Abstract
The effect of light intensity (in the range from 20 to 850 μE/(m2s)) on the change in the main structural, functional, cytometric, and fluorescent parameters of coccolithophorids Emiliania huxleyi (Lohmann) Hay & Mohler 1967 was investigated. The results of microscopic and cytometric analyses showed that E. huxleyi was represented by two cell forms covered with a layer of calcium formations, coccoliths, and cells without coccoliths, the ratio between which varied depending on the light conditions of algal growth: an increase in light intensity from 20 to 850 μE/(m2s) led to a natural decrease in the number of cells covered with coccoliths from 90 to 35%. It is hypothesized that a decrease in the proportion of covered cells in a population with an increase in illumination can be considered as a possible reason for early photoinhibition of growth in E. huxleyi (from 400 μE/(m2 s)). The use of the flow cytometry method made it possible to reveal a number of significant differences in the fluorescent, dimensional, and structural parameters of E. huxleyi cells corresponding to two of its forms: covered and uncovered coccoliths. It was found that uncovered cells are 2.5–3.5 times smaller in volume than covered cells and have low light scattering properties: the light forward scattering index (FS) is two times and the light side scattering (SS) is 25 times lower than in cells covered with coccoliths, which allows one to identify the studied groups of cells of E. huxleyi on the cytograms of a flow cytometer using chlorophyll autofluorescence in the red region of the spectrum (channel FL4) and the SS channel. The noted fluorescence in the green region of the spectrum (channel FL1), which regularly increases with increasing illumination, is also a distinctive feature of cells covered with coccoliths; green autofluorescence is absent in uncovered cells. The results obtained in the work can significantly improve the understanding of E. huxleyi’s physiology as a species that causes massive blooms in the Black Sea and significantly affects the cycle of nutrients and minerals in the marine environment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Coccolithophorids Emiliania huxleyi are planktonic unicellular algae with spherical cells 5–8 µm in diameter. E. huxleyi cells are surrounded by a layer of 10–15 openwork limestone (CaCO3) discs, coccolith 1–2 µm in size; two to three layers of coccoliths can form in some caseson the surface of the cell. The presence of calcium formations—coccoliths causes a number of individual physiological characteristics of E. huxleyi—in particular, leads to an increase in the coefficient of light scattering by cells and a decrease in the specific coefficient of light absorption by pigments [1, 2]. Under favorable growth conditions, E. huxleyi begins to “bloom”, forming colonies of more than 1 million cells/L. After the end of “blooming,” coccoliths partially settle to the bottom and are the main component of calcareous silts, which cover up to 35% of the ocean floor. Thus, these algae play an important role in the transport of inorganic carbon from the atmosphere to bottom sediments, which is of particular importance in connection with the problem of carbon dioxide pollution of the atmosphere [1].
Regular “bloom” in the Black Sea of E. huxleyi is observed both in the summer period with an extremely high level of irradiation during the annual cycle [2] and in the winter months under low illumination conditions [3, 4]. Large-scale blooming areas of coccolithophorids can be detected using satellite sensors based on the brightness of the upward radiation due to the effect of intense backscattering of light by coccoliths in the near-surface layer of water. On the basis of satellite data, the approximate dates of the beginning of flowering and its peak can be determined and the duration of flowering and its spatial characteristics can be established [4, 5].
Ecological and physiological characteristics of Emiliania huxleyi are intensively studied in the context of biogeochemistry (especially in connection with the global carbon cycle), plankton ecology, and biomineralization; special attention is paid to the formation of coccoliths and the assessment of the interaction “calcification–photosynthesis” [1, 5, 6]. Moreover, most of the works are devoted to the study of the “classical” cell shape of E. huxleyi, which is covered with a layer of calcium formations—coccoliths (hereinafter, covered cells). However, it was shown back in the 1970s that, in addition to this form, E. huxleyi can be represented by uncalcified cells without coccoliths (hereinafter, uncovered cells) as well as haploid cells with two long flagella and scales on the surface [6, 7]. It is believed that cells not covered with a layer of coccoliths exhibit the same morphological features as coated cells, but they are not capable of calcification—the synthesis of coccoliths. The formation of these cells is not part of the normal life cycle of E. huxleyi, it is a kind of mutation in response to the adverse effect of an external factor [1]. The reasons for the appearance of uncovered cells in the population are still not clear. On the example of laboratory culture of E. huxleyi, it was shown that the appearance of uncoated cells may be due to the “aging” of the culture and the deficiency of mineral nutrition in the environment [1]. It is noted that uncovered cells are more resistant to the adverse effects of external factors [1, 2, 6]. An important and poorly understood question remains as to how the ecological and physiological characteristics of cells change during the transition from a covered to an uncovered form and how a change in the ratio between covered and uncovered cells in a population will lead to a change in its structural and functional characteristics. Studying these issues in cultivation of E. huxleyi under different illumination was the purpose of the presented work.
