Abstract
Isolated mitochondria from lupine (Lupinus angustifolius L.) cotyledons were used to study the effect of temperature in the range of 20–40°C on oxidation of malate and other NAD-dependent respiratory substrates as well as on the activity of alternative CN-resistant oxidase. The metabolic responses of mitochondria to heat treatment analyzed in vitro were compared with metabolic activities of organelles isolated from seedlings exposed to elevated temperature in vivo. Mild warming of the incubation medium (20–30°C) accelerated oxygen uptake by mitochondria during malate oxidation in the presence of glutamate in the active phosphorylating state (state 3) and, to a lesser extent, in the state 4 (in the absence of ADP). The enhancement of mitochondrial respiration at increasing temperature in this range was entirely due to the activation of the cytochrome pathway in electron-transport chain (ETC). At temperatures of 35–40°С, an appreciable inhibition of the alternative respiration pathway was observed. The successive additions of ADP during malate oxidation under hyperthermia (35–40°С) caused the improvement (phenomenon of conditioning) of oxidative phosphorylation parameters. After the second addition of 100–200 μM ADP, the rate of substrate oxidation in state 3 and the respiratory control ratio (RCR) increased significantly. Similar changes in mitochondrial respiration under hyperthermia were found upon oxidation of other NAD-dependent substrates, but they did not occur during succinate oxidation catalyzed by ETC complex II. In mitochondria isolated from cotyledons of lupine seedlings that were exposed in vivo to high temperature (35°C for 12 h), the malate oxidation at 25 or 35°C was also characterized by a sharp decrease in RCR and the state 3 rate after the first addition of ADP, but these parameters were restored during several cycles of phosphorylation. Oxidation of malate and other NAD-dependent substrates catalyzed by complex I after heating of mitochondria at 40°C in the presence of 10 mM MgCl2 was substantially inhibited and was carried out by rotenone-insensitive NADH dehydrogenases. The results suggest that the suppressed oxidation of NAD-dependent substrates in lupine cotyledon mitochondria at high temperature is probably due to the reversible transformation of complex I from the active to the deactivated form (A/D transition). Possible physiological significance of such regulatory changes in plant respiration in response to adverse environmental conditions is discussed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
The influence of extreme temperatures on respiration and other basic physiological processes in plants is an important issue in fundamental science and also has practical significance. These stress factors, along with drought and salinity, have the strongest negative impact on productivity of the most important agricultural crops. The study of plant respiration as a function of temperature has more than a century-long history, starting from the experiments of F. Foucompre and I.P. Borodin [1] in the second half of the 19th century. The works of Russian scientists, including A.N. Volkov, V.N. Palladin, O.A. Semikhatova, V.N. Zholkevich, and V.K. Voinikov, contributed greatly to clarifying this issue. Based on the sustained scientific interest in the problem, it was found that the action of extreme temperatures on plants is accompanied by profound changes in respiratory metabolism, whose central element is the functioning of mitochondria. Extreme temperatures were shown to inhibit the respiratory metabolism of mitochondria and/or reduce its energy efficiency due to the uncoupling of oxidative phosphorylation, which entrains the negative effect on the rate of seedling growth [2, 3]. In order to survive under these conditions, the plants mobilize a variety of defense mechanisms that modify metabolic reactions, including respiratory metabolism, which allows them to adapt to environmental stresses.
It is known that various abiotic stress factors, including extreme temperature conditions—the impact of hypothermia being the best characterized—induce a fairly uniform response in plant mitochondria aimed at the counteraction of imminent oxidative stress. This includes the activation of enzymes capable of detoxifying reactive oxygen species (ROS), the induction of free oxidation by CN-resistant alternative oxidase (AOX) and rotenone-insensitive NAD(P)H-dehydrogenases, and the energy uncoupling in electron transport chain (ETC) at the level of uncoupling proteins [4, 5]. The main function of AOX and other noncoupled alternative electron transport pathways is the prevention of excessive ROS generation by mitochondria and the maintenance of their oxidative activity under stress conditions [6–8]. Recently, ample evidence has accumulated that such structural and functional flexibility of mitochondria plays an important role in plant adaptation to adverse environmental factors [5–8].
Unlike the extensive studies on hypothermia, the influence of elevated temperatures on functioning of plant mitochondria remains poorly understood. Available literature data are contradictory, partly because of insufficient quality of organelles used in early studies [3]. However, even the present-day works contain conflicting statements that hyperthermia conditions (35°C and above) activate, inhibit, or have no influence on the oxidation of succinate, malate, α-ketoglutarate, and other respiratory substrates in intact plant mitochondria [9–11]. As already noted above, the mitochondrial AOX activity plays an important role in plant resistance to various stress factors, including hypothermia. However, the activity of AOX under hyperthermia is a highly controversial issue [4, 8]. For example, when the temperature of incubation medium was raised from 25 to 42°C, the contribution of AOX to the rate of succinate oxidation by mitochondria of bean seedlings decreased from 38 to 0%, while the rate of oxygen uptake increased almost twofold [11]. On the other hand, the study of succinate oxidation by soybean cotyledon mitochondria revealed no significant differences in the temperature sensitivity of the cytochrome-mediated and alternative oxidation pathways [10]. Apparently, the respiratory and metabolic activity responses of isolated mitochondria to hypothermia and hyperthermia involve many tissue-specific and species-specific aspects. They may also depend on the level of intrinsic or acquired thermotolerance, even in plants belonging to the same species [12]. Furthermore, the respiratory responses would obviously depend on the design of experiments with plants, as well as on the methods for monitoring the respiration of mitochondria under the action of high temperature.
