Abstract
The effects of water deficiency (–0.3 MPa) on plant growth, the water and proline content, fluorescence parameters chlorophyll PSI and II, and CO2/H2O gas exchange in plant leaves were studied in two populations of xero-halophyte Atriplex tatarica L. (C4 NAD-ME) with contrasting productivity. Based on growth parameter analysis, a less-productive population (P1) was more tolerant of osmotic stress, and a more-productive population (P2) was less tolerant. The studied populations demonstrated different ways of maintaining the water balance in leaves. P1 was characterised by an insensitivity of its stomatal apparatus, a significant decrease in water potential of mesophyll cells’ apoplast in substomatal cavity, an increase in proline content, and activation of PSI cyclic electron transport in leaves. In P2, maintaining the water content in leaves under stress conditions was achieved by stomatal closure. The impact of stress was manifested by decreased intensity of photosynthesis, transpiration, PSII efficiency and more intensified of dark respiration in P2. Thus, various ways by which to maintain the water balance in plant leaves in two populations were revealed under weak osmotic stress.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
INTRODUCTION
In contrast to C3 plants, the response of C4 plants to drought has been less well studied in spite of the significant contribution of C4 species to the global carbon budget and food production [1]. C4 plants have a photosynthetic apparatus effective in warm and dry habitats. The C4 species’ high water use efficiency (WUE) led to the general consensus that plants with C4 photosynthesis are drought tolerant [2]; however, some studies have provided more current information on the low drought tolerance of some C4 species [3, 4]. Sage and McKown [5] suggested that some unique functions of C4 photosynthesis, and a more complex biochemical pathway, can reduce the potential for general phenotypic plasticity and the acclimatisation of photosynthesis to environmental changes. Ghannoum [1] suggested that there is a limited capacity for photorespiration or the Mehler reaction to act as significant alternative electron sinks under water stress in C4 photosynthesis. According to Osborne and Sack [4], weak coordination between the carbon-concentrating C4 mechanism and hydraulic functions might lead to low drought tolerance in plants. Most likely, all these factors contribute to significant limitations on C4 plants’ worldwide ecological and geographical distribution.
C4 photosynthesis evolved under low atmospheric CO2 concentrations [6] and seasonally water deficit [4]. According to Osborne and Sack [4], this was accompanied by the plants’ simultaneous improvement in carbon and water metabolism. The lack of coordination between carbon-concentrating mechanism and water loss often leads to suboptimal water-use efficiency (WUE) and photosynthetic losses [7, 8]. The question of changing atmospheric CO2’s influence on the coordination between photosynthetic capacity and hydraulic functions remains unanswered [9]. Further research on the coordination efficiency between hydraulic conductivity and photosynthesis, in leaves and in the entire plant, is important for studying and predicting the physiological effects and performance of C4 photosynthesis [6].
Increasing WUE may be an important step in C4 evolution [4]. At ambient atmospheric CO2, C4 grasses have advantages over C3 grasses because of lower stomatal conductance and low stomatal sensitivity, which results in a WUE advantage [6]. At low atmospheric CO2, the difference in stomata sensitivity to water deficit sharply increases [4]. Taylor et al. [6] have suggested that, in addition to the biochemical advantages supporting higher visible photosynthesis with low stomatal conductivity, an important advantage for C4 grasses is protecting hydraulic functions. Osborne and Sack [4] hypothesised that the role of hydraulic conductance in leaves during the adaptation to drought is greater than stomatal conductance in C4 plants. In the same time there are different points of view [10], thus these suggestions must be researched further.
Atriplex species are widely distributed and of great interest for use in feed production and restoring marginal lands [11]. We conducted a comparative study of the drought and salt tolerance of C3 and C4 xero-halophytes of genus Atriplex. The C4 species A. tatarica significantly accumulated proline and showed a weaker tolerance to PEG-induced osmotic stress (–0.6 МPа) compared with the C3 species [12]. In a previous study it was found some populations of A. tatarica with contrasting productivity in habitats of the South Urals [13]. The aim of this study was to compare the tolerance to weak osmotic stress (–0.3 MPa) of two C4 xero-halophyte A. tatarica populations differing in productivity in their natural habitats.