MATERIALS AND METHODS
As the object of research was used algologically pure culture of marine coccolithophorids Emiliania huxleyi (Lohmann) Hay & Mohler 1967 from the collection of cultures of microalgae and cyanobacteria (IBSS) of the Scientific and Educational Center for Collective Use “Hydrobionts of the World Ocean” (WDCM no. 1201) of Kovalevsky Institute of Biology of the Southern Seas (Russian Academy of Sciences).
Experimental conditions. In experiments, algae were cultivated on a nutrient medium F/2 [8] at a light intensity of 20, 85, 238, 340, 442, 680, and 850 μE/(m2 s) (in duplicate for each illumination) and a temperature of 20°C for 7 days. The light–dark illumination regime in the experiment was maintained in a cycle of 14/10 h. Illumination of flasks (volume 200 mL) was carried out with white light LEDs, various levels of which were achieved by changing the distance to the light source and using neutral filters. The illumination level was determined inside the flasks with a 4П probing sensor of a QSL 2101 quantum meter (Biospherical Instruments, United States); the error in measuring the light intensity was ±1 μE/(m2 s). The pH of the culture medium was in the range 8.2–8.4.
Throughout the experiment, the concentration of culture cells was maintained at approximately the same level in the exponential growth phase by periodic (once a day or less often for poorly growing cultures) dilution with fresh nutrient medium. The adaptation of algae to each studied illumination was carried out for three days (1–3 days of the experiment). During the indicated time interval, the algae performed at least two cell divisions, after which they were considered adapted to the new lighting conditions. Measurement of cell numbers, chlorophyll concentration in samples, relative variable chlorophyll fluorescence a Fv/Fm, as well as cytometric parameters (forward (FS) and side scattering (SS) of light, green and red autofluorescence of cells), were performed on the fourth day of the experiment.
To assess adaptive capabilities of Emiliania huxleyi after the end of the main set of experiments, we studied the dynamics of changes in the structural and functional characteristics of algae when transferred from a high light intensity of 680 μE/(m2 s) in optimal light conditions for growth 85 μE/(m2 s). The experiment lasted 3 days.
Measurement methods. Cytometric analysis of samples was performed on a Cytomics flow cytometerTMFC 500 (BeckmanCoulter, United States) equipped with a 488 nm single-phase argon laser; CXP software was used for data processing. The total number of microalgae in cultures was determined in a cluster on two parametric cytograms by forward light scattering (FS) and fluorescence of individual cells in the red spectral region (FL4, 675 nm) on dimensionless logarithmic scales. The cell concentration was calculated from the sample flow rate (60 μL/min), counting time (33–100 s), and the number of cells recorded during this time interval. Quality control of abundance measurements was performed using calibration fluorospheres (Flow-CheckTM, Beckman Coulter) with a known concentration in the sample.
The growth rate of microalgae was calculated by the following formula [9]:
where:
µ is the rate of algae growth, divisions per day;
N0 and Nt are the initial concentration of cells and their number over time t, cells L–1;
t is time between measurements, days.
The relative size of microalgae cells was determined on a flow cytometer in clusters by forward (FS) and side light scattering (SS) on linear scales [10]. The identification of cells with and without coccoliths was carried out on 2-parametric cytograms by autofluorescence in the red region of the spectrum (FL4, 675 nm) and side scattering (SS), which characterizes the granularity of the cells. Measurement of side light scatter (SS) allowed for reliable cell separation of E. huxleyi, covered and uncovered by coccoliths, into two separate groups. The calculation of the cell volume was carried out according to the calibration dependences presented in [11].
Green autofluorescence of coated and uncoated cells of E. huxleyi was determined on a flow cytometer by the fluorescence of individual cells in the green spectral region (FL1, 525 nm) on dimensionless logarithmic scales.
The content of chlorophyll a and c in the samples were determined spectrophotometrically on an SF‑2000. The calculation of the concentration of pigments was carried out according to the following formulas [12]:
where OD is the optical density of the extracts at the indicated wavelength, taking into account the correction for nonspecific absorption at 750 nm; Vex is the volume of acetone extract, mL; Vf is the volume of the filtered culture, L; Lk is the cuvette length, sm.
Chlorophyll concentration was determined discretely in cells covered and not covered with coccoliths by their fluorescence in the red spectral region (FL4, 675 nm), measured on a flow cytometer. For E. huxleyi, the FL4 values and the total chlorophyll (Chl) content in the suspension, measured spectrophotometrically, were preliminarily calibrated: Chl = 0.0004FL4 + 0.04, R2 = 0.97.