Among the ETC complexes of plant mitochondria, complex I was shown to be the least tolerant to hyperthermia. For example, a 30-min incubation of maize seedling mitochondria at 27°C was followed by an almost complete inactivation of malate oxidation in state 3. At the same time, in the presence of exogenous NAD+, the oxidation of NAD-dependent substrates could proceed by means of rotenone-insensitive NAD(P)H dehydrogenases located on the matrix side of ETC [13]. The bases of low stability of complex I in plant mitochondria at elevated temperature remain poorly understood. However, hyperthermia is known to stimulate the transporter-mediated release of the NAD+ coenzyme from the mitochondrial matrix, thus inhibiting NAD-dependent glycine oxidation [14]. In addition, by analogy with animal mitochondria, it can be assumed that hyperthermia promotes the reversible transition (or transformation of operation mode) of complex I from the active state (A—active) to the inactive state (D—deactivated) [15]. The characteristics of such A/D transformation of complex I operation under stress conditions were extensively studied; in addition to animal mitochondria, the A/D transition was found in mitochondria of fungi and bacteria [16]. The possibility of a similar transformation of the complex I operation mode in higher plants under the action of high temperature or other environmental stress factors has not been studied so far, although its occurrence is considered highly probable, given the similar structure of this complex in the mitochondria of animals and plants [17].
The aim of this work was to study the effect of high temperature on the oxidation of NAD-dependent substrates through various respiratory pathways and on the functional state of complex I in mitochondria of lupine seedling cotyledons.
MATERIALS AND METHODS
Material of study. Mitochondria were isolated from cotyledons of etiolated seedlings of narrow-leaved lupine (Lupinus angustifolius L., cv. Dikaf-14). Lupine seedlings were grown for 5 days on water in a thermostat at 24°C in the dark. In experiments designed to study the effect of elevated temperature on respiration, 5-day-old lupine seedlings were heated in a thermostat at 37°C for 12 h.
Isolation of mitochondria from untreated and heat-treated cotyledons of lupine seedlings was carried out using a differential centrifugation method described earlier [18] with a slight modification. Lupine cotyledons (12 g) were rinsed with distilled water, dried, cooled, and ground in a mortar with a homogenization medium (1 : 4 ratio) containing 0.5 M sucrose, 50 mM Mops buffer (pH 8.0), 10 mM EDTA, 5 mM dithiothreitol (DTT), and 0.1% fatty acid-free BSA. The homogenate was filtered through four layers of Miracloth and centrifuged at 4000 g for 5 min. The resulting supernatant was centrifuged at 15 000 g for 5 min; the pelleted mitochondria were resuspended in a washing medium containing 0.4 M sucrose, 20 mM Hepes buffer (pH 7.2), 5 mM EDTA, and 0.1% BSA and then centrifuged at 4000 g for 5 min. To precipitate mitochondria, the supernatant was centrifuged at 13 000 g for 10 min. The isolated mitochondria were resuspended in a small volume of medium containing 0.3 M sucrose, 20 mM Hepes buffer (pH 7.2), and 0.1% BSA. The suspension of mitochondria (10–15 mg/mL) was stored on ice. All operations were performed in a cold room at 2–4°C. Particular attention was paid to the duration of mitochondrial isolation procedure, which did not exceed 40 min.
Mitochondria with inactivated complex I were obtained by heating in a thermostat the suspension of organelles isolated as described above (protein content 0.6–0.7 mg/mL) for 10 min at 40°C in a medium whose composition is specified in the caption to Fig. 6. After cooling the medium to room temperature, respiratory substrates, cofactors, and inhibitors were added and the rate of oxygen uptake was recorded.
Oxygen uptake by mitochondria at various temperatures of the incubation medium was measured by a polarographic (amperometric) method using the Clark-type oxygen electrode in combination with an Oxytherm Electrode Control Unit (Hansatech Instruments, United Kingdom). The incubation medium (1.2 mL) contained 0.3 M sucrose, 20 mM Hepes buffer (pH 7.4), 5 mM MgCl2, 5 mM KH2PO4 (pH 7.4), and 0.1% BSA. Before measuring the respiration rate, we preincubated mitochondria with the protein content of 0.6–0.7 mg/mL in the medium at a given temperature for various time intervals (from 1 to 3 min), after which the respiratory substrate was introduced into the incubation medium. Unless indicated otherwise in tables or figures, we used 5 mM malate as the oxidation substrate in the presence of 5 mM glutamate (which was needed for the removal of oxaloacetate). Other additives and experimental conditions are specified in the captions to tables and figures. The oxygen content in air-saturated incubation medium at different temperatures was supposed to be equal to O2 concentration in pure water at 760 mm of mercury. It was taken as 250 μM at 25°C, 233 μM at 30°C, 219 μM at 35°C, and 205 μM at 40°C [19]. The rates of oxygen consumption during the oxidation of a respiratory substrate by mitochondria in various metabolic states, the respiratory control ratio (RCR), and the ADP/O ratio were calculated by the method of Chance and Williams [20]. The amount of mitochondrial protein was determined according to Lowry et al. [21] using BSA as a standard.
Inhibitor analysis. The cytochrome respiratory pathway (CP) was inhibited by adding 1.0 mM potassium cyanide (KCN), whereas the alternative pathway (AP) was blocked by means of 3 mM salicylhydroxamic acid (SHAM). The maximal activity of AP respiration was determined from the difference in oxygen uptake by mitochondria during substrate oxidation in state 3 (after the second addition of ADP) in the absence or presence of SHAM on the background of added KCN [22]. The rotenone-sensitive component of mitochondrial respiration during the oxidation of NAD-dependent substrates was determined by the degree of inhibition of oxygen uptake in the presence of 20 μM rotenone. The optimal concentrations of inhibitors were determined in preliminary experiments.
The following reagents were used: MgCl2, KH2PO4, and other salts of the highest purity chemical grade and analytical grade produced in Russia. Respiratory substrates, nucleotides, BSA, Mops, Hepes, rotenone, SHAM, and other chemicals were from Sigma (United States).
