1 : 2000 [59]. The carriers of such rearrangements are phenotypically normal but may have reproductive problems associated with impaired synaptonemal complex formation and chromosome segregation, as well as a possible risk of developing genomic imprinting diseases [6, 17, 25, 30]. It has been suggested that women inheriting, together with the father’s gamete, a derivative autosome containing satDNA III translocated from Y chromosome may be associated with higher risk of ovarian malignancy [67, 68]. Since heterochromatic regions do not contain genes, the pathological effect of such a rearrangement is not entirely clear. It is an open question whether this may be due to the transcription of satDNA, which is activated in many tumors, or its aberrant level. However, the latter is supported by the detection of satDNA transcription in the pericentromeric regions of a number of chromosomes and satDNA III of the Yq12 band, in particular, in the developing tissues of human testes occurring during normal differentiation [62, 69].
Understanding all aspects of satDNA transcription, namely, its strand- and stage specificity, a set of transcribed satDNA classes, changes in their transcription level, and the role of the formed noncoding RNAs, is also of interest from the point of view of the significance of polymorphic variants of heterochromatin blocks, the most frequent of which is a structural rearrangement of chromosome 9, a pericentromeric heterochromatin inversion [6]. Multiple studies do not provide clear answers to questions about the association of polymorphic variants of chromosomes, including changes in the copy number and location of satDNA of heterochromatic blocks, with disturbed reproductive function and clinical or phenotypic manifestations [32]. Despite data on the transcriptional activity of satDNA in human cells [55, 56], including embryonic and extraembryonic tissues [3, 70, 71], the transcriptional status or change in the level of satDNA transcription in the group of carriers of polymorphic variants remains unexplored.
CONCLUSIONS
Structural rearrangements are changes in the chromosome structure, which lead to the disturbed position of loci on chromosomes with or without loss of genetic material. A huge number of combinations of chromosomes involved in the formation of a particular type of rearrangement, as well as break points on the short or long arms of these chromosomes, determine the uniqueness of each chromosome rearrangement [17].
In view of the structural and functional significance of satDNA, structural rearrangements whose formation involves regions of constitutive heterochromatin of chromosomes deserve special attention. Understanding the mechanisms causing structural chromosomal rearrangements involving satDNA has been hampered for a long time by the lack of information about their organization and functioning. Despite the fact that in 2003 the Human Genome international project annotated the completion of the assembly of the reference human genome [72], it actually did not include more than 10% of the human genome [15]. Missing sequences in the assembly were mainly tandem repeats, including regions of nucleolar organizers, as well as satDNA of constitutive heterochromatin [73]. The use of new methods of sequencing and bioinformatics analysis made it possible to recreate the linear structure of repetitive elements for some chromosomes, for example, satDNA of the centromere region of the human Y chromosome [5, 74, 75]. SatDNA transcription is currently a proven fact for both normal and tumor human cells. A change in the transcriptional status of satDNA, and satDNA II, in particular, is a prognostic sign of a number of tumors [60, 64].
The data of current studies indicate the functional significance of satDNA, which normally plays an important role in maintaining the structural and functional integrity of the karyotype. The development of the genome and transcriptome research methods opens the way to further study of the organization and functioning of human satDNA and also contributes to the revision of the long-standing view about this part of the genome as “junk DNA.”
REFERENCES
Hemleben, V., Beridze, T.G., Bakhmanz, L., et al., Satellite DNA, Usp. Biol. Khim., 2003, vol. 43, pp. 267—306.
