Abstract
Zinc (Zn) is an essential micronutrient that is crucial to the health of all organisms. Zn absorption through roots system during the grain-filling period and Zn remobilization from pre-anthesis stores of shoot into grains are two main sources of zinc accumulation in grains. Field and solution experiments were conducted to study the Zn efficiency in two groups of Iranian bread wheat (Triticum aestivum L.) genotypes with high and low Zn content in seeds at two vegetative stages. Among 110 evaluated genotypes, six and seven were selected with high and low seed Zn content, respectively. Assessment of zinc uptake, accumulation and utilization in shoot, root, and shoot dry weight traits showed significant differences between two genotypic groups only at 60 days after planting (DAP). The genotypes with high seed Zn content showed higher uptake, accumulation and utilization of zinc in shoot than those with low seed Zn content. Our results showed Zn efficient genotypes are detected not only by Zn uptake but also by accumulation and utilization of Zn in the shoot. Among the mechanisms involved in Zn efficiency, Zn uptake and/or utilization are likely more influential. The root-to-shoot translocation ratio was at the same rate between these two groups. Our results showed that Zn uptake and accumulation in shoot among genotypes can be considered as a useful parameter for revealing impairments of efficiency in wheat genotypes.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
INTRODUCTION
More than three billion people in all world are suffering from minerals deficiency. It affects labor productivity, health care costs and rate of premature death [1]. People living in developing countries are especially at risk, as they cannot afford to buy foods rich in minerals, such as red and white meat, fresh fruits, and vegetables [2]. Zinc (Zn) is an essential element that has various physiological functions in biological systems [3]. Zn plays an important role in growth and development, function of the immune system, reproductive system health, sensory system function, and neurobehavioral development [1]. Deficiency of Zn after that of iron (Fe), is the most common micronutrient shortage in developing countries [3].
Cereals are the main source of the human diet that are rich in carbohydrates but low in nutrient concentrations especially Fe and Zn [4]. Among cereals wheat is one of the most important crops that is consumed steadily by more than one-third of the world population, contributing more calories and proteins to their diets than other cereal crops. Wheat is nutritious, easy to store and transport, and can be processed into various types of foods [4]. However, more than half of wheat plantation is in Zn-deficient soils and subsequently, the Zn concentration in grain is too low to supply the required Zn for human health [5].
Biofortification is a strategy to overcome Zn deficiency in crops, especially in developing countries [6]. This practice can develop micronutrient-dense food crops using agronomic, breeding, and biotechnological tools [6]. Two main approaches for grain biofortification are breeding and micronutrient fertilization. The latter is a more attainable and cost-effective approach for increasing Zn concentration in grains [6]. However, reports indicate that grain Zn concentration is largely determined by plant genotypes rather than by Zn fertilization, which could be attributed to differences in Zn uptake behavior [7].
Therefore, it seems likely that the nutrient-efficient genotypes would play a major role in the increase of crop quality and yields in the next decades in both organic and inorganic agricultures [8]. On the soils where the effectiveness of fertilizers may be limited by chemical and biological reactions, topsoil drying, and subsoil constraints, using these genotypes would be a practical approach [1]. Also growing nutrient-efficient genotypes of crop plants on poor soils represents an eco-friendly approach that would reduce land degradation by reducing the use of agricultural machinery and minimizing the application of chemicals on agricultural lands [8]. On the other hand, nutrient-efficient genotypes have specific physiological mechanisms allowing them to gain sufficient quantities of a specific nutrient (uptake efficiency) and/or to utilize the absorbed nutrient more effectively (utilization efficiency) [9]. Various genotypes of wheat exhibit a great diversity in their ability to grow on Zn deficient soils [10].
Several mechanisms for increasing Zn efficiency were debated [11]. The interrelated processes include Zn uptake, translocation to shoots, internal utilization efficiency, and when grain yield is measured, remobilization of Zn from shoots to grain [11]. Zn efficient genotypes have been evaluated in monocotyledonous (Triticum aestivum, Zea mays, and Oryza sativa) and dicotyledonous (Lycopersicon esculentum and Phaseolus vulgaris) plants for traits associated with Zn efficiency [12]. Significant differences have been reported in Zn uptake [12]; internal utilization of Zn [9]; root geometry and morphology [13, 14]; and differences in biomass production and distribution [13].