All experiments were made in at least three biological replicates, with three to four assays per replicate. Each series of experiments was repeated at least three times. Figures display the results of representative experiments; tables and graphs show mean values and their standard deviations.
RESULTS
Several experimental designs were applied to investigate the influence of elevated temperature on the oxidation of NAD-dependent substrates in cotyledon mitochondria of etiolated lupine seedlings. First, prompt respiratory responses of isolated mitochondria to elevation of temperature in short-term (10–15 min) experiments were analyzed under in vitro conditions. Second, the metabolic activity of organelles isolated from cotyledons after a prolonged (12 h) exposure of seedlings to elevated temperature under in vivo conditions was examined. Finally, we studied the aftereffects of incubation of a suspension of lupine mitochondria at elevated temperature on the oxidation of NAD-dependent substrates.
Effect of Temperature on Oxidation of NAD-Dependent Substrates by Mitochondria In Vitro
Figure 1 shows representative polarographic curves of oxygen uptake during malate oxidation in the presence of glutamate by mitochondria of lupine cotyledons under control conditions (25°С) and at high temperature (40°С). The oxidation of this NAD-dependent substrate by mitochondria at 25°C was substantially (and approximately to equal extents) stimulated by the first and subsequent additions of ADP (state 3). The depletion of ADP during ATP synthesis was followed by the suppression of substrate oxidation (state 4). The respiratory control ratio (RCR) as defined by Chance (state 3/state 4) was approximately three, thus indicating the tight coupling of oxidative phosphorylation. The ADP/O ratio (2.4–2.5) was close to the theoretical value, which indicated the high efficiency of phosphorylation (Fig. 1a).
Polarographic curves of oxygen uptake during malate oxidation by mitochondria (Mt) of lupine cotyledons at (a) 25°C and (b) 40°C. The incubation medium contained 0.3 M sucrose, 20 mM Hepes buffer (pH 7.4), 5 mM MgCl2, 5 mM KH2PO4 (pH 7.4), and 0.1% BSA; Additional supplements were 5 mM malate, 5 mM glutamate, and 200 μM ADP. Numbers near the curves indicate the rate of oxygen uptake by mitochondria (nmol O2/(min mg protein)).
The increase in temperature of the incubation medium to 40°C led to the acceleration of malate oxidation in state 3. However, the responses of mitochondrial oxidation to the first and subsequent additions of ADP were clearly different. The second addition of ADP markedly accelerated the rate of malate oxidation in state 3 and decreased it in state 4. As a result, the RCR increased after the second addition of ADP (Fig. 1b) and was retained during subsequent phosphorylation cycles (not shown). A similar “conditioning phenomenon” of isolated plant and animal mitochondria after several subsequent additions of ADP is well known [23]. It may have different origins, which will be discussed below.
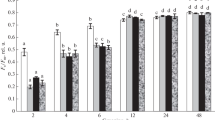
Figure 2 and Table 1 also demonstrate the influence of incubation temperature in the range from 20 to 40°С on the rates of malate oxidation by lupine mitochondria in states 3 and 4, as well as on RCR and ADP/O ratio, as calculated from experiments similar to those in Fig. 1. An increase in temperature in the range from 20 to 30°C was accompanied by an almost linear increase in the rate of substrate oxidation in state 3, while the oxidation rate in state 4 increased nonlinearly with temperature. At the same time, no significant differences were found in the rates of malate oxidation after the first and second additions of ADP (Fig. 2). As already noted above, the mitochondrial malate oxidation at temperatures of 35–40°C differed significantly in state 3 and state 4 after the first and the second addition of ADP. After two additions of ADP at these temperatures, the RCR value increased, while the ADP/O ratio decreased (Fig. 2, Table 1). It should be noted that the ADP quantity added during the first addition (75, 100, 150, or 200 µM) was not critical since the repeated introduction of ADP into the incubation medium was always accompanied by a significant increase in the rate of state 3 oxidation and the RCR value. Thus, at 35–40°C, the initial rate of malate oxidation in state 3 was significantly reduced but increased after several cycles of phosphorylation.
Effect of temperature on the rate of malate oxidation by lupine cotyledon mitochondria in state 3 (curves 1, 2) and state 4 (curves 4, 3) after the first and the second additions of ADP, respectively. Incubation medium contained 0.3 M sucrose, 20 mM Hepes buffer (pH 7.4), 5 mM MgCl2, 5 mM KH2PO4 (pH 7.4), and 0.1% BSA. Additional supple-ments were 5 mM malate, 5 mM glutamate, and 200 μM ADP.
A similar activation of state 3 respiration in lupine mitochondria after the repeated addition of ADP was observed under hyperthermia during the oxidation of other NAD-dependent substrates, such as citrate (Table 2) and pyruvate (not shown). Remarkably, such an effect was not observed during succinate oxidation in mitochondria, which is catalyzed by FAD-dependent succinate dehydrogenase (complex II) (Table 2). It should be noted that the highest rates of succinate oxidation in state 3 at a high temperature after the first addition of ADP could only be achieved in the presence of glutamate and/or ATP in the incubation medium. These additives were needed to remove the inhibition of succinate dehydrogenase by oxaloacetate.