de Koning, A.P., Gu, W., Castoe, T.A., et al., Repetitive elements may comprise over two-thirds of the human genome, PLoS Genet., 2011, vol. 7, no. 12. e1002384. https://doi.org/10.1371/journal.pgen.1002384
Podgornaya, O.I., Ostromyshenskii, D.I., and Enukashvily, N.I., Who needs this junk, or genomic dark matter, Biochemistry (Moscow), 2018, vol. 83, no. 4, pp. 450—466. https://doi.org/10.1134/S0006297918040156
Sullivan, L.L., Chew, K., and Sullivan, B.A., α Satellite DNA variation and function of the human centromere, Nucleus, 2017, vol. 13, pp. 1—9. https://doi.org/10.1080/19491034.2017.1308989
Altemose, N., Miga, K.H., Maggioni, M., et al., Genomic characterization of large heterochromatic gaps in the human genome assembly, PLoS Comput. Biol., 2014, vol. 10, no. 5. e1003628. https://doi.org/10.1371/journal.pcbi.1003628
Liehr, T., Benign and Pathological Chromosomal Imbalances: Microscopic and Submicroscopic Copy Number Variations (CNVs) in Genetics and Counseling, Elsevier, 2014. 220 p.
Alkan, C., Ventura, M., Archidiacono, N., et al., Organization and evolution of primate centromeric DNA from whole-genome shotgun sequence data, PLoS Comput. Biol., 2007, vol. 3, no. 9, pp. 1807—1818. https://doi.org/10.1371/journal.pcbi.0030181
Klein, S.J. and O’Neill, R.J., Transposable elements: genome innovation, chromosome diversity, and centromere conflict, Chromosome Res., 2018, vol. 26, nos. 1—2, pp. 5—23. https://doi.org/10.1007/s10577-017-9569-5
Schueler, M.G. and Sullivan, B.A., Structural and functional dynamics of human centromeric chromatin, Annu. Rev. Genomics Hum. Genet., 2006, vol. 7, pp. 301—313. https://doi.org/10.1146/annurev.genom.7.080505.115613
Peng, J.C. and Karpen, G.H., Epigenetic regulation of heterochromatic DNA stability, Curr. Opin. Genet. Dev., 2010, vol. 18, no. 2, pp. 204—211. https://doi.org/10.1016/j.gde.2008.01.021
Bierhoff, H., Postepska-Igielska, A., and Grummt, I., Noisy silence: non-coding RNA and heterochromatin formation at repetitive elements, Epigenetics, 2014, vol. 9, no. 1, pp. 53—61. https://doi.org/10.4161/epi.26485
Sullivan, B.A. and Karpen, G.H., Centromeric chromatin exhibits a histone modification pattern that is distinct from both euchromatin and heterochromatin, Nat. Struct. Mol. Biol., 2004, vol. 11, no. 11, pp. 1076—1083. https://doi.org/10.1038/nsmb845
Hori, T., Shang, W.H., Toyoda, A., et al., Histone H4 Lys 20 monomethylation of the CENP-A nucleosome is essential for kinetochore assembly, Dev. Cell, 2014, vol. 29, no. 6, pp. 740—749. https://doi.org/10.1016/j.devcel.2014.05.001
Bailey, A.O., Panchenko, T., Shabanowitz, J., et al., Identification of the post-translational modifications present in centromeric chromatin, Mol. Cell. Proteom., 2016, vol. 15, no. 3, pp. 918—931. https://doi.org/10.1074/mcp.M115.053710
Black, E.M. and Giunta, S., Repetitive fragile sites: centromere satellite DNA as a source of genome instability in human diseases, Genes (Basel), 2018, vol. 9, no. 12, p. 615. https://doi.org/10.3390/genes9120615
Fournier, A., Florin, A., Lefebvre, C., et al., Genetics and epigenetics of 1q rearrangements in hematological malignancies, Cytogenet. Genome Res., 2007, vol. 118, nos. 2—4, pp. 320—327. https://doi.org/10.1159/000108316
Gardner, R.J.M. and Amor, D.J., Chromosome Abnormalities and Genetic Counseling, Oxford: Oxford Univ. Press, 2018.