MATERIALS AND METHODS
Plant material and growth conditions. Seeds of Atriplex tatarica L. (C4-NAD) were collected in two different populations (P1 and P2) in the South Urals. Physiological studies were carried out on seedlings in laboratory. Seeds were germinated in distilled water. Three day old seedlings were transplanted to perlite in plastics pots of 24 cm length, 20 cm width and 10 cm depth. The seedlings were grown under circadian illumination (using commercial luminescent white light tubes): 10-h dark/14-h light [200 µmol/m2 s PAR, light meter LI-205A (Li-Cor, USA)] and 25 ± 5°C temperature. Plants of 30 days old were transferred to the experimental solutions (irrigated perlite). Since the purpose of this study was to investigate differences in the adaptation mechanisms in two A. tatarica populations to osmotic stress, rather than shock [14], we used weak osmotic stress (–0.3 MPa), which is not a shock for this xero-halophyte. Solute of 12.5% (m/v) PEG 6000 (–0.3 MPa) were used as the experimental treatment. Osmotic potential of the experimental solution was measured using a freezing-point osmometer Osmomat 030 (Gonotec, Germany). For treatment, PEG solution was added to a plastic tray, and the plastic pots were placed on it. The nutrient solutions 50% Hoagland was used for the control. The treatment lasted 4 days. Plant tolerance to osmotic stress was assessed by the decrease in its productivity compared with the control.
Electrophoresis procedures. Population genetic analyses was done on embryos using starch gel electrophoresis of isozymes. On the basis of starch gel electrophoresis of isozymes from randomly chosen embryos variability of the following enzymatic systems was studied: glutamate oxaloacetate transaminase (GOT (AAT), E.C. 2.6.1.1), diaphorase (DIA, E.C. 1.6.99), glutamate dehydrogenase (GDH, E.C. 1.4.1.2), superoxide dismutase (SOD, E.C. 1.15.1.1), glucose-6-phosphate dehydrogenase (G6PD, E.C. 1.1.1.49), 6-phosphogluconate dehydrogenase (6PGD, E.C. 1.1.1.44), malate dehydrogenase (MDH, Е.С. 1.1.1.37), and malic enzyme (Me, Е.С. 1.1.1.40). The seeds were cleaned of their wings and soaked in water for 12 h, and homogenized in 80 μL of Tris-HCl buffer with KCl, MgCl2, EDTA, Triton X-100, and PVP. Enzymes were separated in 10% starch gel using two buffer systems [15]. Staining of particular enzymes and genetic interpretation of the results followed to Soltis and Soltis [16]. Nei’s [17] genetic distances (D) were estimated between populations to generate average clustering using the UPGMA methods (modified from NEIGHBOR procedure of PHYLIP v. 3.5) [18].
Water content. At the end of the experiment, water content (g/g DW) was assessed for the shoots of plants. Biomass was estimated for dry shoots (DW). Plant samples were dried at 80°C for 2 days until reaching a constant mass in order to measure quantitatively the dry shoot matter. The water content in the shoots for each treatment and control group was calculated as WC = (FW – DW)/DW.
Proline content. Free proline was determined according to [19]. Dry shoot samples (0.2 g) from each group were homogenized in 2 mL of boiling distilled water, heated at 100°C for 10 min in a water bath and then the homogenates were centrifuged. The mixtures were heated at 100°C for 1 h in a water bath after adding acid ninhydrin and glacial acetic acid. Reaction was stopped by submerging the sample into an ice bath. The mixtures were read at 520 nm using a Genesis 10 UV Scanning spectrophotometer (Thermo Scientific, United States). Proline concentrations were determined using a calibration curve and expressed as mg/g DW.