The activity of alternative pathways of mitochondrial oxidation was determined by means of respiratory inhibitors. The oxidation of malate in the mitochondria of lupine cotyledons at normal temperature was inhibited by 65–70% with rotenone (complex I inhibitor) and by 80–85% with cyanide (cytochrome oxidase inhibitor) (Fig. 3). In the presence of rotenone, the addition to the incubation medium of exogenous NAD+, which is known to be transported into intact plant mitochondria, did not stimulate oxygen uptake. Hence, the activity of rotenone-insensitive NADH dehydrogenases, whose substrate-binding centers are located on the inner (matrix) side of the inner mitochondrial membrane, was not limited by the level of this coenzyme (Fig. 3a). Furthermore, we failed to significantly promote the AOX activity by supplementing the reaction medium with pyruvate and DTT (Fig. 3b) as effectors known to stimulate the AOX operation in plant mitochondria [4]. Thus, the activity of alternative “matrix-located” rotenone-insensitive NADH dehydrogenases in lupine mitochondria was 35% of the total rate, and the maximum activity of CN-resistant AOX did not exceed 20% of the respiration rate in state 3.
Effect of inhibitors on malate oxidation by mitochondria (Mt) of lupine cotyledons at 25°C. The incubation medium contained 0.3 M sucrose, 20 mM Hepes buffer (pH 7.4), 5 mM MgCl2, 5 mM KH2PO4 (pH 7.4), and 0.1% BSA. Additional supplements were 5 mM malate, 5 mM glutamate, 0.3 mM pyruvate, 200 µM ADP, 0.3 mM NAD+, 1 mM DTT, 1 mM KCN, 3 mM SHAM, and 20 µM rotenone. Numbers near the curves indicate the oxygen uptake rate by mitochondria (nmol O2/(min mg protein)).
We found that the state 3 rate of malate oxidation in lupine mitochondria (as recorded after the second addition of ADP) was increased by elevated temperature predominantly (at 20–35°C) or exclusively (at 35–40°C) due to the increased activity of the cytochrome pathway in ETC. The activity of AOX decreased significantly under hyperthermia but was not completely inactivated (Fig. 4).
Oxidation of Malate by Mitochondria Isolated from Cotyledons of Lupine Seedlings Subjected to Heat Treatment In Vivo
Figure 5 shows that the exposure of lupine seedlings to hyperthermia (35°C for 12 h) caused an apparent inhibition of malate oxidation by mitochondria, which persisted after the isolation of organelles. While measuring malate oxidation by mitochondria at 25°C, we observed that the phosphorylating (state 3) oxidation of the substrate was inhibited and the RCR was reduced after the first addition of ADP. The second addition of ADP significantly enhanced or “improved” the oxidative and phosphorylating activity of mitochondria. Specifically, the state 3 oxidation rate and the RCR were found to increase (Fig. 5a). At the same time, the efficiency of oxidative phosphorylation, as characterized by the ADP/O ratio, remained substantially lower than the theoretically possible level. Interestingly, the reversible suppression of mitochondrial malate oxidation was most pronounced at the incubation temperature of 35°C, at which the organelles functioned in vivo in cotyledon cells. In this case, the rate of the substrate oxidation in state 3 and the RCR value after the first addition of ADP were found to decrease. However, the ADP/O ratio remained quite high and sharply decreased after the second addition of ADP as compared with the increased rate of malate oxidation in state 3 and the increased RCR (Fig. 5b). Thus, the observed similarity in the responses to ADP additions in experiments in vitro and in vivo (Fig. 1b, Fig. 5) seem to indicate the common mechanism that controls the malate oxidation in lupine cotyledon mitochondria under the action of high temperature.
Oxidation of malate at (a) 25°C and (b) 35°C by mitochondria (Mt) isolated from lupine cotyledons after heating the seedlings at 35°C. The incubation medium contained 0.3 M sucrose, 20 mM Hepes buffer (pH 7.4), 5 mM MgCl2, 5 mM KH2PO4 (pH 7.4), and 0.1% BSA; additional supplements were 5 mM malate, 5 mM glutamate, and 200 μM ADP. Numbers near the curves indicate the rate of oxygen uptake by mitochondria (nmol O2/(min mg protein)).
Influence of High Temperature and Mg2+ Ions on Oxidation of NAD-Dependent Substrates by Lupine Cotyledon Mitochondria
Since mitochondrial complex I is known to play a key role in ETC operation in animal mitochondria, its structure and function has long remained in the focus of researchers' attention. Experiments with alamethicin-permeabilized rat heart mitochondria and bovine heart submitochondrial particles (SMP) revealed that complex I in these samples exists as a mixture of active (A) and deactivated (D) forms or states. The reversible A/D transformation of complex I was found to be sensitive to some environmental factors, including high temperature [24, 25]. For animal SMP and mitochondria, several diagnostic tests have been proposed to reveal the functioning of complex I in the deactivated state. These tests comprise the use of divalent cations (Mg2+, Ca2+). At high concentration of these ions, the D/A transformation of complex I was significantly slowed down, which provides a simple means to determine the degree of complex I inactivation under hyperthermia. For example, after heating rat heart mitochondria at 38°C for 20 min in the presence of 10 mM MgCl2, the catalytic activity of complex I was inhibited by 40–80% depending on the pH of incubation medium [25].
