Abstract
Azospirillum brasilense may regulate MN052803-LTP expression and the activity of defensive enzymes in wheat (Triticum aestivum L.) cultivars to improve salinity tolerance. In a primary experiment, germination indexes of 18 wheat cultivars were measured and Sorkhtokhm and Qods were selected as tolerant and sensitive cultivars to salinity, respectively. Selected cultivars inoculated with A. brasilense (Sp245 produce more ABA and Sp7 as standard strain) and grown-up to five days, then salinity (200 mM NaCl) was applied to seedling via Hoagland’s nutrient solution. The relative expression of MN052803-LTP (authors recorded in the Gen Bank) of roots and shoots was measured at 12, 24, and 48 hours after salinity applied. The results showed that MN052803-LTP expression increased in the order of salinity, inoculation, and inoculation plus salinity. Meanwhile, phenylalanine ammonia-lyase (PAL) and tyrosine ammonia-lyase (TAL) activity increased in the same order in 12 days-old seedlings. In a similar experiment, 10 mM dithiothreitol (DTT) was used as a reducer and inoculation as a stimulator of MN052803-LTP expression, then the relative expression and phosphatidylcholines (PC) content were measured. Although the MN052803-LTP expression and PC was reduced due to the application of DTT, inoculation eliminates its inhibitory effect. The highest amount of PC was observed in inoculated plants, and the lowest in the plants treated with DTT. Probably, A. brasilense improves salt tolerance of wheat cultivars through MN052803-LTP expression, and PC content via repairing the membrane damages by supplying the membrane phospholipids, such as phosphatidylcholine, and accumulation of antioxidant compounds by activating PAL and TAL via membrane lipid-dependent signaling cascades.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Unwanted salt in the rhizosphere decreases the plant’s water uptake and consequently with the usual leaf transpiration result in a reduction in the plant’s water content. Following dehydration, cells shrink and lose their turgor pressure leading to changes in morphology, physiology, and gene expression of plants. The consequence of the adverse effect of salinity is a reduction of quality and quantity of plant production. One alternative strategy for improving the tolerance of a plant to stress is using the beneficial microorganisms present in the rhizosphere. Azospirillum brasilense is one of the plant growth-promoting rhizobacteria (PGPRs) that can associate with wheat and increase crop quality and quantity through suitable physiological and biochemical modification in plants [1].
Accumulation of antioxidant compounds such as flavonoids and other phenolic compounds is one of the general responses to abiotic stresses including salinity, which induce oxidative stress due to the formation of ROS and free radicals [2]. The activation of PAL and TAL enzymes stimulates the biosynthesis of the phenolic compounds of the phenylpropanoid pathway that have strong ROS scavenging activity and assist plants to overcome oxidative stress [2]. Besides, phenolic compounds are important in plant defensive mechanisms and play a crucial role in the interactions of plants with microbes [2]. PAL also plays an important role in the synthesis of chemical signals such as phenols, phytoalexins, and lignin that they decrease cell wall expansion and cell extensibility as well as limit water loss and prevent cell collapse due to dehydration. The activation of the plant defense system against bacteria as a physiological response of a host plant is so important. In this regard, the utilization of plant defense systems (PAL and TAL enzymes) would facilitate or limit the anchoring process of bacteria on the root surface. Benizri et al. [3] reported that the anchoring procedure in the inoculation condition is an important step in a successful association between microorganisms and plant cells.
One of the major inducible plant defense responses is the accumulation of plant defense proteins. In this regard, plant lipid transfer protein (LTPs), previously thought to be involved only in the transfer of a broad range of lipids between membranes [4], while they also implicate in the plant defense system. The defensive role of the plant LTPs was found because of the response of LTP genes expression to biotic and abiotic stresses [5]. Overexpression of LTP genes in different plants indicated that they significantly enhance tolerance of rice (OsDIL) and pepper (CALTP1) to drought as well as Arabidopsis (AZI1), Tamarix hispida (ThLTP), Nicotiana tabacum (NtLTP4), and rice (Os11g24070, Os04g33920 and Os05g06780) to salinity. Interestingly, LTP in N. tabacum regulating transcription levels of NHX1 and HKT1 to alleviate the toxicity of salinity stress [6].