Cyclic electron transport around photosystem I (PSI). The redox potential changes of chlorophyll P700 were measured by monitoring the leaf absorbance at 820 nm using a dual-wavelength ED-P700DW pulse modulated system (Walz, Germany) in combination with a PAM 101 fluorometer (Walz) [22]. The kinetics of P700 oxidation was measured under far-red light (720 nm, 17.2 W/m2). The maximum oxidation of P700 was determined using a xenon discharge lamp (50 ms, 1500 W/m2; Walz) in the presence of far-red light. PSI cyclic electron transport activity was measured as the P700 oxidation kinetics in response to far-red illumination by monitoring changes in leaf absorbance at 820 nm [23].
Photosystem II (PSII). The quantum yield of PSII photoreaction in dark adapted (20 min) leaf was determined with a pulse-amplitude-modulated chlorophyll fluorometer (PAM 101, Walz) [24]. The ratio of variable to maximum chlorophyll a fluorescence (Fv/Fm) was used as a measure of the maximum quantum yield of PSII reaction. During measurements, the sample was illuminated with weak modulated red light. The output signal of PAM 101 was processed with an analog-digital convertor (PDA-100, Walz) and displayed on a computer. The potential photosynthetic efficiency of dark-adapted leaves was estimated from the values of minimal (F0) and maximal (Fm) fluorescence using an expression: Fv/Fm= (Fm – F0)/Fm.
CO2/H2O gas exchange. The CO2/H2O gas exchange was analyzed at two CO2 concentrations: ambient CO2 (400 ppm) and low CO2 (200 ppm). Low CO2 concentration allows to artificially control the opening of stomata during an experiment [7], to assess the sensitivity of the stomatal apparatus to external influences [4, 6], and to differentiate the stomatal and metabolic contributions to drought tolerance. The CO2/H2O exchange was analyzed by placing a leaf segment into a temperature controlled leaf chamber where the sample was illuminated through a fiber-optic light guide from a KL 1500LCD light source (Schott, Germany). A halogen lamp (150 W, Philips, Netherlands) installed in this illuminator provided PAR intensity of 2000 μmol/m2 s). The steady-state CO2/H2O exchange rates at the leaf–air interface were measured with a single-channel LI-820 infrared gas analyzer (LI-COR, United States) in the open-circuit mode. Apparent photosynthesis (A) expressed as µmol (CO2)/m2 s. The leaf transpiration (E, mmol (H2O)/m2 s) was calculated from the difference in gas humidity at the inlet and outlet from the leaf chamber. In this experimental system, the humidity of gas flow at the entrance to the leaf chamber was kept constant at a known level using a LI-610 dew point generator (LI-COR). Humidity at the exit of the leaf chamber was determined with a HMP50 psychrometric sensor (Vaisala INTERCAP, Finland). Water use efficiency (WUE) was calculated as the ratio of apparent photosynthetic assimilation to the transpiration rate (A/E). After CO2/H2O gas exchange measuring the light was turned off and after steady state the dark respiration (Rd, µmol (CO2)/m2 s) was measured.
Water potential of mesophyll cells’ apoplast in substomatal cavity (Ψwa). Ψwa was determined in attached leaf by means of a new method using the instruments to assess photosynthetic CO2/H2O gas exchange (a single-channel infrared gas analyzer LI-820 (LI-COR) [20, 21]. The method is based on the determination of relative humidity (RH) above the leaf surface that reduces E to zero. This value is equal to RH in the substomatal cavity. Determination of RH values makes it possible to calculate Ψwa at the interface between aqueous and gaseous phases of mesophyll cells’ apoplast in substomatal cavity. RH at the air stream entering the leaf chamber was determined using a psychrometric sensor HMP50 (Vaisala INTERCAP, Finland). Equilibrium pressure of vapor over the surface of an aqueous solution is related to the chemical potential of water by the following equation: е = eo exp[ΨwV/(RT)], where e is equilibrium pressure of vapor over aqueous solution, eo is pressure of saturated vapor over the surface of pure water (Ψw = 0) at absolute temperature T, R is absolute gas constant of 8.31441 J/mol K, T is absolute temperature (K), and V is molar volume of water (18 cm3/mol). Therefore, Ψw = (RT/V)ln(e/eo). Water potential is expressed in J/m3 or Pa. By definition, relative humidity is described with the formula: RH [%] = (e/eo) × 100, where eo is pressure of saturated vapor [Pa] and e is real pressure of vapor [Pa] at temperature t [°C]. The value of Ψwa was expressed in MPa. It takes <1 h to measure leaf Ψwa [21].