Dimitri, P., Corradini, N., Rossi, F., et al., The paradox of functional heterochromatin, Bioessays, 2005, vol. 27, no. 1, pp. 29—41. https://doi.org/10.1002/bies.20158
Scheuermann, M.O., Tajbakhsh, J., Kurz, A., et al., Topology of genes and nontranscribed sequences in human interphase nuclei, Exp. Cell Res., 2004, vol. 301, no. 2, pp. 266—279. https://doi.org/10.1016/j.yexcr.2004.08.031
Giunta, S. and Funabiki, H., Integrity of the human centromere DNA repeats is protected by CENP-A, CENP-C, and CENP-T, Proc. Natl. Acad. Sci. U.S.A., 2017, vol. 114, no. 8, pp. 1928—1933. https://doi.org/10.1073/pnas.1615133114
Amor, D.J., Bentley, K., Ryan, J., et al., Human centromere repositioning “in progress”, Proc. Natl. Acad. Sci. U.S.A., 2004, vol. 101, no. 17, pp. 6542—6547. https://doi.org/10.1073/pnas.0308637101
Jagannathan, M. and Yamashita, Y.M., Function of junk: pericentromeric satellite DNA in chromosome maintenance, Cold Spring Harb. Symp. Quant. Biol., 2017, vol. 82, pp. 319—327. https://doi.org/10.1101/sqb.2017.82.034504
Grewal, S.I. and Jia, S., Heterochromatin revisited, Nat. Rev. Genet., 2007, vol. 8, no. 1, pp. 35—46. https://doi.org/10.1038/nrg2008
Eichler, E.E., Duplication, structure and the evolution of the human genome, Mol. Cytogenet., 2017, vol. 10, no. 1, p. L27.
Metzler-Guillemain, C., Mignon, C., Depetris, D., et al., Bivalent 15 regularly associates with the sex vesicle in normal male meiosis, Chromosome Res., 1999, vol. 7, pp. 369—378.
Crosetto, N., Mitra, A., Silva, M.J., et al., Nucleotide-resolution DNA double-strand break mapping by next-generation sequencing, Nat. Methods, 2013, vol. 10, no. 4, pp. 361—365. https://doi.org/10.1038/nmeth.2408
Tomasetti, C., Li, L., and Vogelstein, B., Stem cell divisions, somatic mutations, cancer etiology, and cancer prevention, Science, 2017, vol. 355, no. 6331, pp. 1330—1334. https://doi.org/10.1126/science.aaf9011
Jackson, S.P. and Bartek, J., The DNA-damage response in human biology and disease, Nature, 2009, vol. 461, no. 7267, pp. 1071—1078. https://doi.org/10.1038/nature08467
Barra, V. and Fachinetti, D., The dark side of centromeres: types, causes and consequences of structural abnormalities implicating centromeric DNA, Nat. Commun., 2018, vol. 9, no. 1, p. 4340. https://doi.org/10.1038/s41467-018-06545-y
Markova, Zh.G., Min’zhenkova, M.V., Musatova, E.V., et al., Unbalanced Y-autosomal translocations without phenotypic manifestations, Med. Genet., 2018, vol. 11, pp. 7—10. https://doi.org/10.25557/2073-7998
Kolomiets, O.L., Lelekova, M.A., Kashintsova, A.A., et al., Detection of disorders of meiosis and spermatogenesis using light, electron and fluorescence microscopy, Androl. Genital.Khir., 2018, vol. 19, no. 1. https://doi.org/10.17650/2070-9781-2018-19-1-00-00
Baranov, V.S. and Kuznetsova, T.V., Tsitogenetika embrional’nogo razvitiya cheloveka (Cytogenetics of Human Embryonic Development), St. Petersburg: N-L, 2007.