Kader [4] reported that LTP has a main role in the transport of phospholipids (that are a major component of the plasma membrane, PM) between membranes within the cell. They maintain cell function and mediating responses to stress during plant growth and development. Furthermore, the LTP could activate the plant phenylpropanoid pathway genes [7] that they defend against free radicals and may also provide tolerance to a wide array of stresses. Not only LTPs are involved in stress conditions, but they were also transiently expressed during the inoculation process [4]. In Chinese milk vetch (Astragalus sinicus), LTPAsE246 has been shown to participate in the transport of plant lipids to symbiosome membranes and nodule organogenesis associated with infection thread formation [8]. In the inoculation of Oryza sativa roots with mycorrhizal, the expression of LTP and Pal was increased, these genes are involved in the plant response to the environment stress [7]. This simply means that PGPRs can affect the expression of stress-responsive genes and modulate plant responses to stress.
LTP transfer activity inactivated with increasing specific concentration of reduced-dithiothreitol [9] while this inactivation-transfer was accompanied by the change of the protein conformation. Dithiothreitol (DTT) reduces the expression of LTPs, via the change in the α-helix proportion of disulfide bridges [4]. Kader [4] reported that the α-helix proportion of disulfide bridges of LTPs decreased in wheat and maize from 40 to 25% under treatment with dithiothreitol (DTT) which confirms the effect of DTT on LTPs indirectly. In addition, the reduction of maize LTPs was absorbed by DTT, which prevents lipid transfer activity [9].
Azospirillum brasilense reduces the adverse effects of salinity of wheat cultivar [10]. Meanwhile, the effect of some PGPRs such as Rhizobium spp. on LTP expression and improvement of plant tolerance to abiotic stress has been reported [8]. Be noted, the effect of A. brasilense on the expression of MN052803-LTP (Authors recorded in the Gen Bank) of wheat (T. aestivum L.) cultivars under salinity condition and also the effect of dithiothreitol on MN052803-LTP is not yet reported. Therefore, the objective of this study was (a) to evaluate the effects of A. brasilense strains and/or salinity on PAL and TAL enzyme activities (two enzymes involved in the phenylpropanoid pathway), (b) to assay the effects of A. brasilense strains and/or salinity on the expression of MN052803-LTP of tolerant and sensitive wheat cultivars, and (c) to check the effects of A. brasilense strains as a stimulator and dithiothreitol as a reducer on the MN052803-LTP expression, and PC content under salinity condition.
MATERIALS AND METHODS
Plant and bacteria materials. Seeds of 18 common wheats (Triticum aestivum L.) cultivars (Bezostia, Hamun, Sivand, Kaveh, Sardary, Kaskogen, Azady, Gaspard, Karaj, Sorkhtokhm, Qods, Dez, Sepahan, Roshan, Zarin, Shoele, Bam, and Navid) were obtained from Institute of Agricultural and Research, Isfahan, Iran. Sterilized seeds were transferred into an autoclaved Petri dish containing 8 mL of saline water (0, 100, 150, and 200 mM NaCl) in each Petri dish. During 12 days, germination rate, seedling vigor, salinity tolerance index, and germination stress index were measured or calculated every day. On day 12th the average root length, shoot length, and the weight of roots and shoots were measured. According to the results of germination indexes, the concentration of 200 mM NaCl was the more effected concentration, and two cultivars of wheat named Sorkhtokhm and Qods were the most tolerant and sensitive cultivars to salinity, respectively. Therefore, according to the result of the preliminary experiment, two cultivars of Qods and Sorkhtokhm were selected as the sensitive and tolerant cultivars, respectively to salinity, and the concentration of 200 mM NaCl (as the salt stress condition), to perform the main experiments.
Bacteria culture. Two strains of Azospirillum brasilense including Sp7 (standard), and Sp245 (produce more ABA) were obtained from NCIMB Ltd, Germany. A. brasilense strains (Sp7 and Sp245) cultured in Nitrogen free basal (NFb) medium [11] supplemented with NH4Cl (0.25 g/L) at 30°C in Erlenmeyer flasks for 48 h and shaken in a rotary shaker at 0.56 g. The growth was harvested by centrifugation (1000 g, 10 min), washed with sterile saline phosphate buffer and then re-suspended in phosphate buffer at a concentration of 108 CFU/mL of A. brasilense [1].