Statistical analysis. The physiological measurements were performed 5–6 times for each treatment and control group, and the means and standard errors (SEs) are calculated using Sigma Plot 12.0 statistical program. Comparisons of parameters were made between treatments using analysis of variance (ANOVA) with the Tukey test. Differences were considered significant at P < 0.05. The principal component analysis (PCA) was accomplished with the R version 3.6.1.
RESULTS
Genetic Distance and Growth Parameters
Population genetic analysis based on eight enzymes (13 loci) showed that A. tatarica populations P1 and P2 are genetically different with a genetic distance (D) 0.0545. At experiment there were differences in dry biomass accumulation by control plants of P1 and P2 populations: P2 plants were 2-fold more productive than P1 plants (Table 1). Under weak osmotic stress, the dry weight of P1 plants decreased by 25%, and P2 plants decreased by 45%.
Water Exchange and Proline Content
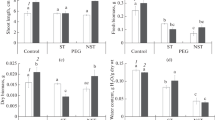
The water potential of mesophyll cells’ apoplast in substomatal cavity (Ψwa) in P1 and P2 plants were not different under control conditions. Under osmotic stress, Ψwa in P1 plants was more labile and decreased 1.3-fold when compared with that in the control (Fig. 1a). There were no differences in water content in the studied plants from different populations (Fig. 1b). There was significant increase in proline accumulation in the P1 plants under osmotic stress (Table 1).
Water potential of mesophyll cells’ apoplast in substomatal cavity (a) and water content (b) in plant shoots of populations P1 (1) and P2 (2) of xero-halophyte Atriplex tatarica under weak osmotic stress (–0.3 MPa). The values are means (±SE). Different letters above the bars represent significant differences at the P < 0.05 (Tukey’s pairwise comparison).
Efficiency of Photosystems I and II
PSII function analyses showed that the maximum quantum yield (Fv/Fm) was the same (0.80–0.82) in both P1 and P2 populations under control conditions; however, under osmotic stress, Fv/Fm decreased to 0.76 in P2 plants (Table 1).
PSI cyclic electron transport activity was investigated in both populations. We measured the P700 oxidation kinetics in response to far-red illumination by monitoring changes in leaf absorbance at 820 nm (Table 1). Plants were dark adapted for 2 h, and then illuminated with far-red light. In both populations, it took 20–25 s to reach the maximum oxidation level under far-red light under control. Under osmotic stress, the time required to reach the maximum P700 oxidation in P1 plants increased 1.6-fold over that of the control (Table 1).
CO2 /H2O Gas Exchange
P1 and P2 plants exhibited differences in CO2/H2O gas exchange at an ambient atmospheric concentration of CO2 (400 ppm). The intensities of apparent photosynthesis (A) and transpiration (E) were 1.4-fold and 1.6-fold higher, respectively, in P2 plants than those in P1 plants under control conditions (Figs. 2a, 2b). Under osmotic stress, P2 plants had a 1.3-fold decrease in A, a 1.7-fold decrease in E and a 2-fold increase in dark respiration (Rd) (Fig. 2c). CO2/H2O gas exchange in both plant populations was also measured at low CO2 (200 ppm): intensity of A decreased in both populations (compared with 400 ppm) and E increased in P2, which indicated that P2 plants’ stomata were highly sensitive (Figs. 3a, 3b compared with Fig. 2a). Differences in WUE between two populations were also observed. At ambient CO2 WUE values were higher in P1 plants than those in P2 plants (Fig. 2d), and at low CO2, these differences increased (Fig. 3c).