Kovaleva, N.V., Homologous Robertsonian translocations: spectrum, sex ratios, and reproductive risks, Russ. J. Genet., 2019, vol. 55, no. 1, pp. 10—23. https://doi.org/10.1134/S1022795419010095
McFarlane, R.J. and Humphrey, T.C., A role for recombination in centromere function, Trends Genet., 2010, vol. 26, no. 5, pp. 209—213. https://doi.org/10.1016/j.tig.2010.02.005
Lee, H., Hayden, K.E., and Willard, H.F., Organization and molecular evolution of CENP-A associated satellite DNA families in a basal primate genome, Genome Biol. Evol., 2011, vol. 3, pp. 1136—1149. https://doi.org/10.1093/gbe/evr083
Arias, E.E. and Walter, J.C., Strength in numbers: preventing rereplication via multiple mechanisms in eukaryotic cells, Genes Dev., 2007, vol. 21, no. 5, pp. 497—518. https://doi.org/10.1101/gad.1508907
Archambault, V., Ikui, A.E., Drapkin, B.J., et al., Disruption of mechanisms that prevent rereplication triggers a DNA damage response, Mol. Cell. Biol., 2005, vol. 25, no. 15, pp. 6707—6721. https://doi.org/10.1128/MCB.25.15.6707-6721.2005
Iwata-Otsubo, A., Dawicki-McKenna, J.M., Akera, T., et al., Expanded satellite repeats amplify a discrete CENP-A nucleosome assembly site on chromosomes that drive in female meiosis, Curr. Biol., 2018, vol. 27, no. 15, pp. 2365—2373. https://doi.org/10.1016/j.cub.2017.06.069
Müller, S. and Almouzni, G., Chromatin dynamics during the cell cycle, Nat. Rev. Genet., 2017, vol. 18, no. 3, pp. 192—208. https://doi.org/10.1038/nrg.2016.157
Choo, K.H.A., Why is the centromere so cold?, Genome Res., 1998, vol. 8, pp. 81—82. https://doi.org/10.1101/gr.8.2.81
Kasinathan, S. and Henikoff, S., Non-B-form DNA is enriched at centromeres, Mol. Biol. Evol., 2018, vol. 35, no. 4, pp. 949—962. https://doi.org/10.1093/molbev/msy010
Aze, A., Sannino, V., Soffientini, P., et al., Centromeric DNA replication reconstitution reveals DNA loops and ATR checkpoint suppression, Nat. Cell Biol., 2016, vol. 18, no. 6, pp. 684—691. https://doi.org/10.1038/ncb3344
Lai, X., Broderick, R., Bergoglio, V., et al., MUS81 nuclease activity is essential for replication stress tolerance and chromosome segregation in BRCA2-deficient cells, Nat. Commun., 2016, vol. 8. 15983. https://doi.org/10.1038/ncomms15983
Jaco, I., Vera, E., and Blasco, M.A., Centromere mitotic recombination in mammalian cells, J. Cell Biol., 2008, vol. 181, no. 6, pp. 885—892. https://doi.org/10.1083/jcb.200803042
Miniou, P., Jeanpierre, M., Blanquet, V., et al., Abnormal methylation pattern in constitutive and facultative (X inactive chromosome) heterochromatin of ICF patients, Hum. Mol. Genet., 1994, vol. 3, no. 12, pp. 2093—2102.
Jeanpierre, M., Turleau, C., Aurias, A., et al., An embryonic-like methylation pattern of classical satellite DNA is observed in ICF syndrome, Hum. Mol. Genet., 1993, vol. 2, no. 6, pp. 731—735.