There have been several studies reported about Zn efficiency in bread wheat [13–15]. However, there is no comprehensive study about Zn efficiency in early vegetative growth which can save both money and time in breeding programs [16]. In the current study, the Zn efficiency was assessed using a combination of field and solution experiments. In addition, the different manners of genotypes with high and low seed Zn content in respect to Zn efficiency parameters such as Zn uptake and utilization in the shoot along with Zn accumulation in the shoot were evaluated at two vegetative growth stages of 30 and 60 DAPs in the solution experiment.
MATERIALS AND METHODS
Field experiment. A total of 110 bread wheat (Triticum aestivum L.) genotypes (76 local varieties and 34 cultivars), collected from different parts of Iran, were kindly provided by Seed and Plant Improvement Institute in Karaj, Iran (Table 1). All genotypes were field grown during the cropping season at the experimental farm of Faculty of Agricultural Sciences, Shahed University, Tehran, Iran (35°55′ N, 51°34′ E, 1032.6 m above sea level). Some of the soil characteristics in the topsoil layer (0–30 cm) were pH 7.45, electrical conductivity of a saturated soil extract 7.4 dS/m, organic matter 0.78%, CaCO3 15.59%, total S, K, P, Mn, Cu, B, Fe, and Zn – 29, 533, 40.83, 1.73, 0.81, 1.15, 7.57, and 0.7 mg/kg respectively.
All genotypes were harvested at maturity and seed were analyzed for Zn content. Atomic absorption spectrometry (Shimadzu AA-670, Japan) following Campbell and Plank [17] was used to measure the grain Zn concentration. Based on grain Zn content, genotypes were sorted into two groups of high and low Zn content.
Solution experiment. From high and low Zn groups, six and seven genotypes were respectively selected for conducting the solution experiment [14]. For vernalization of winter wheat genotypes in these two groups seeds were located in a refrigerator at 4°C for four weeks. Seeds were sown in pots (25 cm height, 22 cm top, and 18 cm bottom diameters) containing perlite and fed with modified one-quarter-strength Johnson’s nutrient solution [18]. The solution contained 2 mM KH2PO4, 2 mM K2SO4, 1 mM MgSO4, 4 mM Ca provided as CaSO4 and/or Ca(NO3)2 and the micronutrients 50 μM Cl (KCl), 25 μM B (H3BO3), 20 μM Fe (as Fe-EDTA), 2 μM Mn (as MnSO4·H2O), 2 μM Zn (as ZnSO4·7H2O), and 0.5 μM Cu (as CuSO4·5H2O) [23]. In the first seven days, plants were fed with this solution every day and subsequently once in every three days. Plants were grown in controlled conditions with light/dark periods: 16/8 h; temperature (light/dark): 22/18°C; relative humidity (light/dark): 60/70%; and photosynthetic flux density: 400 μmol/(m2 s) during 60 days.
The solution experiment was set up according to a randomized complete block design (RCBD) in three biological replicates. Two pots and four plants per pot were considered in each replicate (six pots per genotype). All plants were harvested at two vegetative growing periods of 30 and 60 DAPs (days after planting). All tissue samples (in both growth stages of 30 and 60 DAPs) were well washed with deionized water before any measurement to remove any metal that may be stuck. Root and shoot samples were weighed and processed for tissue nutrient analysis after oven drying at 70°C for 72 h. Dried samples of root and shoot were ground and burned to ash at 550°C for 8 h, and the ash was dissolved in 2 M HCl [17]. The root and shoot Zn concentration in digestion solutions was determined by atomic absorption spectrometry (Shimadzu AA-670, Japan).
According to equality of Zn concentration in solution experiment for all treatments, sum of root and shoot Zn contents were reported as plant total Zn uptake. Also, root-to-shoot Zn translocation ratio was calculated by the percentage of Zn in the shoot to the total Zn uptake. Root and shoot Zn content were calculated as the total accumulation of Zn per plant. Utilization efficiency of Zn (the dry matter (DM) produced per mg of Zn in the specific dry weight (DW) (of plant tissue), along with root and shoot DW, were used for recognizing the efficiency of wheat cultivars differing in their responses to zinc deficiency [13–15].