Apparent photosynthesis (a), transpiration (b), dark respiration (c) and water use efficiency (d) in plant leaves of populations P1 (1) and P2 (2) of xero-halophyte A. tatarica under weak osmotic stress (–0.3 MPa) at ambient atmospheric CO2 (400ppm). The values are means (±SE). Different letters above the bars represent significant differences at the P < 0.05 (Tukey’s pairwise comparison).
Apparent photosynthesis (a), transpiration (b) and water use efficiency (c) in plant leaves of populations P1 (1) and P2 (2) of xero-halophyte A. tatarica under weak osmotic stress (–0.3 MPa). Measurements of CO2/H2O gas exchange were carried out at low CO2 (200 ppm). The values are means (±SE). Different letters above the bars represent significant differences at the P < 0.05 (Tukey’s pairwise comparison).
DISCUSSION
Two different populations of C4 xero-halophyte A. tatarica, with differences in their productivity in natural habitats [13], were chosen for studying their tolerance to weak PEG-induced osmotic stress. Population genetic analysis confirmed that the plants studied were from two different populations (D = 0.0545) of A. tatarica. The growth parameter differences between populations were also obtained in the laboratory experiment (Table 1). Under weak PEG-induced water deficiency the studied plants were stressed, as evidenced by decrease in growth parameters and increase in respiration intensity in both populations (Table 1, Fig. 2c). Plant tolerance to osmotic stress was assessed by degree of growth reduction under osmotic stress compared with those in the control variant. Based on this, the less productive (under control conditions) P1 population was more tolerant to osmotic stress, and the more productive (control conditions) P2 population was less tolerant. At the same time, under osmotic stress, the water content in both populations’ leaves remained at the same level as in the control variants (Fig. 1b). We can suggest the presence of two different mechanisms to maintain leaf water balance in these two populations. Increased transpiration due to highly sensitive and open stomata in response to low CO2 (200 ppm), which is more typical for C3 species [7], was observed only in P2 (Fig. 3b) and led to a significant decrease in WUE (up to 3-fold) (Figs. 2d, 3c). This suggests that the studied populations differ greatly in stomatal sensitivity and regulation of water metabolism. Under weak osmotic stress, the more tolerant P1 population was characterised by stomatal insensitivity, as indicated by their constant values of transpiration intensity under ambient CO2 (400 ppm) (Fig. 2b) and low CO2 (200 ppm) (Fig. 3b). Further, in P1 plants there was significant decrease in the water potential of mesophyll cells’ apoplast in substomatal cavity and increase in the leaves’ proline content (Fig. 1a, Table 1). In A. halimus populations it was revealed that proline was the main osmolyte, which was synthesised during the first 24 h under osmotic stress [25]. In the P2 population, Ψwa was retained (Fig. 1a) by closing the stomata, and this was accompanied by decreasing intensities of A and E under weak osmotic stress (Figs. 2a, 2b). As result, the growth parameters decreased two-fold over those in the control (Table 1). Wherein P2 plants WUE increased due to more significant decrease in transpiration than that in apparent photosynthesis (Fig. 2d). This indicates the weakening coordination between СО2 and Н2О exchange. Under weak osmotic stress, the populations differed in Rd values also. In P2, there was a two-fold increase in Rd (Fig. 2c) accompanied by a decrease in growth. It suggested an increase in the maintenance respiration associated with energy dissipation, a factor that is especially important during limited photorespiration activity in C4 plants [1].