Measuring the relative variable fluorescence of chlorophyll a Fv/Fm (maximum quantum efficiency of PS II) was carried out on a Mega-25m fluorometer with pulse modulation of exciting light at a wavelength of ~455 nm. The calculation was carried out according to the following formula [13]:
where:
Fv/Fm is relative variable chlorophyll fluorescence a;
F0 is the value of chlorophyll fluorescence at open reaction centers;
Fm is maximum fluorescence at closed reaction centers.
Statistical data processing was carried out using standard software packages Microsoft Excel 7.0, Statistica-5, and Grapher-9 for a personal computer. The points indicated on the graphs represent the average of four measurements: two series of biological replicates of the experiments performed, two replicates of the determination of the studied parameters in each. The bars on the charts denote the standard deviations of the described values.
RESULTS
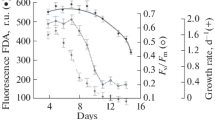
According to the results of the experimental study, the regularities of changes in the growth rate of coccolithophorids of E. huxleyi depending on light conditions were established (Fig. 1). It was shown that the optimal illumination for the growth of algae varies from 85 to 440 μE/(m2s). Within the specified range, algae maintain a maximum growth rate of approximately 0.8–1 div/day. At a light intensity of 20 μE/(m2 s), E. huxleyi grows at a low rate of 0.3 div/day, which is due to the light limitation of algal growth under these conditions. At an illumination above 440 μE/(m2s) a progressive decrease in the rate of cell growth is observed up to a complete cessation of growth at an illumination of 680 μE/(m2s). The low functional activity of algae at high illumination is also indicated by the values of the coefficient of quantum efficiency of the PS II reaction centers (Fv/Fm). Thus, under optimal growth conditions, the values of the Fv/Fm coefficient are 0.6–0.7, while an increase in illumination to 440 and 680 μE/(m2s) leads to a decrease in the described parameter to 0.4 and 0.2, respectively. Intracellular chlorophyll concentration (a + c) at E. huxleyi decreases linearly from 0.3 pg/cell at a light intensity of 20 μE/(m2 s) up to 0.08 pg/cell at 680 μE/(m2 s). Note that the ratio between chlorophylls a and c in E. huxleyi cells did not depend on illumination and remained relatively constant throughout the experiment: the content of chlorophyll c was approximately 22–25% of the chlorophyll a content.
Using the technical capabilities of the flow cytometer, it was possible to reliably separate the E. huxleyi cells covered and not covered with coccoliths (Fig. 2a) to analyze their structural, functional, and fluorescent parameters and also to calculate the ratio between these groups of cells depending on the lighting conditions. The results showed that the ratio of covered and uncovered cells in the population strongly differs depending on the light conditions of algal growth: with an increase in light intensity, a pronounced tendency towards a decrease in the percentage of covered cells in culture is observed (Fig. 2b). Thus, in the light range from 20 to 100 μE/(m2 s), the number of covered cells was approximately 80–90%; with an increase in irradiation up to 440 μE/(m2 s), their percentage decreased to values of 50–55% and up to 35% at extremely high illumination of 850 μE/(m2 s). It can be assumed that the photoinhibition of the growth rate of E. huxleyi, observed at light intensities above 400 μE/(m2 s), is associated with a significant increase in the number of uncovered cells in culture, which, due to the absence of coccoliths on the surface, are more exposed to high illumination. However, growth stops E. huxleyi and a significant increase in the number of cells uncovered with coccoliths in culture at high light intensity (680–850 μE/(m2 s)) does not indicate the lethal effect of light on the growth of algae. When transferring a culture with a high illumination of 680 μE/(m2s) in optimal light conditions of 85 μE/(m2s) within 2 days, the growth rate of algae is restored in full, the intracellular concentration of chlorophyll (a+c) changes according to the new light conditions of growth, and the index of the relative variable fluorescence of chlorophyll Fv/Fm reaches values of 0.6–0.7 (Fig. 3).
(a) Visualization of the method of population division of Emiliania huxleyi into two cell forms—covered with a layer of calcium formations, coccoliths (region A), and cells without coccoliths (region B)—by measuring the side light scattering of cells (SS) and chlorophyll autofluorescence in the red region of the spectrum (FL4); (b) change in the percentage of cells of Emiliania huxleyi covered with coccoliths depending on the light intensity at which the algae were grown.
Dynamics of growth rate (○), intracellular chlorophyll concentration a + c (●) and coefficient of variable chlorophyll fluorescence, Fv/Fm (◇), in Emiliania huxleyi when the light conditions of growth are changed. The vertical dashed line marks the moment when the illumination in the experiment changes from 680 to 85 μE/(m2 s).