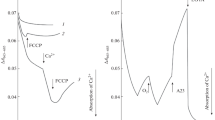
We applied a similar test to detect the thermal inactivation of rotenone-sensitive oxidation of NAD-dependent substrates in intact cotyledon mitochondria. In these experiments, we studied the action of preliminary 10-min heating at 40°C of the lupine mitochondria suspended in the Mg2+-free reaction medium and in the medium supplemented with 10 mM MgCl2 on the oxidation of malate or pyruvate. Figure 6a shows that the malate oxidation at 25°C was strongly inhibited (though not completely) after heating of mitochondria in the presence of Mg2+ and did not respond to the addition of ADP. At the same time, no spontaneous reactivation of respiration was observed in the course of the polarographic experiment (7–10 min). The addition of exogenous NAD+ partially stimulated oxygen uptake that was insensitive to rotenone (Fig. 6a). Unlike the oxidation of malate, mitochondrial oxidation of succinate catalyzed by ETC complex II proceeded at a high rate under these conditions and was accompanied by phosphorylation, which was evidenced by the presence of respiratory control (Fig. 6a). A similar inhibition of rotenone-sensitive oxygen uptake after heating of mitochondria in the presence of 10 mM MgCl2 was observed during the oxidation of pyruvate by mitochondria (Fig. 6b). If MgCl2 was added to the incubation medium of preheated mitochondria simultaneously with or after the addition of the respiratory substrate, the rate of malate oxidation in state three was restored fairly fast (though not completely) during several phosphorylation cycles. At the same time, malate oxidation was inhibited by rotenone (Fig. 6c) and was weakly stimulated by exogenous NAD+ (data not shown). Thus, the heating of lupine mitochondria in the presence of a high Mg2+ concentration was characterized by the following features. First, the rotenone-sensitive malate oxidation (i.e., complex I activity) was almost completely inhibited and did not recover for a long lag period after the substrate addition. Next, it was accompanied by the partial loss of NAD+ pool from the mitochondrial matrix, which restricted the rate of this substrate oxidation mediated by alternative rotenone-insensitive “matrix” NADH dehydrogenases.
Effect of Mg2+ and the preheating of mitochondria (Mt) on the oxidation of NAD-dependent substrates. Mitochondria were heated at 40°C for 10 min in a standard incubation medium in the (a, b) presence or (c) absence of 10 mM MgCl2. The incubation medium contained 0.3 M sucrose, 20 mM Hepes buffer (pH 7.4), 5 mM KH2PO4 (pH 7.4), and 0.1% BSA; additional supplements were 5 mM malate, 5 mM glutamate, 5 mM pyruvate (in the presence of 0.6 mM thiamin pyrophosphate), 5 mM succinate, 1 mM NADH, 10 mM MgCl2, 100 μM ADP, 0.35 mM NAD+, and 20 μM rotenone. Numbers near the curves indicate the rates of oxygen uptake by mitochondria (nmol O2/(min mg protein)).
DISCUSSION
Experimental data on plant growth and metabolic activity of mitochondria at elevated temperature vary greatly [11, 26, 27]. The effects of hyperthermia on mitochondrial respiration depend possibly on plant material, duration of heat treatment, and the quality of isolated organelles [3].
Our experiments in vitro have shown that the oxidation of malate by mitochondria of lupine cotyledons in states 3 and 4 was activated with temperatures in the range of 20–30°C. The rate of substrate oxidation in the active state (state 3 or V3) increased approximately 1.7 times per 10°C of temperature increment (coefficient Q10). During state 3 oxidation of malate at 30–40°C, similar Q10 coefficients could only be achieved after adding several ADP aliquots into the incubation medium, which was needed to relieve the initial inhibition of oxidation of NAD-dependent substrate under hyperthermia (35–40°C) (Fig. 1). These results obtained from in vitro experiments were consistent with the data on malate oxidation under the action of elevated temperature in vivo. The 12-h heating of lupine seedlings at 35°C was followed by the reversible inhibition of oxidation of a NAD-dependent substrate in state 3, and this inhibition was most pronounced at elevated temperature of organelle incubation (Fig. 5). Thus, lupine cotyledon mitochondria exhibit sufficiently high thermal stability and are able to withstand (overcome) the effects of hyperthermia in vivo and in vitro. Most importantly, these mitochondria retain the potential ability to restore their oxidative and phosphorylating activity after the end of heat treatment, which is generally consistent with the published data [3, 13].
It should be also noted that malate oxidation under hyperthermia occurred predominantly via the cytochrome pathway (CP) respiration, with an extremely low contribution from the AOX activity (Fig. 4). These results seem to indicate a higher sensitivity of AP in cotyledon mitochondria to hyperthermia compared to CP, which was also noted by other authors [11, 27]. For example, the temperature optimum of AOX activity in isolated mitochondria of maize seedlings was 15–20°C; the AOX activity decreased gradually at higher temperatures and was almost completely lost at 41°C [27]. The origin of thermal inhibition of AP activity under in vitro conditions remains unknown. Importantly, this inhibition can be reversible. In particular, a drop-in temperature of the incubation medium from 42 to 25°C restored the maximum activity of AOX in mitochondria of bean hypocotyls [11]. The inconsistency of published data on the role of AOX in respiration under hyperthermia might be explained by the fact that the activity of mitochondrial AOX depends not only on temperature but also on the reduction level of the ubiquinone pool, the tightness of respiratory control by the adenylate system, and the extent of AOX activation under the influence of keto acids and other effectors [10].
The mechanisms underlying the discovered inhibitory action of hyperthermia on oxidation of NAD-dependent substrates, which is catalyzed by rotenone-sensitive ETC complex I, deserve more thorough consideration. Although the inhibition of complex I activity in plant mitochondria at elevated temperature was described quite a long time ago [13, 14], its mechanism and functional role remain the matter of interest, because the inhibition is highly labile and may originate from different causes. For example, in vitro experiments revealed a rapid decrease in the rate of NAD-dependent glycine oxidation in pea leaf mitochondria at 40°C, which was prevented by the addition of NAD+ coenzyme to the incubation medium. This finding was ascribed to an almost complete depletion of the NAD+ pool in the matrix after a 5-min incubation of organelles under hyperthermia [14]. The authors concluded that high temperature induces a rapid and massive release of NAD+ coenzyme from the mitochondrial matrix by means of a specific transporter [14]. It is not yet known how widespread this phenomenon is among mitochondria isolated from other plant materials. Our in vitro experiments showed that the heat treatment was apparently accompanied by the partial loss of NAD+ pool from the organelles since exogenous NAD+ had some stimulating effect on respiration. However, this pool depletion had little effect on the rate of malate oxidation mediated by alternative rotenone-insensitive NADH dehydrogenases that are known to have a lower affinity for the substrate than complex I (Fig. 6). In addition, the exposure of etiolated lupine seedlings for several hours at 35°C did not impair the ability of isolated mitochondria to oxidize malate in state 3 at a high rate after several additions of ADP (Fig. 5). Thus, in addition to possible changes in the level of NAD+ coenzyme in the matrix, another mechanism apparently functions in lupine mitochondria, which regulates the oxidation of NAD-dependent substrates not only during hyperthermia but also after the end of the thermal treatment.