Statistical analysis. Normality test based on the Shapiro-Wilk method, analysis of variance (ANOVA), Student’s t-test, and Pearson correlation coefficient were performed using SPSS software version 21.0 (SPSS, United States). The least significant difference (LSD), was calculated for mean comparison with SAS 9.2 software at P ≤ 0.05 and 0.01. The heatmap was utilized for the studied traits and genotypes clustering at 60 DAP using the squared Euclidean distance and ward linkage by MetaboAnalyst 2.0 web server (http://www.metaboanalyst.ca/).
RESULTS
Seed Zn Content in Different Wheat Genotypes
The average grain Zn concentration in 110 bread wheat genotypes ranged from 30.45 to 85 mg/kg (Table 1). Also, the seed Zn content was calculated in the 110 bread wheat genotypes (Table 1). The six genotypes selected among the 110 field studied genotypes for high Zn showed a range of Zn content from 2.28 to 2.82 µg/seed. In contrast, the seven low Zn genotypes were in the range of 0.79–0.99 µg/seed. The average zinc content in high Zn genotypes was nearly 3-folds higher than those of low Zn genotypes (Table 1).
Zn Uptake, Accumulation and Utilization in Shoot
Our results showed that the Zn uptake by the plants along with shoot Zn content (accumulation) and utilization were significantly different among two groups of genotypes at 60 DAP, whereas no significant difference was observed at 30 DAP (Table 2). Zinc uptake, accumulation and utilization in shoot of genotypes with high seed Zn content were 1.44-, 1.53-, and 1.94-fold higher than those with low seed Zn content at 60 DAP, respectively (Table 2). In contrast, root Zn accumulation, root and shoot Zn concentrations, and root-to-shoot translocation ratio were not significant among genotypes at two growth stages of 30 and 60 DAPs (Table 2).
The results showed that the Zn efficiency parameters in the efficient genotypes are indicated not only by Zn uptake but also by Zn accumulation and utilization in shoot. It seems different impairment types were found in Zn inefficient genotypes. The Mashhad 5 genotype showed low Zn uptake (3.617 µg/plant), low Zn accumulation (2.522 µg/plant), and low utilization in the shoot (3.737 mg DM/(mg Zn/kg DW)) (Table 3). Although the Molavi Khorramabad genotype showed high Zn uptake (7.120 µg/plant) and root Zn accumulation (3.516 µg/plant), the detected root-to-shoot translocation ratio (50.617%), shoot Zn accumulation (3.604 µg/plant), and Zn utilization in shoot (5.450 mg DM/(mg Zn/kg DW)) were low (Table 3). Though the lowest Zn utilization (3.100 mg DM/(mg Zn/kg DW)) belonged to Zarrin genotype, considerable Zn uptake (5.888 µg/plant), shoot Zn accumulation (4.470 µg/plant), and root-to-shoot Zn translocation ratio (75.917%) were detected in this genotype.
Root and Shoot Dry Weight
Significant differences in root and shoot DW were observed between the two high and low Zn content groups of bread wheat genotypes at 60 DAP, whereas these differences were not significant at 30 DAP (Table 2). In this study, root and shoot DW in genotypes with high seed Zn content was 1.90- and 1.67-fold higher than genotypes with low seed Zn content, respectively (Table 2).
Statistical analysis showed significant differences in root DW among 13 selected genotypes at both 30 and 60 DAPs. Shahabad Ashtareh and Mashhad 2 genotypes with 0.031 and 0.007 g had the highest and the lowest root DW at 30 DAP, respectively. Also, the shoot DW was statistically different only at 60 DAP. At 60 DAP, root and shoot DWs of Khorramabad were the highest (0.320 and 0.300 g), and in Zarrin were the lowest (0.080 and 0.110 g), respectively (Table 4).