Table 1 shows the main cytometric and structural parameters of covered and uncovered E. huxleyi cells in different light conditions of algae growth. From the presented data, it can be seen that the studied groups of cells E. huxleyi differ significantly in the magnitude of forward (FS) and side (cell granularity) (SS) light scattering. Thus, the values of FS in covered cells is twice as high as in uncovered cells, while SS in cells covered with coccoliths has values 25 times higher than in cells without coccoliths at all irradiation levels. The obtained differences are obviously due to the light scattering properties of coccoliths. Conversion from relative units (FS) to absolute (cell volume, μm3) showed that the volume of cells covered with coccoliths is approximately 2.5–3.5 times the value of the described parameter in cells without coccoliths.
Cells covered and not covered with coccoliths differ in terms of autofluorescence in the red region of the spectrum (675 nm, channel FL4), and, accordingly, in the content of chlorophyll in them. For both groups of E. huxleyi cells, the same type of light dependences of red autofluorescence are noted: a decrease in FL4 with an increase in illumination. However, for cells covered with a layer of coccolith, the FL4 values were significantly higher than those for uncovered cells in the entire studied light range. The observed difference is probably due to differences in the amount of light quanta absorbed by cells since the presence of calcium formations, coccoliths, leads to an increase in the scattering coefficient and a decrease in the specific absorption coefficient of light by pigments. Therefore, the number of light quanta absorbed by uncovered cells will exceed that of covered cells and, consequently, the chlorophyll content in them will be lower.
It was noted that nonspecific autofluorescence is recorded in the green region of the spectrum (525 nm) in cells covered with a layer of coccoliths, while this fluorescence is absent in cells without coccoliths. The emission of covered cells in this region of the spectrum is probably associated with cellular fluorophores that are part of the coccoliths themselves, in particular, polysaccharides [14–16], chitin [17], or other organic substances [18]. In addition, autofluorescence in the green region of the spectrum is not only an exclusive property of individual taxa but can manifest itself to one degree or another in cells of different nature and origin, depending on their physiological state [19]. It is an indicator of the intensity of oxidative processes in the cells of bacteria and eukaryotes [20] and grows in cells under extreme conditions [21] and is subject to destructive changes: with degradation of the photosynthetic apparatus, decreased enzymatic activity, increased membrane permeability, and denaturation of proteins [19] as well as during apoptosis [22, 23]. This perhaps explains the increase in green autofluorescence values in cells covered with coccoliths of E. huxleyi with increasing light intensity (see Table 1). However, it is not possible at the present to specifically answer whether the increase in green autofluorescence is associated with a change in the functional state of E. huxleyi in response to the action of light or due to an increase in the glow intensity of fluorescent molecules and compounds that make up coccoliths and this requires additional research.
DISCUSSION
Today, one of the main theories explaining the massive development of E. huxleyi coccolithophorids in the waters of the World Ocean under conditions of extremely high levels of illumination is that there is a decrease in the degree of light penetration into the cell due to the special light-scattering properties of coccoliths that cover the cell membrane [1, 2, 17]. Thus, the cell has a competitive advantage over other phytoplankton representatives under high irradiation conditions. Nevertheless, the results of our experimental study showed that growth photoinhibition of E. huxleyi begins to appear at a light intensity above 400 μE/(m2 s) up to a complete stop of algae growth at an illumination of 680 μE/(m2 s). A light-dependent decrease in the growth rate occurs against the background of a decrease in the specific content of chlorophyll in cells and the values of the quantum efficiency coefficient of PS II reaction centers. It should be noted that illumination of cells with light intensity of 400–850 μE/(m2 s) does not have a lethal effect on microalgae; the functional characteristics of cells are quickly restored when transferred to favorable growth conditions (Fig. 3). We believe that the observed growth inhibition of E. huxleyi is associated with a significant increase in the percentage of uncovered cells in culture at high illumination (approximately 65% at 850 μE/(m2 s)), which, due to the absence of coccoliths on the surface, are more exposed to high illumination. This is confirmed by the difference in the intracellular concentration of chlorophyll, the value of which was significantly higher in the cells covered with coccoliths in the entire light range under study.
A huge number of works are devoted to questions regarding calcification of E. huxleyi, the data of which were obtained using a wide arsenal of experimental methods [1, 24, 25]. Due to the distinctive feature associated with the ability of cells surrounded by coccoliths to scatter light, it becomes possible to use a modern methodological base, in particular, flow cytometry. Side-scatter (SS) flow cytometers allow for cell identification of E. huxleyi with their different morphotypes [25, 26]. We noted that the SS values are 25 times higher in the cells covered with coccoliths, which is probably due to the high granularity of the surface of the outer coccoliths. Using this approach of cell separation, we were able to identify a number of differences in the fluorescent, dimensional, and structural parameters of E. huxleyi cells corresponding to two of its forms: covered and uncovered coccoliths.