The gradual restoration of energy-linked functions in isolated plant mitochondria (state 3 oxidation rate and RCR values) after the addition of respiratory substrates, including NAD-dependent ones, is not a new phenomenon. For example, the fast (within a few minutes) acceleration of state 3 oxidation of substrates and the increase in RCR after several additions of ADP were described and analyzed in early polarographic studies with isolated plant mitochondria after aging the suspensions at 0°C (the effect termed “conditioning”). A similar functional restoration of the organelles was observed after a long (for several hours) incubation of mitochondria at 25°C in the absence of a respiratory substrate (this effect was called “self-restoration”) [22, 28]. Hung and Romani [28] analyzed all possible causes for the reversible inhibition of α‑ketoglutarate oxidation in avocado fruit mitochondria after prolonged exposure of the suspended organelles under deenergized conditions. Specifically, the authors considered a diminished permeation of respiratory substrates, a significant loss of the NAD+ pool, the reduced activity of adenine nucleotide translocase or ATP synthetase, and the suppression of NADH dehydrogenase; the latter reason was regarded as the most probable one.
A mechanism of inhibiting the oxidation of NAD-dependent substrates under various stressful conditions, including hyperthermia, might be similar to the spontaneous reversible transformation of complex I from the active (A) to the deactivated (D) state, which was found in animals, some yeasts, and bacteria [16]. In animal mitochondria, the deactivation of complex I arises when the reaction catalyzed by rotenone-sensitive NADH-ubiquinone oxidoreductase becomes impossible due to the absence of a substrate or in the presence of NADH under complete reduction of the ubiquinone pool. Furthermore, the experiments with submitochondrial particles (SMP) isolated from various sources revealed that the A/D transformation of complex I was accelerated with temperature and was completed very fast (<1 min) under hyperthermia (at 37–38°C) [16]. The backward transition of complex I from the inactive to the active state (D/A) also proceeded rapidly at normal temperature after the substrate addition and the restoration of electron transport in the respiratory chain. However, the rate of D/A transformation of complex I can be substantially slowed down in the presence of divalent cations (Mg2+ or Ca2+) [15, 16].
The results of the present work suggest that plant mitochondria, the mitochondria of lupine cotyledons in particular, possess a similar mechanism of regulating the complex I activity under the action of high temperature. For example, our in vitro experiments showed that a short-term (several minutes) preincubation of mitochondria at 40°C significantly suppressed the maximum rate of malate oxidation in state 3, and this suppression was apparent after the first addition of ADP (Fig. 1). It should be noted that the oxidation of a NAD-dependent substrate was not completely inhibited, primarily, due to the presence of rotenone-insensitive NADH dehydrogenases in the matrix of plant mitochondria whose activity was not suppressed under hyperthermia [13]. Furthermore, the activation of complex I functioning after the appearance of NADH in the matrix upon malate oxidation should apparently be accompanied by the rapid transition of complex I from the deactivated to the active state (D/A transformation) and by the acceleration of oxygen uptake. It is also reasonable to assume that the ADP supplements additionally stimulated such a transition of complex I due to the activation of the respiratory chain, which decreased the reduction level of electron carriers, including the ubiquinone pool.
Evidence indicating the transition of complex I to an inactive state under conditions of hyperthermia in the mitochondria of lupine cotyledons was obtained by us when studying the effect of divalent cations on this process. We found that the presence of 10 mM MgCl2 in the suspension had no adverse impact on respiration of unstressed mitochondria but was accompanied by an almost complete inhibition of complex I-catalyzed malate oxidation after short-term heating of the suspension (for 10 min at 40°C), which was entirely mediated under given conditions by rotenone-insensitive NADH dehydrogenases (Fig. 6a). At the same time, the elevated temperature had little effect in the posttreatment period on the oxidation of succinate (in the presence of glutamate) and exogenous NADH (Figs. 6a, 6b). Similar results were obtained under the action of hyperthermia on the oxidation of other NAD-dependent substrates, e.g., pyruvate by lupine mitochondria (Fig. 6b). The inactivation of complex I during heating of mitochondria at a high MgCl2 concentration was most probably caused by Mg2+ uptake and accumulation in the matrix. The Mg2+ entry could be a passive diffusion driven by the concentration gradient or may result from the active transport in the presence of a residual membrane potential across the inner mitochondrial membrane due to incomplete washing of respiratory substrates during the isolation of organelles. It should be emphasized that, in mitochondria preheated in the presence of magnesium, no spontaneous reactivation of malate oxidation was observed during the polarographic experiment (Figs. 6a, 6b). At the same time, the restoration of rotenone-sensitive malate oxidation in preheated mitochondria proceeded quite fast if the NAD-dependent substrate was added simultaneously or before magnesium (Fig. 6c) but it took much longer time or was entirely absent if Mg2+ was added to the incubation medium a few minutes before the addition of malate (data not shown). Such significant changes in the rate of restoration of complex I activity depending on the sequence of additions of magnesium and NAD-dependent substrate to the incubation medium might be explained by different rates of transport and accumulation in the matrix of two effectors exerting opposite influence on this process.