In C4 plants, both C3 and C4 cycles are functional, thereby increasing the energetic cost of CO2 assimilating compared with C3 plants. As a consequence, two additional ATP molecules are needed for each CO2 fixed to drive the C4 cycle. These additional ATP has been suggested to be produced by PSI cyclic electron transport, which contributes to the generation of a pH gradient across the thylakoid membrane without the net production of NADPH [23, 26]. Comparative analysis within genus Flaveria, which includes C3, intermediate C3–C4 and C4 species, showed that C4 plants have higher expression levels of proteins that are involved in PSI cyclic electron transport and changes in thylakoid structure contribute to the high activity of cyclic electron flow C4 photosynthesis [23]. In P1 A. tatarica plants, PSI cyclic electron transport was activated under weak osmotic stress (Table 1).
Principal component analysis (PCA) of physiological and biochemical parameters of two A. tatarica populations confirmed a significant difference between populations under control conditions (Fig. 4a). Under osmotic stress, the parameters of two populations overlapped. The greatest changes in parameters under stress (compared with control) were observed in less tolerant P2 population. First principal component (PC1) represented 37% of the pattern variation, PC2—25%. The main parameters of population clustering were physiological parameters determining CO2/H2O exchange (transpiration, apparent photosynthesis, water use efficiency, dark respiration) and PSII (Table 2). For identification the processes that contribute more to adaptation, and demonstrate the differences in plants’ responses to drought, PCA of each studied populations A. tatarica (under control and stress conditions) was carried out. P1 plants were clearly divided into two groups – those growing under control conditions and those growing under weak osmotic stress (Fig. 4b). These plants were clearly distinguished by PC1, which represented 50% of the total variation (Fig. 4b). PSI cyclic electron transport was the main element that contributed to PC1, as well as proline content and Ψwa. These parameters had the highest values of factor loading (Table 2). WUE, water content and apparent photosynthesis in leaves of P1 plants were major contributors to PC2. First two principal components (PC1 and PC2) were enough to explain 81% of the pattern variation. P2 plants are clearly distinguished into two groups—control and weak osmotic stress—by PC1, representing 64% of the total variation (Fig. 4c), and the main elements, which contributed to PC1 were Rd, WUE and PSII (Table 2). The water content, Ψwa and transpiration in leaves were major contributors to PC2. First two principal components were sufficient to explain 79% of the pattern variation. According to PCA, in P1 plants the proline content and water potential regulation were largely contributed in adaptation to weak osmotic stress. In P2 plants the dark respiration (dissipation) and WUE played significant roles in adaptation to weak osmotic stress. Moreover, PCA showed that increase in PSI cyclic electron transport was the significant factor for P1 plants, as decrease in PSII was the significant factor for P2 plants under weak osmotic stress (Fig. 4, Table 2).
Principal component analysis (PCA) score plot results comparing parameters of A. tatarica from P1 and P2 populations: (a) both P1 and P2, (b) P1 and (c) P2 to identify processes that contribute more to adaptation to weak osmotic stress (–0.3 MPa). Light squares—P1 plants under control condition; dark squares—P1 plants under osmotic stress; light triangles—P2 plants under control condition; dark triangle—P2 plants under osmotic stress.
The study of two populations of C4 xero-halophyte A. tatarica with different productivity and tolerance to weak osmotic stress, allowed to identify various ways of maintaining water balance in the plant leaves. In more tolerant P1 population, a decrease in the water potential of mesophyll cells’ apoplast in substomatal cavity with participation by proline and activation of cyclic electron transport around PSI, were the main factors in maintaining leaf water balance and constant transpiration under osmotic stress. In less tolerant P2 population, stomata sensitivity resulted in their closure to maintain leaf water balance under osmotic stress. Probably the stomata closure contributed to weakening coordination between the carbon-concentrating C4 mechanism and hydraulic functions and led to low drought tolerance and decrease in plant productivity.