Relationship between Zn Efficiency and Biomass
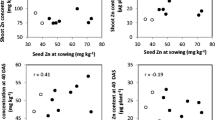
A significant positive correlation was observed between the Zn uptake and root or shoot DW at 30 DAP (r = 0.65 and 0.65, P ≤ 0.05, respectively) and at 60 DAP (r = 0.61 and 0.58, P ≤ 0.05, respectively) (Fig. 1). Also, the Zn utilization in shoot was significantly correlated with the root or shoot DW at 60 DAP (r = 0.70 and 0.76 P ≤ 0.01, respectively) (Fig. 2). Above all, the Zn utilization showed a positive correlation with Zn uptake and accumulation in shoot at 60 DAP (r = 0.83 and 0.83, P ≤ 0.01, respectively) (Fig. 2).
Associations between Zn Efficiency and Biomass
The studied traits in 13 bread wheat genotypes at 60 DAP were exposed by means of heatmap and two-dimensional dendrograms (Fig. 3). Clustering analysis of all traits at 60 DAP developed three main groups. The Zn uptake, accumulation and utilization in shoot were different between these clustered genotypes. The first group including four genotypes with high ability in Zn uptake, accumulation and utilization in shoot. The genotypes in the second group showed a lower average in the studied traits than those in the first. Molavi Khorramabbad was placed in this cluster. Also, genotypes in the third group showed a low ability in Zn uptake, accumulation and utilization in shoot with Mashhad 5 and Zarrin located in this group (Fig. 3).
DISCUSSION
Crop improvement through plant breeding strategies is based on genetic variation. Results of previous studies indicated genetic variations could help to identify genotypes with inherently high Zn concentration in grain [19]. The average of Zn concentrations in bread wheat grain in previous studies were 16.4–39.5 mg/kg [20], 16.2–32.4 mg/kg [21], and 24–36 mg/kg [2]. The sufficient concentration of Zn for plant growth is 1–3 mg/kg [22]. So, it appears that the used soil type, in this field experiment (0.7 mg/kg), was classified as zinc deficient soil. In the present study, while the soil suffered from the deficiency of zinc, the Zn concentration in 110 Iranian bread wheat grains was 30.45–85 mg/kg (Table 1). This shows a high variation for Zn concentration in these genotypes compared to the previous studies. These variations can be used in breeding programs for producing elite genotypes with high yields and Zn concentrations in grains.
Accumulation of zinc in wheat grains is supplied principally from two sources: concurrent uptake during the grain-filling stage and net remobilization of Zn from the pre-anthesis stores of source tissues [2]. The relative importance of these two sources can change under Zn-deficient conditions. So there is no unanimous agreement about the relative contributions of these two sources to grain zinc deposition. According to studies on genotypes of different cereal species, the concentration of Zn in plant tissues is not a reliable parameter for distinguishing the sensitivity of genotypes to zinc deficiency. In contrast, the total content of Zn per plant (portion seed, shoot, or root) is better related with the sensitivity of cereals to Zn deficiency [13]. Also, in the wheat genotypes, distinctive differences of sensitivity to Zn deficiency among the genotypes are closely related to the Zn content per plant tissue but not to the Zn concentration [23]. In the presented study, the genotypes were selected based on Zn content in seed for farther analyses.
The variation in Zn efficiency between these two groups was observed in the 60 DAP and not in 30 DAP. So, in the massive experiments, it would be possible to evaluate the Zn efficiency genotypes in the 60 DAP as vegetative growth stages. In the early growth stage, mineral deficiency occurred in plant tissues. Gradually, total minerals uptake was increased when plants approach maturity [24]. Same capabilities in zinc uptake, accumulation, and utilization parameters were found among all young plants at 30 DAP, whereas with growing plants the genetic differences were appeared between studied genotypes at 60 DAP [25].
In the solution experiment at 60 DAP, the efficiency parameters of Zn uptake, and utilization along with Zn accumulation (content) in shoot showed higher in genotypes with high seed Zn content than those with low seed Zn content (Table 3). In this regard, the results of the field experiment showed different levels of Zn in seed between two groups of genotypes. The field and solution experiments both indicated differences in Zn efficiency between the two groups. However, low seed Zn content in inefficient genotypes occurred because of the impairments in all or one of the Zn efficiency parameters. We found inefficient genotypes in different types, the first one, Mashhad 5 genotype which has low Zn uptake, shoot Zn accumulation and utilization, the second, Molavi Khoramabad, that has high Zn uptake with the lowest shoot Zn accumulation and subsequently, the last one Zarrin that has low Zn utilization (Table 3).