Dimensional structure analysis of E. huxleyi showed that covered cells are 2.5–3.5 times larger than uncovered cells in volume. Similar calculation of cell volumes of E. huxleyi with and without coccoliths using flow cytometry and calibration beads was carried out in [27]. The authors have shown that the volumes of the studied groups of cells differ by two timeson average: the volume of cells with coccoliths varies from 115 to 155 μm3, while its value in uncovered cells is approximately 57–77 μm3 depending on growing conditions. The authors explain the increase in the volume of cells covered with coccoliths up to 155 μm3 in unfavorable growth conditions by an increase in the size of coccospheres (protoplasts and calcium carbonate plates) or the number of coccoliths on the cell surface. The hypothesis put forward by the authors explains the regular increase in the volume of cells covered with coccoliths with an increase in illumination that we noted. Thus, the difference in the volume of E. huxleyi cells covered and uncovered by coccoliths was 2.5–3 times with a limiting and optimal light intensity for growth, while this parameter differed by almost four times at an inhibiting light level. Note that the hypothesis of “overproduction” of coccoliths under unfavorable growth conditions and the formation of several layers of calcareous discs on the cell surface of E. huxleyi was noted in a number of works [1, 28–30] devoted to the study of this type of algae. In [31], an increase in the volume of cells, both with and without coccoliths, with an increase in illumination, was also noted but not as significant as in our study. However, the authors give a different interpretation of the data obtained and associate the change in cell volume with an increase in the rate of photosynthesis and the concomitant accumulation of carbon inside the cell [32]. It should be noted that Müller et al. obtained a significantly smaller difference between the volumes of cells with and without coccoliths: the cells differed by a factor of 1.5on average. This is perhaps due to different instrumental approaches to calculating sizes or different strains of E. huxleyi used in research.
I would like to pay special attention to the issue of differences in the values of green autofluorescence (FL1) in the studied groups of cells of E. huxleyi. We have found that cells without coccoliths are characterized by the absence of fluorescence in this region of the spectrum, while green autofluorescence is recorded in covered cells E. huxleyi, and its value regularly increases with increasing illumination. The observed difference can be explained by two reasons. First, emission in the green region of the spectrum can be associated with fluorophores that are part of coccolith-containing cells of E. huxleyi, in particular polysaccharides [14–16] and calcite [33], or with a change in the content of intracellular pigments [34]. If we consider the assumption that the green autofluorescence of the cells covered with the coccosphere is associated with polysaccharides, then there are data in [35] that contradict this hypothesis. The authors have shown that polysaccharides are present both in cells covered with coccoliths and without them, and they hardly differ in the composition of their monosaccharides. We believe that the observed green autofluorescence is due to the luminescence of fibrillar material or “glue,” through which coccoliths attach to the cell membranes [36]. In other sources, this “glue” is described as an acidic polysaccharide or “coccolite polysaccharide” [17, 37], which is capable of binding with calcium ions [38] and with the surface of CaCO3 crystals [39]. Acidic polysaccharide is produced in the intracellular vesicles of coccoliths, deposited on the cell surface, integrated into CaCO3 crystals and then transported to the cell surface with coccoliths [40]. It is assumed that the rate of calcification of the covered cells increases with increasing light intensity, which is probably the reason for an increase in green fluorescence and cell volumes with coccoliths under these conditions.
CONCLUSIONS
The results of the experimental study made it possible to identify new aspects of the physiology of coccolithophorids of E. huxleyi as a species that causes massive “blooms” in the Black Sea and significantly affects the cycle of nutrients and minerals in the marine environment. It is shown that the population of E. huxleyi is represented by two cellular forms covered with a layer of calcium formations, coccoliths, and cells without coccoliths, the ratio between which depends on the light conditions of algal growth. An increase in light intensity leads to a natural decrease in the number of cells covered with coccoliths in culture, which is probably the reason for the photoinhibition of growth of E. huxleyi at an illumination above 400 μE/(m2 s).