REFERENCES
Ghannoum, O., C4 photosynthesis and water stress, Ann. Bot., 2009, vol. 103, p. 635. https://doi.org/10.1093/aob/mcn093
Haxeltine, A. and Prentice, I.C., BIOME3: An equilibrium terrestrial biosphere model based on ecophysiological constraints, resource availability, and competition among plant functional types, Global Biogeochem. Cycle, 1996, vol. 10, p. 693. https://doi.org/10.1029/96GB02344
Taylor, S.H., Ripley, B.S., Woodward, F.I., and Osborne, C.P., Drought limitation of photosynthesis differs between C3 and C4 grass species in a comparative experiment, Plant Cell Environ., 2011, vol. 34, p. 65. https://doi.org/10.1111/j.1365-3040.2010.02226.x
Osborne, C.P. and Sack, L., Evolution of C4 plants: a new hypothesis for an interaction of CO2 and water relations mediated by plant hydraulics, Philos. Trans. R. Soc., B, 2012, vol. 367, p. 583. https://doi.org/10.1098/rstb.2011.0261
Sage, R.F. and McKown, A.D., Is C4 photosynthesis less phenotypically plastic than C3 photosynthesis? J. Exp. Bot., 2006, vol. 57, p. 303. https://doi.org/10.1093/jxb/erj040
Taylor, S.H., Aspinwall, M.J., Blackman, C.J., Choat, B., Tissue, D.T., and Ghannoum, O., CO2 availability influences hydraulic function of C3 and C4 grass leaves. J. Exp. Bot., 2018, vol. 69, p. 2731. https://doi.org/10.1093/jxb/ery095
Lawson, T. and Blatt, M.R., Stomatal size, speed, and responsiveness impact on photosynthesis and water use efficiency, Plant Physiol., 2014, vol. 164, p. 1556. https://doi.org/10.1104/pp.114.237107
Bellasio, C., Quirk, J., Buckley, T.N., and Beerling, D.J., A dynamic hydro-mechanical and biochemical model of stomatal conductance for C4 photosynthesis, Plant Physiol., 2017, vol. 175, p. 104. https://doi.org/10.1104/pp.17.00666
Domec, J.C., Smith, D.D., and McCulloh, K.A., A synthesis of the effects of atmospheric carbon dioxide enrichment on plant hydraulics: implications for whole-plant water use efficiency and resistance to drought, Plant Cell Environ., 2017, vol. 40, p. 921. https://doi.org/10.1111/pce.12843
Kocacinar, F., McKown, A.D., Sage, T.L., and Sage, R.F., Photosynthetic pathway influences xylem structure and function in Flaveria (Asteraceae), Plant Cell Environ., 2008, vol. 31, p. 1363. https://doi.org/10.1111/j.1365-3040.2008.01847.x
Le Houérou, H.N., The role of saltbushes (Atriplex spp.) in arid land rehabilitation in the Mediterranean Basin: a review, Agrofor. Syst., 1992, vol. 18, p. 107. https://doi.org/10.1007/BF00115408
Rakhmankulova, Z.F., Shuyskaya, E.V., Voronin, P.Yu., and Usmanov, I.Yu., Comparative study on resistance of C3 and C4 xero-halophytes of the genus Atriplex to water deficit and salinity, Russ. J. Plant Physiol., 2019, vol. 66, p. 250. https://doi.org/10.1134/S1021443719020109
Rakhmankulova, Z.F., Shuyskaya, E.V., Shcherbakov, A.V., Fedyaev, V.V., Biktimerova, G.Ya., Khafisova, R.R., and Usmanov, I.Yu., Content of proline and flavonoids in the shoots of halophytes inhabiting the South Urals, Russ. J. Plant Physiol., 2015, vol. 62, p. 71. https://doi.org/10.1134/S1021443715010112
Shavrukov, Y., Salt stress or salt shock: which genes are we studying? J. Exp. Bot., 2013, vol. 64, p. 119. https://doi.org/10.1093/jxb/ers316
Muona, O. and Szmidt, A., A multilocus study of natural populations of Pinus sylvestris, Population Genetics in Forestry, Lecture Notes Biomath. Ser. vol. 60, Berlin: Springer-Verlag, 1985, p. 226.