Hagleitner, M.M., Lankester, A., Maraschio, P., et al., Clinical spectrum of immunodeficiency, centromeric instability and facial dysmorphism (ICF syndrome), J. Med. Genet., 2008, vol. 45, no. 2, pp. 93—99. https://doi.org/10.1136/jmg.2007.053397
Ehrlich, M., The ICF syndrome, a DNA methyltransferase 3B deficiency and immunodeficiency disease, Clin. Immunol., 2003, vol. 109, no. 1, pp. 17—28. https://doi.org/10.1016/S1521-6616(03)00201-8
Wijmenga, C., Hansen, R.S., Gimelli, G., et al., Genetic variation in ICF syndrome: evidence for genetic heterogeneity, Hum. Mutat., 2000, vol. 16, no. 6, pp. 509—517. https://doi.org/10.1002/1098-1004(200012)16:6<509::AID-HUMU8>3.0.CO;2-V
Thijssen, P.E., Ito, Y., Grillo, G., et al., Mutations in CDCA7 and HELLS cause immunodeficiency-centromeric instability—facial anomalies syndrome, Nat. Commun., 2015, vol. 6, p. 7870. https://doi.org/10.1038/ncomms8870
Unoki, M., Funabiki, H., and Velasco, G., CDCA7 and HELLS mutations undermine nonhomologous end joining in centromeric instability syndrome, J. Clin. Invest., 2018, vol. 129, no. 1, pp. 78—92. https://doi.org/10.1172/JCI99751
Mills, R.E., Bennett, E.A., Iskow, R.C., et al., Which transposable elements are active in the human genome?, Trends Genet., 2007, vol. 23, no. 4, pp. 183—191. https://doi.org/10.1016/j.tig.2007.02.006
McNulty, S.M., Sullivan, L.L., and Sullivan, B.A., Human centromeres produce chromosome-specific and array-specific satellite transcripts that are complexed with CENP-A and CENP-C, Dev. Cell, 2017, vol. 42, no. 3, pp. 226—240. https://doi.org/10.1016/j.devcel.2017.07.001
Lemoine, F.J., Degtyareva, N.P., Lobachev, K., et al., Chromosomal translocations in yeast induced by low levels of DNA polymerase a model for chromosome fragile sites, Cell, 2005, vol. 120, no. 5, pp. 587—598. https://doi.org/10.1016/j.cell.2004.12.039
Enukashvily, N.I. and Ponomartsev, N.V., Mammalian satellite DNA: a speaking dumb, Adv. Protein Chem. Struct. Biol., 2013, vol. 90, pp. 31—65. https://doi.org/10.1016/B978-0-12-410523-2.00002-X
Biscotti, M.A., Canapa, A., Forconi, M., et al., Transcription of tandemly repetitive DNA: functional roles, Chromosome Res., 2015, vol. 23, pp. 463—477. https://doi.org/10.1007/s10577-015-9494-4
Trofimova, I. and Krasikova, A., Transcription of highly repetitive tandemly organized DNA in amphibians and birds: a historical overview and modern concepts, RNA Biol., 2016, vol. 13, no. 12, pp. 1246—1257. https://doi.org/10.1080/15476286.2016.1240142
Fournier, A., McLeer-Florin, A., and Lefebvre, C., 1q12 chromosome translocations form aberrant heterochromatic foci associated with changes in nuclear architecture and gene expression in B cell lymphoma, EMBO Mol. Med., 2010, vol. 2, no. 5, pp. 159—171. https://doi.org/10.1002/emmm.201000067
Alitalo, T., Tiihonen, J., Hakola, P., et al., Molecular characterization of Y;15 translocation segregating in a family, Hum. Genet., 1988, vol. 79, no. 1, pp. 29—35. https://doi.org/10.1007/bf00291705
Ferreira, D., Meles, S., Escudeiro, A., et al., Satellite non-coding RNAs: the emerging players in cells, cellular pathways and cancer, Chromosome Res., 2015, vol. 23, no. 3, pp. 479—493. https://doi.org/10.1007/s10577-015-9482-8
Ting, D.T., Lipson, D., Paul, S., et al., Aberrant overexpression of satellite repeats in pancreatic and other epithelial cancers, Science, 2011, vol. 331, no. 6017, pp. 593—596. https://doi.org/10.1126/science.1200801
Eymery, A., Horard, B., Atifi-Borel, M.El., et al., A transcriptomic analysis of human centromeric and pericentric sequences in normal and tumor cells, Nucleic Acids Res., 2009, vol. 37, no. 19, pp. 6340—6354. https://doi.org/10.1093/nar/gkp639