According to the results of the study, significant physiological differences in cells were revealed in E. huxleyi covered and not covered with coccoliths. Thus, uncovered cells are characterized by a small size, low values of forward and side light scattering, a lower specific content of chlorophyll, and also the absence of green autofluorescence (FL1). In view of this, a change in the ratio between covered and uncovered cells in a population will lead to a change in its structural and functional characteristics as a whole. Thus, the culture is dominated by cells covered with coccoliths in low light conditions (in natural conditions typical for the winter season). At high values of illumination (600–800 μE/(m2 s)), the contribution of covered cells is reduced to 30–40%. Based on this, it should be assumed that the estimate of the number of cells of E. huxleyi in the sea in summer (at an extremely high level of irradiation), based on satellite measurements, gives underestimated results relative to real values since it registers only cells covered with coccoliths. In addition, the absence of solid calcium formations (coccoliths) in E. huxleyi and, as a result, a significant reduction in the size of uncovered cells makes microalgae more susceptible to being eaten by zooplankton, thereby influencing the dynamics of the food chain.
REFERENCES
Paasche, E., A review of the coccolithophorid Emiliania huxleyi (Prymnesiophyceae), with particular reference to growth, coccolith formation, and calcification-photosynthesis interactions, Phycologia, 2001, vol. 40, p. 503. https://doi.org/10.2216/i0031-8884-40-6-503.1
Churilova, T.Ya. and Suslin, V.V.,The reasons of dominance of Emiliania huxleyi in phytoplankton of the deep-water part of the Black Sea in early summer, Ekol. Bezpeka Pribrezhnoi Shel’fovoi Zon Kompl. Nevikoristannya Resur. Shel’fu, 2012, vol. 26, p. 195.
Stel’makh, L.V., Senicheva, M.I., and Babich, I.I., Ecological-physiological principles of water “blooming” caused by Emiliania huxleyi in the Sevastopol Bay, Ekol. Morya, 2009, vol. 77, p. 28.
Kubryakova, E.A., Kubryakov, A.A., and Mikaelyan, A.S., Winter blooms of coccolithophorids in the Black Sea: interannual variability and causing factors, Materialy VI Vserossiiskoi nauchnoi konferentsii molodykh uchenykh “Kompleksnye issledovaniya Mirovogo okeana” (Proc. VI All-Russ. Sci. Conf. of Young Scientists “Complex Studies of the World Ocean”), Moscow: Inst. Okeanol. im. P.P. Shirshova, Ross. Akad. Nauk, 2021, p. 507.
Cokacar,T., Oguz,T., and Kubilay, N., Satellite-detected early summer coccolithophore blooms and their interannual variability in the Black Sea, Deep Sea Res., Part I, 2004, vol. 51, p. 1017. https://doi.org/10.1016/j.dsr.2004.03.007
Klaveness, D.and Paasche, E., Two different Coccolithus huxleyi cell types incapable of coccolith formation, Arch. Microbiol., 1971, vol. 75, p. 382. https://doi.org/10.1007/BF00407700
Klaveness, D.,Coccolithus huxleyi (Lohmann) Kamptner. I. Morphological investigations on the vegetative cell and the process of coccolith formation, Protistologica, 1972, vol. 8, p. 335.
Guillard, R.and Ryther, J., Studies of marine planktonic diatoms. I. Cyclotella nana Hustedt, and Detonula confervacea (cleve) Gran., J. Can. Microbiol., 1962, vol. 8, p. 229. https://doi.org/10.1139/m62-029
Finenko, Z.Z. and Lanskaya, L.A., Growth and rate of algae division in limited water volumes, in Ekologicheskaya fiziologiya morskikh planktonnykh vodoroslei (v usloviyakh kul’tur) (Ecological Physiology of Marine Planktonic Algae (in Vitro Conditions)), Khailov, K.M., Ed., Kiev: Naukova Dumka, 1971, p. 22.
Solomonova, E. and Mukhanov, V., Evaluation of the part of physiologically active cells in accumulative cultures Phaeodactylum tricornutum and Nitzschia sp. using flow cytometry, Morsk. Ekol. Zh., 2011, vol. 10, p. 67.
Solomonova, E. and Akimov, A., The ratio of the dead and living components of suspension in microalgae cultures depending on the growth stage and illumination, Morsk. Ekol. Zh., 2014, vol. 13, p. 73.
Jeffrey, S.W.and Humphrey, G.F., New spectrophotometric equations for determining chlorophylls a, b, c 1 and c 2 in higher plants, algae and natural phytoplankton, Biochem. Physiol. Pflanzen, 1975, vol. 167, p. 191.
Matorin, D.N., Osipov, V.A., Yakovleva, O.V., and Pogosyan, S.I., Opredelenie sostoyaniya rastenii i vodoroslei po fluorestsentsii khlorofilla: uchebno-metodicheskoe posobie (Determination of State of Plantsand Algae by Fluorescence of Chlorophyll: Practical Manual), Moscow: MAKS Press, 2010.