Inoculation of wheat seedlings and induction of salt stress. Seeds of tolerant and sensitive wheat cultivars (Sorkhtokhm and Qods) were sterilized, then the sterilized seeds were incubated at room temperature for 3 h. Wheat seeds were shaken in high phosphate NFb liquid medium enriched with 0.1% ammonium chloride contain 108 CFU/mL of A. brasilense strains and was shaken at 0.28 g for 3 h (Shaker Model INFORS AG, BOTTMINGEN, Japan). The inoculated and none inoculated seedlings were transferred into the sterilized pots filled with perlite. The pots (with 6 seedlings) irrigated with 1/4 strength of Hoagland’s nutrient solution [12]. The pots kept at 25/18°C (day/night) and 16/8 h (light/dark) photoperiod using white light (photon density 650 μmol/(m2 s)) for 5 days. Then, Hoagland’s nutrient solution with two levels of salinity (0 and 200 mM) applied to pots as irrigated water. After 0, 12, 24, and 48 h of exposure of plants to salt stress, the roots and the shoots of inoculated and non-inoculated cultivars were collected and stored at -80°C in ultra-freezer for real-time quantitative PCR. Furthermore, some of the treated plants kept for 7 more days, and then PAL and TAL enzyme activity of shoots were measured.
Determination of PAL and TAL enzymatic activities. The activities of PAL and TAL in the shoots of wheat cultivars were determined using the method of Beaudoin-Eagan and Thorpe [13]. All steps of enzyme extraction were carried out at 0 to 4oC. One gram fresh tissues of shoots were homogenized with 3 mL of 0.05 M Tris-HCl buffer (pH 8) (Sigma-Aldrich, Germany) containing 15 mM of 2-mercaptoethanol (Sigma-Aldrich). Then the homogenates were centrifuged for 20 min at 4025 g. The protein content of each extract was measured. The enzymatic activities of PAL and TAL of shoots were assayed by measuring the amount of trans-cinnamic acid at 290 nm and p-coumarate at 333 nm for PAL and TAL, respectively.
RNA extraction and real-time quantitative PCR. The total RNA was isolated from frozen shoots and roots, using Trizol reagent (RNA Biotech, Iran). The extracted RNA was treated with DNase (Fermentas, United States). Then, the first stranded cDNA was synthesized using the M-MLV reverse transcriptase (Fermentas). Real-time PCR was performed in triplicate using SYBER Green Master Mix (RNA biotech). Gene-specific primers were designed for a 101 bp fragment of LTP2. The primer pair was 5'-CTCGTGCTGGTCGCCCTGGTG-3' in sense direction and 5'‑TGGGAATCAAGGGTGGACG-3' in anti-sense direction. The primers pair for the housekeeping gene, actin, (Gen Bank Accession No. GI:48927617) were designed as 5'-GTTCCAATCTATGAGGGATACACGC-3' in sense direction and 5'-GAACCTCCACTGAGAACAACATTACC-3' in anti-sense direction [14]. Serial dilutions of cDNA were used to obtain optimized standard curve amplification efficiency and the best cDNA concentration for real-time PCR was obtained. The relative expression ratio of target and reference genes was calculated based on its real-time efficiencies (E) and crossing point difference (∆Cp) of the sample versus control as well as reference versus control, respectively [15]. Finally, cDNA of LTPs was sequencing and the results were examined by CLC sequences viewer software. The final file was prepared for gene registration, which was ultimately recorded at Gene Bank as MN052803-LTP (https://www.ncbi.nlm. nih.gov/nuccore/MN052803). Homology searches were carried out using the NCBI blast Email server. The total volume of the real-time qPCR was 12.0 µL, containing 6.25 mL SYBER Green Master Mix (RNA Biotech), 0.25 mL of 10 mM each primer (forward and reverse), 1.0 mL cDNA (1 : 25 dilutions, as previously defined), and 4.25 mL ultra-pure water. The protocol for PCR was 30 cycles of denaturation at 95°C for 30 s, annealing at 55°C for 1 min and elongation at 72°C for 1 min. Three technical repetitions were performed (triplicate) for each biological repetition. The relative quantification of expression (RQ) was calculated using the comparative threshold cycle method [16], using the equation RQ = 2–∆∆Ct, based on the RQ values.