In our results, the same ratio of root-to-shoot translocation was detected between two groups of genotypes with high and low Zn content. Also, no significant differences were observed in the root and shoot Zn concentration of two groups genotypes. The results of similar study on Zn deficiency of durum and bread wheat showed that the durum wheat genotypes were more sensitive to Zn deficiency than bread wheat genotypes in a solution culture [23]. The different sensitivity to Zn deficiency among the durum and bread wheat genotypes were not related with the Zn concentration in the seeds or in the shoot dry matter. These genotypes showed similar concentrations of Zn in the shoot dry matter. The Zn concentration in bread wheat genotypes was similar to the durum wheat genotypes. Besides, the Zn content (accumulation) per shoot was more correlated with the sensitivity of genotypes to Zn deficiency. These results showed that less sensitivity of bread wheat genotypes compared to the durum wheat, were characterized by higher Zn uptake and higher root-to-shoot transport capacity of Zn [23]. On the other hand, among different mechanisms of Zn efficiency in some genotypes of durum and bread wheat in soil and solution culture suggested that uptake is the main mechanism which has an important physiological effect on Zn utilization in shoot. These results showed that the root-to-shoot partitioning was not associated with Zn efficiency in genotypes [15].
Moreover, clustering of genotypes in three different subgroups in the heatmap dendrogram indicated different physiological events with respect to Zn efficiency in these genotypes (Fig. 3). In general, different enzymes and proteins are involved in the uptake and transport of Zn and impaired synthesis of each of them can lead to reduced uptake and translocation of Zn [26]. Molecular analyses of three genotypes can show important factors and enzymes involving in this process. The data from these molecular analyses of these three genotypes could use in genetic engineering to biofortified genotypes with a high yield but low levels of micronutrients.
The importance of dry weight in plant tissues for recognizing genotypic efficiency has already been demonstrated. Efficiency is defined as the ability of plants to obtain higher dry matter yields and/or grain yields under moderate and deficient nutrient levels [15, 11]. Genc et al. [15] reported that high Zn concentration in root and shoot resulted in increased root and shoot DW, and the efficient genotypes had greater dry matter than inefficient ones. Similar results in rice [7], maize [5], and wheat [27] have been reported. In this study, root and shoot DW was detected higher in the genotypes with high Zn content than those with low Zn content (Table 3). These traits had positive correlations with Zn uptake and utilization in shoot (Figs. 1 and 2).
According to our results, Zn uptake and utilization in shoot had a positive correlation with root and shoot DW (Figs. 1 and 2). In addition, Zn utilization in shoot had a positive correlation with Zn uptake and Zn accumulation in shoot (Fig. 2). Also, all measured traits had higher values in Zn efficient genotypes compared to Zn inefficient ones (Table 3).
The plant’s growth and development are controlled by signals that depend on the adequate uptake of micronutrients from soil to root and root to shoot. Zinc has many important roles in the plant’s growth, i.e., photosynthesis, enzyme activity such as carbonic anhydrase [4], chlorophyll concentration [28], stomatal conductance [29], and auxin biosynthesis [30]. So adequate uptake of Zn is necessary for optimum plant growth and yield. Zn efficient genotypes have a greater ability to absorb and utilize Zn and can use Zn in critical processes in cells. They also have greater growth and dry matter than inefficient genotypes. Besides, the positive correlation of Zn uptake and utilization in shoot with root and shoot DW indicated this idea.
To conclude, results revealed that there was Zn efficiency differences between the two groups of genotypes with high and low seed Zn content. Field and solution experiments indicated the difference in Zn efficiency between the two mentioned groups. These differences were clearly observed at 60 DAP of growth stages. While there are several parameters play a role in Zn efficiency, Zn uptake and shoot Zn accumulation could be the most important parameters which reveal impairments in Zn inefficiency. The shoot Zn accumulation and utilization in the shoot along with Zn uptake were important factors to identify Zn efficient genotypes. Different impairment types was observed among the Zn inefficient genotypes and these genotypes can reveal important information in future studies. This study was based on vegetative growth and our findings need to be verified under field conditions with different levels of Zn in the soil where efficiency is based on yield.