Fichtinger-Schepman, A.M.J., Kamerling, J.P., Versluis, C., and Vliegenthart, J.F., Structural studies of the methylated, acidic polysaccharide associated with coccoliths of Emiliania huxleyi (Lohmann) Kamptner, Carbohydr. Res., 1981, vol. 93, p. 105. https://doi.org/10.1016/S0008-6215(00)80756-9
Borman, A.H., de Jong, E.W., Thierry, R., Westbroek, P., Bosch, L., Gruter, M., and Kamerling, J.P., Coccolith-associated polysaccharides from cells of Emiliania huxleyi (Haptophyceae)., J. Phycol., 1987, vol. 23, p. 118. https://doi.org/10.1111/j.1529-8817.1987.tb04433.x
Marsh,M.E., Regulation of CaCO3 formation in coccolithophores, Comp. Biochem. Physiol., Part B: Biochem. Mol. Biol., 2003, vol. 136, p. 743. https://doi.org/10.1016/S1096-4959(03)00180-5
Kayano, K., Saruwatari, K., Kogure, T., and Shiraiwa, Y., Effect of coccolith polysaccharides isolated from the coccolithophorid, Emiliania huxleyi, on calcite crystal formation in vitro CaCO3 crystallization, Mar. Biotechnol., 2011, vol. 13, p. 83. https://doi.org/10.1007/s10126-010-9272-4
Takahashi, J., Fujiwara, S., Kikyo, M., Hirokawa, Y., and Tsuzuki, M., Discrimination of the cell surface of the coccolithophorid Pleurochrysis haptonemofera from light scattering and fluorescence after fluorescein-isothiocyanate-labeled lectin staining measured by flow cytometry, Mar. Biotechnol., 2002, vol. 4, p. 94. https://doi.org/10.1007/s10126-001-0083-5
Tang, Y.Z.and Dobbs, F.C., Green autofluorescence in dinoflagellates, diatoms, and other microalgae and its implications for vital staining and morphological studies, Appl. Environ. Microbiol., 2007, vol. 73, p. 2306. https://doi.org/10.1128/AEM.01741-06
Price, P.B.and Bay, R.P., Marine bacteria in deep Arctic and Antarctic ice cores: a proxy for evolution in oceans over 300 million generations, Biogeosciences, 2012, vol. 9, p. 3799. https://doi.org/10.5194/bg-9-3799-2012
Roldán, M., Ascaso, C., and Wierzchos, J., Fluorescent fingerprints of endolithic phototrophic cyanobacteria living within halite rocks in the Atacama Desert, Appl. Environ. Microbiol., 2014, vol. 80, p. 2998. https://doi.org/10.1128/AEM.03428-13
Veldhuis, M.J.W., Kraay, G.W., and Timmermans, K.R., Cell death in phytoplankton: correlation between changes in membrane permeability, photosynthetic activity, pigmentation and growth, Eur. J. Phycol., 2001, vol. 36, p. 167. https://doi.org/10.1017/S0967026201003110
Franklin, D.J.and Berges, J.A., Mortality in cultures of the dinoflagellate Amphidinium carterae during culture senescence and darkness, Proc. R. Soc. B, 2004, vol. 271, p. 2099. https://doi.org/10.1098/rspb.2004.2810
Paasche, E., Brubak, S., Skattebøl, S., Young, J.R., and Green, J.C., Growth and calcification in the coccolithophorid Emiliania huxleyi (Haptophyceae) at low salinities, Phycologia, 1996, vol. 35, p. 394. https://doi.org/10.2216/i0031-8884-35-5-394.1
Fuertes, M.Á., Flores, J.A., and Sierro, F.J., The use of circularly polarized light for biometry, identification and estimation of mass of coccoliths, Mar. Micropaleontol., 2014, vol. 113, p. 44. https://doi.org/10.1016/j.marmicro.2014.08.007
von Dassow, P., van den Engh, G., Iglesias-Rodriguez, D., and Gittins, J.R., Calcification state of coccolithophores can be assessed by light scatter depolarization measurements with flow cytometry, J. Plankton Res., 2012, vol. 34, p. 1011. https://doi.org/10.1093/plankt/fbs061
Iglesias-Rodriguez, M.D., Halloran, P.R., Rickaby, R.E., HallI. R., Colmenero-Hidalgo, E., Gittins, J.R., Green, D.R.H., Tyrrell, T., Gibbs, S.J., von Dassow, P., Rehm, E., Armbrust, E.V., and Boessenkool, K.P., Phytoplankton calcification in a high-CO2 world, Science, 2008, vol. 320, p. 336. https://doi.org/10.1126/science.1154122
Garde, K.and Cailliau, C., The impact of UV-B radiation and different PAR intensities on growth, uptake of 14C, excretion of DOC, cell volume, and pigmentation in the marine prymnesiophyte, Emiliania huxleyi, J. Exp. Mar. Biol. Ecol., 2000, vol. 247, p. 99. https://doi.org/10.1016/S0022-0981(00)00145-3