Determination of effective DTT concentration and use of DTT as a treatment. According to Miroliaei et al. [17] different concentrations of dithiothreitol (DTT) had different effect on the amount of protein activity, therefore, as a primary experiment the effect of different concentrations of DTT (5, 10 and 15 mM) on the expression of MN052803-LTP (Authors recorded at the Gen Bank) was evaluated. DTT applied in Hoagland’s nutrient solution as irrigated water. After 0, 12, 24, and 48 h that wheat cultivars (tolerant and sensitive to salinity) exposed to DTT, the roots and the shoots of both cultivars were collected and stored at –80°C in ultra-freezer for real-time quantitative PCR. The result showed that 10 mM DTT reduced the expression of MN052803-LTP. In contrast, the other concentrations of DTT increased the gene expression. Therefore, 10 mM DTT was selected as a reducer of MN052803-LTP expression for further experiment. Then the effect of 10 mM DTT on the expression of MN052803-LTP and phosphatidylcholine (PC) content of inoculation and non-inoculated cultivars were evaluated in a separate experiment. To do so, inoculated and none inoculated seedlings irrigated with Hoagland’s nutrient solution for 5 days, and then 10 mM of DTT (as a reducer-concentration) added to nutrient media. After 0, 12, 24, and 48 h of exposure to DTT, the roots and the shoots of both cultivars were collected and stored at –80°C in an ultra-freezer for real-time quantitative PCR. Furthermore, some of the treated plants kept for 7 more days after the application of DTT and phosphatidylcholine (PC) content were measured in 12 days old seedlings.
Chromatographic assay. Phospholipid extracts were obtained using the method reported by Folch et al. [18] which modified to extract phospholipid from whole plant (roots and shoots), the homogenate tissue was transferred to a graduated glass tube. Subsequently, chloroform-methanol (2 : 1, v/v) was added to the glass tube at twice the volume as that of the used extract. Then strongly oscillated for 1 min and centrifuged at 2500 g for 10 min. After centrifugation, the supernatant was discarded, but the boundary layer was not. The methanol-water solution (1 : 1, v/v) was added to the glass tube at a quarter of the volume as that of the subnatant, and strongly oscillated for 1 min then, centrifuged at 2500 g for 10 min. The supernatant and the boundary layer were then discarded. Finally, the subnatant was transferred to another glass tube, dried under a stream of the nitrogen, and stored at –20°C. Before HPLC analysis the extracted phospholipid was dissolved in a mobile phase solvent containing 20% chloroform. HPLC analyses were performed on a KNAUER/AZURA using 100 mm Agilent Zorbax C18H10 5 µm particle size analytical UV-vis spectra recorded on a Lambda 25 UV-Vis spectrometer using a 1 cm quartz cuvette. The mobile phase solvent acetonitrile, methanol, and 85% phosphoric acid (90 : 3 : 1, v/v) was thoroughly mixed in advance, filtered through a microporous membrane (0.2 μm) and degassed, and then, delivered to the column by a computerized solvent delivery system at the flow rate of 0.80 mL/min. The sample volume injected for HPLC analysis was 20 μL. The effluent was detected by a UV detector at 203 nm. The data were analyzed by computer-based on the model DL-800 chromatographic workstation.
Statistical analysis. All experiments were carried out with three replicates. The biochemical and gene expression parameters were statistically analyzed using ANOVA and the mean values were compared using Duncan’s multiple range tests. Excel 2016 was used to draw the necessary graphs. Sequence data and its analyses were carried out with CLC sequences viewer programs and then Sequin software was used to record the gene in the NCBI gene bank.
RESULTS
A brief explanation of the results of the primary experiment (germination of 18 common wheat cultivars) indicated that the seedling vigor, germination percentage, and germination rate in all cultivars were significantly affected by salinity stress (details in Supplementary Tables S1-S4). However, the lowest and the highest seedling vigor, germination percentage, and the rate of germination were observed in Qods and Sorkhtokhm cultivars, respectively under 200 mM of NaCl of the growth media. Therefore, Qods and Sorkhtokhm cultivars were selected as sensitive and tolerant cultivars, respectively to salinity for further experiments.
The Symbiotic Effects of A. brasilense Strains on Growth Parameters
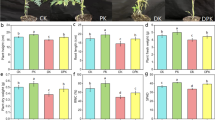
The results of growth parameters in wheat cultivars, including the fresh, and dry weight of plants as well as the shoot length, and the average plant root lengths, showed that salt stress (200 mM) reduced these parameters, while inoculation of wheat seedlings with A. brasilense (Sp245 and Sp7) improved these parameters, even under salt stress. The results also revealed there were significant differences between the two cultivars. In fact, the levels of measured parameters in salt-tolerant cultivar (Sorkhtokhm) were higher than salt-sensitive one (Qods) in all salinity levels (Fig. 1).