Isozymes in Plant Biology, Soltis, D.E. and Solis, P.S., Eds., Portland, OR: Dioscorides, 1989.
Nei, M., Molecular Evolutionary Genetics, New York: Columbia Univ. Press, 1987.
Yeh, F.C., Yang, R.C., and Boyle, T. PopGene, Version 1.32. Microsoft Windows-Based Freeware for Population Genetic Analysis, Edmonton: Univ. of Alberta, 1999.
Bates, S., Waldren, R.P., and Tear, I.D., Rapid determination of free proline for water stress studies, Plant Soil, 1973, vol. 39, p. 205.
Voronin, P.Yu., Rakhmankulova, Z.F., Shuyskaya, E.V., Maevskaya, S.N., Nikolaeva, M.K., Maksimov, A.P., Maximov, T.Chr., Myasoedov, N.A., Balnokin, Yu.V., Rymar, V.P., Valdayskih, V.V., and Kuznetsov, Vl.V., New method for quantitative determination of water potential of mesophyll cell’ apoplast in substomatal cavity of the leaf, Russ. J. Plant Physiol., 2017, vol. 64, p. 452. https://doi.org/10.1134/S1021443717020133
Voronin, P.Yu., Maevskaya, S.N., and Nikolaeva, M.K., Physiological and molecular responses of maize (Zea mays L.) plants to drought and rehydration, Photosynthetica, 2019, vol. 57, p. 850. https://doi.org/10.32615/ps.2019.101
Klughammer, C. and Schreiber, U., Measuring P700 absorbance changes in the near infrared spectral region with a dual wavelength pulse modulation system, in Photosynthesis: Mechanisms and Effects, Garab G., Ed., Dordrecht: Springer-Verlag, 1998, p. 4357.
Nakamura, N., Iwano, M., Havaux, M., Yokota, A., and Munekage, Y.N., Promotion of cyclic electron transport around photosystem I during the evolution of NADP-malic enzyme-type C4 photosynthesis in the genus Flaveria, New Phytol., 2013, vol. 199, p. 832. https://doi.org/10.1111/nph.12296
Schreiber, U., Chlorophyll Fluorescence and Photosynthetic Energy Conversion: Simple Introductory Experiments with the TEACHING-PAM Chlorophyll Fluorometer, Effeltrich: Heinz Walz, 1997.
Ben Hassine, A., Bouzid, S., and Lutts, S., Does habitat of Atriplex halimus L. affect plant strategy for osmotic adjustment? Acta Physiol. Plant, 2010, vol. 32, p. 325. https://doi.org/10.1007/s11738-009-0410-4
Kanai, R. and Edwards, G.E., The biochemistry of C4 photosynthesis, in C 4 Plant Biology, Sage, R.F. and Monson, R.K., Eds., San Diego: Academic, 1999, p. 215.
Funding
This work was supported by Ministry of Science and Higher Education of the Russian Federation (theme FFES-2021-0010).
Author information
Authors and Affiliations
Contributions
Z.F. Rakhmankulova, E.V. Shuyskaya contributed equally to this work.
Corresponding authors
Ethics declarations
Conflict of interests. The authors declare that they have no conflicts of interest.
Statement on the welfare of humans or animals. This article does not contain any studies involving animals performed by any of the authors.
Rights and permissions
About this article
Cite this article
Rakhmankulova, Z.F., Shuyskaya, E.V. & Voronin, P.Y. Different Ways of Maintaining Water Balance in Leaves in Two Populations of C4 Atriplex tatarica Differing in Productivity and Drought Tolerance. Russ J Plant Physiol 68, 1143–1151 (2021). https://doi.org/10.1134/S1021443721060170
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1021443721060170