Hall, L.L., Byron, M., Carone, D.M., et al., Demethylated HSATII DNA and HSATII RNA foci sequester PRC1 and MeCP2 into cancer-specific nuclear bodies, Cell Rep., 2017, vol. 18, no. 12, pp. 2943—2956. https://doi.org/10.1016/j.celrep.2017.02.072
Bersani, F., Lee, E., Kharchenko, P.V., et al., Pericentromeric satellite repeat expansions through RNA-derived DNA intermediates in cancer, Proc. Natl. Acad. Sci. U.S.A., 2015, vol. 112, no. 49, pp. 15148—15153. https://doi.org/10.1073/pnas.1518008112
Eymery, A., Callanan, M., and Vourc’h, C., The secret message of heterochromatin: new insights into the mechanisms and function of centromeric and pericentric repeat sequence transcription, Int. J. Dev. Biol., 2009, vol. 53, nos. 2—3, pp. 259—268. https://doi.org/10.1387/ijdb.082673ae
Alexiadis, V., Ballestas, M.E., Sanchez, C., et al., RNAPol-ChIP analysis of transcription from FSHD-linked tandem repeats and satellite DNA, Biochim. Biophys. Acta, 2007, vol. 1769, no. 1, pp. 29—40. https://doi.org/10.1016/j.bbaexp.2006.11.006
Hoshi, N., Fujita, M., Mikuni, M., et al., Seminoma in a postmenopausal woman with a Y;15 translocation in peripheral blood lymphocytes and a t(Y;15)/45,X Turner mosaic pattern in skin fibroblasts, J. Med. Genet., 1998, vol. 35, no. 10, pp. 852—856. https://doi.org/10.1136/jmg.35.10.852
Gravholt, C.H., Fedder, J., Naeraa, R.W., et al., Occurrence of gonadoblastoma in females with Turner syndrome and Y chromosome material: a population study, J. Clin. Endocrinol. Metab., 2000, vol. 85, no. 9, pp. 3199—3202. https://doi.org/10.1210/jcem.85.9.6800
Jehan, Z., Vallinayagam, S., Tiwari, S., et al., Novel noncoding RNA from human Y distal heterochromatic block (Yq12) generates testis-specific chimeric CDC2L2, Genome Res., 2007, vol. 17, no. 4, pp. 433—440. https://doi.org/10.1101/gr.5155706
Kuznetsova, T.V., Enukashvili, N.I., Trofimova, I.L., et al., Localization and transcription of the centromeric heterochromatin of chromosome 1 in human embryonic and extraembryonic tissues, Med. Genet., 2012, vol. 11, no. 4(118), pp. 19—24.
Trofimova, I.L., Enukashvili, N.I., Kuznetsova, T.V., et al., Transcription of satellite DNA in human embryogenesis: a review of the literature and our own data, Med. Genet., 2018, vol. 17, no. 3, pp. 3—7. https://doi.org/10.25557/2073-7998.2018.03.3-7
Collins, F.S., Green, E.D., and Guttmacher, A.E., A vision for the future of genomics research, Nature, 2003, vol. 422, no. 6934, pp. 835—847. https://doi.org/10.1038/nature01626
Miga, K.H., Completing the human genome: the progress and challenge of satellite DNA assembly, Chromosome Res., 2015, vol. 23, no. 3, pp. 421—426. https://doi.org/10.1007/s10577-015-9488-2
Miga, K.H., Newton, Y., Jain, M., et al., Centromere reference models for human chromosomes X and Y satellite arrays, Genome Res., 2014, vol. 24, no. 4, pp. 697—707. https://doi.org/10.1101/gr.159624.113
Jain, M., Koren, S., Miga, K.H., et al., Nanopore sequencing and assembly of a human genome with ultra-long reads, Nat. Biotechnol., 2018, vol. 36, no. 4, pp. 338—345. https://doi.org/10.1038/nbt.4060
Funding
This work was financially supported by the Russian Foundation for Basic Research (no. 18-34-00279).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflict of interest. This article does not contain any studies involving animals or human participants performed by any of the authors.
Additional information
Translated by K. Lazarev
Rights and permissions
About this article
Cite this article
Puppo, I.L., Saifitdinova, A.F. & Tonyan, Z.N. The Role of Satellite DNA in Causing Structural Rearrangements in Human Karyotype. Russ J Genet 56, 41–47 (2020). https://doi.org/10.1134/S1022795419080155
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1022795419080155