Growth parameters (plant length (a), fresh (b) and dry (c) weight) of sensitive (Qods) and tolerant (Sorkhtokhm) wheat cultivars to salinity when inoculated with Azospirillum brasilense (Sp245 and Sp7) strains under salt stress (200 mM NaCl). The values related to non-inoculated (1) and inoculated wheat cultivars (using 107 CFU/mL) with Sp245 (2) and Sp7 (3) strains. Each value represents the mean of three replicates ± SD.
The Activities of PAL and TAL Enzymes
Analysis of variance (details in Supplementary Table S5) showed that salinity and inoculation had a significant effect on the PAL and TAL enzyme activity of the shoots, while cultivar and their interactions did not affect PAL activity. Under the control condition, the highest activity of PAL in the shoots of tolerant and sensitive cultivars was 4.04 and 3.14 U/mg proteins, respectively. Simultaneously, TAL activity was lower in different cultivars (1.9 U/mg proteins) but almost the same in the sensitive and tolerant cultivars (Fig. 2). Inoculation increased the activity of PAL in both cultivars by almost 40% but TAL activity increased differently in the tolerant and sensitive cultivars (22 and 10%, respectively). Although salinity increased PAL and TAL enzymes activity, the dual effect of inoculation and salinity increased much higher the activity of these enzymes (50% more for PAL in both cultivars, and 16 and 8% more for TAL activity in the tolerant and sensitive cultivars, respectively) compared to non-inoculated plants under salinity condition.
PAL (a) and TAL (b) shoots enzymes values of sensitive (Qods) and tolerant (Sorkhtokhm) wheat cultivars to salinity when inoculated with Azospirillum brasilense (Sp245 and Sp7) strains under salt stress (200 mM NaCl). The values related to non-inoculated (1) and inoculated wheat cultivars (using 107 CFU/mL) with Sp245 (2) and Sp7 (3) strains. Each value represents the mean of three replicates ± SD.
Effect of Salinity and Inoculation on MN052803-LTP Expression
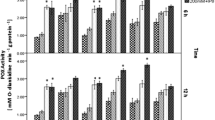
The highest MN052803-LTP expression was observed at 12 hours during the course of the experiment in all cases. This means that the MN052803-LTP expression in the roots and the shoots were increased after the plants subjected to salinity up to 12 hours then decline or stay as constant in the sensitive and tolerant cultivars (Fig. 3). Twelve hours after salinity imposed, MN052803-LTP expression of non-inoculated plants was increased in the roots and the shoots (6 and 2.6-fold in the tolerant and 3 and 1.2-fold in the sensitive cultivars, respectively), and afterward their values were reduced but still was higher than that in control plants. Meanwhile, inoculation caused a significant increase in the expression of MN052803-LTP and reached its values to 6 and 2.9-fold in the tolerant and 3.9 and 2.4-fold in the sensitive cultivars for the roots and the shoots, respectively. Under dual treatments (salinity and inoculation), the maximum relative expression of MN052803-LTP was observed as compared to other treatments. The MN052803-LTP expressions of the roots were 7.7 and 5.9-fold in the tolerant and sensitive cultivars, respectively, and 3‑fold in both cultivars for the shoots when compared to their corresponded control plants at 12 h. However, the relative expression was higher in the tolerant cv. than the sensitive one.
MN052803-LTP expression of roots (a, b) and shoots (c, d) of the sensitive and tolerant (Qods and Sorkhtokhm, respectively) wheat cultivars to salinity when inoculated with Azospirillum brasilense (Sp245 and Sp7) strains under salt stress (200 mM NaCl). The values related to the relative expression of MN052803-LTP of non-inoculated (NI) and inoculated wheat cultivars (using 108 CFU/mL) with Sp7 and Sp245 strains at 0, 12, 24 and 48 hours after salinity started. (1) NI-0 mM NaCl, (2) NI–200 mM NaCl, (3) Sp7-0 mM NaCl, (4) Sp245-0 mM NaCl, (5) Sp7-200 mM NaCl, (6) Sp245-200 mM NaCl. Each value represents the mean of three replicates ± SD.
Effect of Dithiothreitol (DTT) and Inoculation on MN052803-LTP Expression
Different concentrations (5, 10, and 15 mM) of DTT (as described in part 2 of the primary experiment) on the expression of MN052803-LTP had different effects (Fig. 4). Although the trend of the relative expression of MN052803-LTP was similar for 5 and 15 mM of DTT in roots and shoots of both cultivars, the MN052803-LTP expression was much higher, and looks likes that these concentrations stimulated this gene to express more. In contrast, 10 mM of DTT caused a reduction of MN052803-LTP expression in the roots and the shoots (on average 0.7 and 0.5-fold, respectively) of both cultivars. Although the positive or negative effect of DTT concentrations was much higher in the tolerant cultivar than the sensitive one, the concentration of 10 mM of DTT was considered as a reducer of MN052803-LTP activity.