REFERENCES
Welch, R.M. and Graham, R.D., Breeding for micronutrients in staple food crops from a human nutrition perspective, J. Exp. Bot., 2004, vol. 55, p. 353.
Hussain, S., Maqsood, M.A., Rengel, Z., and Khan, M.K., Mineral bioavailability in grains of Pakistani bread wheat declines from old to current cultivars, Euphytica, 2012, vol. 186, p. 153.
Cakmak, I. and Kutman, U., Agronomic biofortification of cereals with zinc: a review, Eur. J. Soil Sci., 2018, vol. 69, p. 172.
Gupta, N., Ram, H., and Kumar, B., Mechanism of zinc absorption in plants: uptake, transport, translocation and accumulation, Rev. Environ. Sci. Biotechnol., 2016, vol. 15, p. 89.
Wang, H., Liu, R., and Jin, J., Effects of zinc and soil moisture on photosynthetic rate and chlorophyll fluorescence parameters of maize, Biol. Plant., 2009, vol. 53, p. 191.
Rehman, A., Farooq, M., Ozturk, L., Asif, M., and Siddique, K.H., Zinc nutrition in wheat-based cropping systems, Plant Soil, 2018, vol. 422, p. 283.
Wissuwa, M., Ismail, A.B., and Graham, R.D., Rice grain zinc concentrations as affected by genotype, native soil-zinc availability, and zinc fertilization, Plant Soil, 2008, vol. 306, p. 37.
Rengel, Z. and Damon, P.M., Crops and genotypes differ in efficiency of potassium uptake and use, Physiol. Plant., 2008, vol. 13, p. 624.
Sattelmacher, B., Horst, W.J., and Becker, H.C., Factors that contribute to genetic variation for nutrient efficiency of crop plants, Z. Pflanzenernaehr. Bodenkd., 1994, vol. 157, p. 215.
Hacisalihoglu, G., Hart, J.J., and Kochian, L.V., High-and low-affinity zinc transport systems and their possible role in zinc efficiency in bread wheat, Plant Physiol., 2001, vol. 125, p. 456.
Rengel, Z., Physiological mechanisms underlying differential nutrient efficiency of crop genotypes, in Mineral Nutrition of Crops: Fundamental Mechanisms and Implications, New York: Food Products Press, 1999, p. 227.
Riseman, A. and Craig, R., Physiological and morphological traits associated with zinc efficiency in Exacum, Plant Soil, 2000, vol. 219, p. 41.
Cakmak, I., Torun, B., Erenoğlu, B., Öztürk, L., Marschner, H., Kalayci, M., Ekiz, H., and Yilmaz, A., Morphological and physiological differences in the response of cereals to zinc deficiency, Euphytica, 1998, vol. 100, p.349.
Fathi, S., Sabet, M.S., Lohrasebi, T., Razavi, K., Karimzadeh, G., and Gholami Malekroudi, M., Effect of root morphological traits on zinc efficiency in Iranian bread wheat genotypes, Acta Agric. Scand., Sect. B, 2016, vol. 66, p. 575.
Genc, Y., McDonald, G.K., and Graham, R.D., Contribution of different mechanisms to zinc efficiency in bread wheat during early vegetative stage, Plant Soil, 2006, vol. 281, p. 353.
Ru, S., Main, D., Evans, K., and Peace, C., Current applications, challenges, and perspectives of marker-assisted seedling selection in Rosaceae tree fruit breeding, Tree Genet. Genomes, 2015, vol. 11, p. 8.
Campbell, C. and Plank, C.O., Preparation of plant tissue for laboratory analysis, in Handbook of Reference Methods for Plant Analysis, Boca Raton, FL: CRC Press, 1997, p. 37.
Siddiqi, M.Y., Glass, A.D., Ruth, T.J., and Fernando, M., Studies of the regulation of nitrate influx by barley seedlings using 13NO3 −1, Plant Physiol., 1989, vol. 90, p. 806.