Müller, M.N., Antia, A.N., and LaRoche, J., Influence of cell cycle phase on calcification in the coccolithophore Emiliania huxleyi, Limnol. Oceanogr., 2008, vol. 53, p. 506. https://doi.org/10.4319/lo.2008.53.2.0506
Müller, M.N., Trull, T.W., and Hallegraeff, G.M., Independence of nutrient limitation and carbon dioxide impacts on the Southern Ocean coccolithophore Emiliania huxleyi, ISME J., 2017, vol. 11, p. 1777. https://doi.org/10.1016/S0022-0981(00)00145-3
Müller, M.N., Brandini, F.P., Trull, T.W., and Hallegraeff, G.M., Coccolith volume of the Southern Ocean coccolithophore Emiliania huxleyi as a possible indicator for palaeo-cell volume, Geobiology, 2021, vol. 19, p. 63. https://doi.org/10.1111/gbi.12414
Aloisi, G., Covariation of metabolic rates and cell size in coccolithophores, Biogeosciences, 2015, vol. 12, p. 4665. https://doi.org/10.5194/bg-12-4665-2015
Fox, E., Meyer, E., Panasiak, N., and Taylor, A.R., Calcein staining as a tool to investigate coccolithophore calcification, Front. Mar. Sci., 2018, vol. 5, p. 326. https://doi.org/10.3389/fmars.2018.00326
Sayanova, O., Haslam, R.P., Calerón, M.V., López, N.R., Worthy, C., Rooks, P., and Napier, J.A., Identification and functional characterization of genes encoding the omega-3 polyunsaturated fatty acid biosynthetic pathway from the coccolithophore Emiliania huxleyi, Phytochemistry, 2011, vol. 72, p. 594.
Nanninga, H.J., Ringenaldus, P., and Westbroek, P., Immunological quantitation of a polysaccharide formed by Emiliania huxleyi, J. Mar. Syst., 1996, vol. 9, p. 67. https://doi.org/10.1016/0924-7963(96)00017-6
Billard, C.and Inouye,I., What is new in coccolithophore biology? in Coccolithophores: From Molecular Processes to Global Impact, Berlin: Springer-Verlag, 2004, p. 1.
Tsuji, Y., Yamazaki, M., Suzuki, I., and Shiraiwa, Y., Quantitative analysis of carbon flow into photosynthetic products functioning as carbon storage in the marine coccolithophore, Emiliania huxleyi, Mar. Biotechnol., 2015, vol. 17, p. 428. https://doi.org/10.1007/s10126-015-9632-1
De Jong, E.W., Bosch, L., and Westbroek, P., Isolation and characterization of a Ca2+ binding polysaccharide associated with coccoliths of Emiliania huxleyi (Lohmann) Kamptner, Eur. J. Biochem., 1976, vol. 70, p. 611. https://doi.org/10.1111/j.1432-1033.1976.tb11052.x
Henriksen, K., Stipp, S.L.S., Young, J.R., and Marsh, M.E., Biological control on calcite crystallization: AFM investigation of coccolith polysaccharide function, Am. Miner., 2004, vol. 89, p. 1709. https://doi.org/10.2138/am-2004-11-1217
van Emburg, P.R., De Jong, E.W., and Daems, W.T., Immunochemical localization of a polysaccharide from biomineral structures (coccoliths) of Emiliania huxleyi, J. Ultrastruct. Mol. Struct. Res., 1986, vol. 94, p. 246. https://doi.org/10.1016/0889-1605(86)90071-6
ACKNOWLEDGMENTS
We thank the O.S. Alatartseva, employee of the Department of Ecological Physiology of Algae of Kovalevsky Institute of Biology of the Southern Seas (Russian Academy of Sciences) for providing the E. huxleyi culture.
Funding
The work was carried out within the framework of the topic of the state assignment of Kovalevsky Institute of Biology of the Southern Seas of Russian Academy of Sciences no. 121041400077−1“Functional, Metabolic, and Toxicological Aspects of Hydrobionts and Their Populations in Biotopes with Different Physical and Chemical Regimes.”
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interests. The authors declare that they have no conflicts of interest.
Statement on the welfare of humans or animals. This article does not contain any studies involving humans or animals performed by any of the authors.
Rights and permissions
About this article
Cite this article
Shoman, N.Y., Solomonova, E.S. & Akimov, A.I. Influence of Light on Change in Morphophysiological Characteristics of Coccolithophorids Emiliania huxleyi. Russ J Plant Physiol 69, 40 (2022). https://doi.org/10.1134/S1021443722020170
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1134/S1021443722020170