It is not excluded that the transition of complex I to the inactive state in isolated plant mitochondria also occurs at a lower temperature (30–35°C) but it requires a longer exposure in this case, as observed with animal mitochondria [16]. We obtained indirect evidence of such transformation of complex I under in vivo conditions at a relatively low temperature (35°C). Clarifying this issue is important, because the A/D transition of complex I under hyperthermia was mostly studied in vitro using isolated mitochondria, SMP, or an isolated enzyme. More research is clearly needed to clarity the issues under discussion. At the same time, the results of this work indicate for the first time the possibility of A/D transformation of complex I in intact plant mitochondria under the influence of high temperature.
As for the possible physiological role of the discussed changes in the activity of complex I, this question remains open even for animal mitochondria in which they were discovered about 30 years ago [15]. The functional role of inhibition of complex I under hyperthermia in animal mitochondria seems hard to explain because the normal temperature of many animals exceeds 36°C. Nevertheless, a proposal was put forward that the temporary inactivation of complex I can prevent the excessive generation of superoxide and hydrogen peroxide by mitochondria under stress conditions [29]. If the supposed “antioxidant” property of the D form of complex I is experimentally confirmed, it will be of great importance for plant mitochondria, especially under conditions of elevated temperature. The temperature of plant organisms, unlike that in animals, varies over a wide range; therefore, its rapid rise (up to 35°C and above) accompanied by sharp activation of respiration would have obviously lead to a rapid exhaustion of respiratory substrates and the depletion of oxygen content in tissues and cells. Furthermore, the substrate oxidation via the main cytochrome pathway of ETC is activated under hyperthermia and the mitochondrial ROS formation in plant organs, including lupine cotyledons, is accelerated. These heating-induced changes are often accompanied by the inhibition of AOX that plays a key role in maintaining redox homeostasis in mitochondria and cells by preventing oxidative stress [8]. Therefore, the temporary inhibition of NADH dehydrogenase (complex I), which is the main supplier of reducing equivalents to ETC and one of the main sources of ROS [17, 30], could be an important protective mechanism preventing the excessive generation of superoxide and hydrogen peroxide in plant mitochondria under conditions of hyperthermia.
REFERENCES
Semikhatova, O.A. and Chirkova, T.V., Fiziologiya dykhaniya rastenii (Physiology of Plant Respiration), St. Petersburg: S.-Peterb. Gos. Univ., 2003.
Voinikov, V.K., Mitokhondrii rastenii pri temperaturnom stresse (Plant Mitochondria under Temperature Stress), Novosibirsk: GEO, 2011.
Semikhatova, O.A., Energetika dykhaniya rastenii pri povyshennoi temperature (Energetics of Plant Respiration at Higher Temperatures), Leningrad: Nauka, 1974.
Grabelnych, O.I., Borovik, O.A., Tauson, E.L., Pobezhimova, T.P., Katyshev, A.I., Pavlovskaya, N.S., Koroleva, N.A., Lyubushkina, I.V., Bashmakov, V.Yu., Popov, V.N., Borovskii, G.B., and Voinikov, V.K., Mitochondrial energy-dissipating systems (alternative oxidase, uncoupling proteins, and external NADH dehydrogenase) are involved in development of frost-resistance of winter wheat seedlings, Biochemistry (Moscow), 2014, vol. 79, p. 506.
Atkin, O.K. and Macherel, D., The crucial role of plant mitochondria in orchestrating drought tolerance, Ann. Bot., 2009, vol. 103, p. 581. https://doi.org/10.1093/aob/mcn094
Liberatore, K.L., Dukowic-Schulze, S., Miller, M.E., Chen, C., and Kianian, S.F., The role of mitochondria in plant development and stress tolerance, Free Radical Biol. Med., 2016, vol. 100, p. 238. https://doi.org/10.1016/j.freeradbiomed.2016.03.033
Jacoby, R.P., Li, L., Huang, S.B., Lee, C., Millar, A.H., and Taylor, N.L., Mitochondrial composition, function and stress response in plants, J. Integr. Plant Biol., 2012, vol. 54, p. 887. https://doi.org/10.1111/j.1744-7909.2012.01177.x
Vanlerberghe, G.C., Alternative oxidase: a mitochondrial respiratory pathway to maintain metabolic and signaling homeostasis during abiotic and biotic stress in plants, Int. J. Mol. Sci., 2013, vol. 14, p. 6805. https://doi.org/10.3390/ijmc14046805
Varakina, N.N., Pobezhimova, T.P., and Voinikov, V.K., The effect of hyperthermia on the energy activity of mitochondria and the growth of maize seedlings, Fiziol. Rast., 1991, vol. 38, p. 304.
Atkin, O.K., Zhang, Q., and Wiskich, J.T., Effect of temperature on rates of alternative and cytochrome pathway respiration and their relationship with the redox poise of quinone pool, Plant Physiol., 2002, vol. 128, p. 212. https://doi.org/10.1104/pp.010326
Lin, T.-Y. and Markhart, A.H., Temperature effects on mitochondria respiration in Phaseolus acutifolius A. Gray and Phaseolus vulgaris L., Plant Physiol., 1990, vol. 94, p. 54. https://doi.org/10.1104/pp.94.1.54
Hu, W.H., Xiao, Y.A., Zeng, J.J., and Hu, X.N., Photosynthesis, respiration and antioxidant enzymes in pepper leaves under drought and heat stresses, Biol. Plant., 2010, vol. 54, p. 761.
Pobezhimova, T.P., Voinikov, I.K., and Varakina, N.N., Thermal resistance and functional stability of single complexes of the respiratory chain of maize mitochondrial incubated in vitro, Fiziol. Rast., 1997, vol. 44, p. 873.