Xu, Y., An, D., Li, H., and Xu, H., Review: Breeding wheat for enhanced micronutrients, Can. J. Plant Sci., 2011, vol. 91, p. 231.
Oury, F.X., Leenhardt, F., Remesy, C., Chanliaud, E., Duperrier, B., Balfourier, F., and Charmet, G., Genetic variability and stability of grain magnesium, zinc and iron concentrations in bread wheat, Eur. J. Agron., 2006, vol. 25, p. 177.
Liu, Z., Wang, H., Wang, X., Zhang, G., Chen, P., and Liu, D., Genotypic and spike positional difference in grain phytase activity, phytate, inorganic phosphorus, iron, and zinc contents in wheat, J. Cereal Sci., 2006, vol. 44, p. 212.
Evans, I. and Solberg, E., Minerals for Plants, Animals and Man. Agri-Facts (Agdex 531-3), Alberta: Alberta Agric. Food Rural Dev., 1998.
Cakmak, I., Sari, N., Marschner, H., Kalayci, M., Yilmaz, A., Eker, S., and Gülüt, K.Y., Dry matter production and distribution of zinc in bread and durum wheat genotypes differing in zinc efficiency, Plant Soil, 1996, vol. 180, p. 173.
Weih, M., Hamnér, K., and Pourazari, F., Analyzing plant nutrient uptake and utilization efficiencies: comparison between crops and approaches, Plant Soil, 2018, vol. 430, p. 7.
Sarić, M.R., Theoretical and practical approaches to the genetic specificity of mineral nutrition of plants, Proc. First Int. Symp. on Genetic Aspects of Plant Nutrition, Serbian Academy of Sciences and Arts, Belgrade, August 30–September 4, 1982, Dordrecht: Springer-Verlag, 1983, p. 1.
White, P.J. and Broadley, M.R., Physiological limits to zinc biofortification of edible crops, Front. Plant Sci., 2011, vol. 2, p. 80.
Koleli, N., Eker, S., and Cakmak, I., Effect of zinc fertilization on cadmium toxicity in durum and bread wheat grown in zinc-deficient soil, Environ. Pollut., 2004, vol. 131, p. 453.
Aravind, P. and Prasad, M., Zinc protects chloroplasts and associated photochemical functions in cadmium exposed Ceratophyllum demersum L., a freshwater macrophyte, Plant Sci., 2004, vol. 166, p.1321.
Hu H. and Sparks, D., Zinc deficiency inhibits chlorophyll synthesis and gas exchange in stuart pecan, HortScience, 1991, vol. 26, p. 267.
Idris, E.E., Iglesias, D.J., Talon, M., and Borriss, R., Tryptophan-dependent production of indole-3-acetic acid (IAA) affects level of plant growth promotion by Bacillus amyloliquefaciens FZB42, Mol. Plant Microbe Interact., 2007, vol. 20, p. 619.
ACKNOWLEDGMENTS
We sincerely thank Prof. Agha Fakhr Mirlohi from Isfahan University of Technology for reading this manuscript and advice for improving it before submission.
Funding
This research was granted by Tarbiat Modares University and National Institute of Genetic Engineering and Biotechnology [grant number 451M from Wheat Missiondriven Project] of Iran.
Author information
Authors and Affiliations
Contributions
Conceptualization: Fathi, S.; Sabet, M.S.; Lohrasebi, T. Data acquisition: Fathi, S.; Sabet, M.S. Data analysis: Fathi, S.; Sabet, M.S. Design of methodology: Fathi, S.; Sabet, M.S.; Lohrasebi, T. Writing and editing: Fathi, S.; Sabet, M.S.; Lohrasebi, T.
Corresponding author
Ethics declarations
Conflict of interests. The authors declare that they have no conflicts of interest.
Statement on the welfare of humans or animals. This article does not contain any studies involving animals performed by any of the authors.
Rights and permissions
About this article
Cite this article
Fathi, S., Sabet, M.S. & Lohrasebi, T. Distribution of Zinc in Bread Wheat Genotypes with High and Low Zn-content. Russ J Plant Physiol 68 (Suppl 1), S51–S60 (2021). https://doi.org/10.1134/S1021443721070037
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1021443721070037