Lenne, C., Neuburger, M., and Douce, R., Effect of high physiological temperature on NAD+ content of green leaf mitochondria, Plant Physiol., 1993, vol. 102, p. 1157. https://doi.org/10.1104/pp.1024.1157
Grivennikova, V.G., Gladyshev, G.V., and Vinogradov, A.D., Deactivation of mitochondrial NADH:ubiquinone oxidoreductase (respiratory complex I): Extrinsically affecting factors, Biochim. Biophys. Acta, Bioenerg., 2020, vol. 1861, p. 1. https://doi.org/10.1016/j.bbabio.2020.148207
Maklashina, E., Kotlyar, A.B., and Cecchini, G., Active/de-active transition of respiratory complex I in bacteria, fungi, and animals, Biochim. Biophys. Acta, Bioenerg., 2003, vol. 1606, p. 95. https://doi.org/10.1016/S0005-2728(03)00087-2
Braun, H.-P., Binder, S., Brennike, A., Eubel, H., Fernie, A.R., Finkemeier, I., Klodman, J., Konig, A.-C., Kuhn, K., Meyer, E., Obata, T., Schwarzlnder, M., Takenaka, M., and Zehrmann, A., The life of plant mitochondrial complex I, Mitochondrion, 2014, vol. 19, p. 295. https://doi.org/10.1016/j.mito.2014.02.006
Shugaev, A.G., Butsanets, P.A., Andreev, I.M., and Shugaeva, N.A., Effect of salicylic acid on the metabolic activity of plant mitochondria, Russ. J. Plant Physiol., 2014, vol. 61, p. 520. https://doi.org/10.1134/S1021443714040189
Rukovodstvo po izucheniyu biologicheskogo okisleniya polyarograficheskim metodom (Handbook for the Analysis of Biological Oxidation by Polarographic Method), Frank, G.M., Kondrashova, M.N., and Mokhova, E.N., Eds., Moscow: Nauka, 1973.
Chance, B. and Williams, G.R., The respiratory chain and oxidative phosphorylation, Adv. Enzymol., 1956, vol. 17, p. 65.
Lowry, O.H., Rosebrough, N.J., Farr, A.L., and Randall, R.J., Protein measurement with the folin phenol reagent, J. Biol. Chem., 1951, vol. 193, p. 265.
Moller, I.M., Berzi, A., van der Plas, L.H., and Lambers, H., Measurement of the activity and capacity of the alternative pathway in intact plant tissues. Identification of problems and possible solutions, Physiol. Plant., 1988, vol. 72, p. 642. https://doi.org/10.1111/J.1399-3054.1988.TB09176.X
Romani, R. and Ozelkok, S., “Survival” of mitochondria in vitro, Plant Physiol., 1973, vol. 57, p. 702. https://doi.org/10.1104/pp.51.4.702
Kotlyar, A.B. and Vinogradov, A.D., Slow active/inactive transition of the mitochondrial NADH-ubiquinone reductase, Biochim. Biophys. Acta, Bioenerg., 1990, vol. 1019, p. 151. https://doi.org/10.1016/0005-2728(90)90137-s
Grivennikova, V.G., Kapustin, A.N., and Vinogradov, A.D., Catalytic activity of NADH-ubiquinone oxidoreductase (complex I) in intact mitochondria, J. Biol. Chem., 2001, vol. 276, p. 9038. https://doi.org/10.1074/jbc.M009661200
Hemrika-Vagner, A.M., Kreuk, K.C.M., and van der Plas, L.H.W., Influence of growth temperature on respiratory characteristics of mitochondria from callus-forming potato tuber discs, Plant Physiol., 1982, vol. 70, p. 602. https://doi.org/10.1104/pp.70.2.602
Stewart, C.R., Martin, B.A., Reding, L., and Cerwick, S., Seedling growth, mitochondrial characteristics, and alternative respiratory capacity of corn genotypes differing in cold tolerance, Plant Physiol., 1990, vol. 92, p. 761. https://doi.org/10.1104/pp.92.3.761
Hung, L.-S. and Romani, R.J., Metabolically driven self-restoration of energy-linked functions by avocado mitochondria, Plant Physiol., 1991, vol. 95, p. 1096. https://doi.org/10.1104/pp.95.4.1096
Drose, S., Stepanova, A., and Galkin, A., Ischemic A/D transition of mitochondrial complex I and its role in ROS generation, Biochim. Biophys. Acta, Bioenerg., 2016, vol. 1857, p. 946. https://doi.org/10.1016/j.bbabio.2015.12.013
Murphy, M.P., How mitochondria produce reactive oxygen species, Biochem. J., 2009, vol. 417, p. 1. https://doi.org/10.1042/BJ20081386
Funding
The work was performed within the framework of the state assignment of the Ministry of Education and Science of the Russian Federation, project no. 121040800153-1 “Mechanisms of Plant Adaptation to the Factors of Aridization of the Global Climate and Anthropogenic Pollution of the Environment.”
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflicts of interest. This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Translated by A. Bulychev
Abbreviations: A/D—active/deactivated forms of mitochondrial complex I; AOX—alternative oxidase; AP—alternative oxidation pathway; CP—cytochrome oxidation pathway; DTT—dithiothreitol; ETC—electron transport chain; RCR—respiratory control ratio; ROS—reactive oxygen species; SHAM—salicylhydroxamic acid; SMP—submitochondrial particles; TPP—thiamine pyrophosphate; V3—substrate oxidation rate in the presence of ADP (state 3); V4—substrate oxidation rate after ADP depletion (state 4).
Rights and permissions
About this article
Cite this article
Shugaev, A.G., Butsanets, P.A. & Shugaeva, N.A. Effect of High Temperature on Oxidation of NAD-Dependent Substrates and Alternative Oxidase Activity in Mitochondria of Lupine Cotyledons. Russ J Plant Physiol 69, 69 (2022). https://doi.org/10.1134/S102144372204015X
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1134/S102144372204015X