MN052803-LTP expression of sensitive (Qods) and tolerant (Sorkhtokhm) wheat cultivars to salinity when treated with different concentration of DTT ((1) 10 mM DTT, (2) 0 mM DTT, (3) 5 mM DTT, (4) 15 mM DTT). The values related to the relative expression of MN052803-LTP of roots (a, b) and shoots (c, d). Each value represents the mean of three replicates ± SD.
Using 10 mM of DTT (as a reducer of MN052803-LTP expression) and inoculation (as stimulator) on the MN052803-LTP expression showed that the MN052803-LTP expression changed according to the type of treatment applied (Fig. 5). The higher expression of MN052803-LTP was observed in the roots and shoots of inoculated plants. Meanwhile, 10 mM of DTT caused a reduction of MN052803-LTP expression in the roots and the shoots (on average 0.7 and 0.5-fold, respectively) of both cultivars. In the dual effect of treatments, inoculation reduced the inhibitory effect of DTT on the MN052803-LTP expression. In another point of view, it looks like 10 mM DTT also unaffected the role of A. brasilense in an association system on the MN052803-LTP expression.
MN052803-LTP expression of inoculated (Sp7 and Sp245) and non-inoculated (NI) wheat cultivars when treated with 10 mM DTT. The values related to the relative expression of MN052803-LTP of roots (a, b) and shoots (c, d) of the sensitive and tolerant (Qods and Sorkhtokhm, respectively) wheat cultivars to salinity. (1) NI—10 mM DTT, (2) Sp7—10 mM DTT, (3) Sp245—10 mM DTT, (4) NI—0 mM DTT. Each value represents the mean of three replicates ± SD.
Variation of Phosphatidylcholine Content
The result showed that phosphatidylcholine (PC) content was significantly linear with the peak area within the wide linear range. The optimum resolution (separation and detection) of the PC was achieved within 6.7 min (Fig. 6a). The results (Fig. 6b) showed that phosphatidylcholine content increased in the order of salinity (200 mM NaCl), A. brasilense (Sp245 strain), and their dual effects (salinity and inoculation) in comparison to the control plants. Moreover, there were significant differences between the effect of salinity and inoculation on the PC content of the salt-sensitive and salt-tolerant cultivars. The PC content of the salt-tolerant cultivar (Sorkhtokhm) was higher than the salt-sensitive cultivar (Qods). In contrast, adding DTT led to a reduction in the amount of PC. Therefore, the highest PC content (3.4 µg/g fr wt) was measured in the inoculated salt-tolerant cultivar treated with 200 mM NaCl, while the minimum amount of PC (0.3 µg/g fr wt) was observed in the plants exposed to 10 mM DTT.
The curve of standard chromatogram of phosphatidylcholine (a), and phosphatidylcholine (PC) content (b) of the salt-sensitive (Qods, 1) and salt-tolerant (Sorkhtokhm, 2) cultivars under inoculation with Azospirillum brasilense stain (Sp245), DTT (10 mM) and salt stress (200 mM NaCl). The values related to the phosphatidylcholine content of whole plant. The abbreviated letters C, B, S, and DTT stand for cultivar, Sp245, salinity, and DTT, respectively. Each value represents the mean of three replicates ± SD.
DISCUSSION
Salt stress is one of the abiotic stresses that negatively affect plant growth and development. Our results showed that 200 mM NaCl could reduce the growth parameters of wheat cultivars, including the average plant root lengths, shoot length, fresh, and dry weight of plants. Results of different studies also reported a significant reduction of root length, dry and fresh weight under salinity conditions [1]. However, inoculation with A. brasilense (Sp245 and Sp7) could reduce the adverse effects of salinity and consequently improved growth parameters. This positive effect of A. brasilense under salinity conditions and inducing a better growth environment for plants could be related to the useful substances produced by symbiotic bacteria such as growth regulators [1].
There was a positive correlation between the measured parameters and the compatibility of tolerant and sensitive wheat cultivars with the A. brasilense strains. Inoculation of wheat cultivars with A. brasilense Sp245 and Sp7 strains showed different physiological responses to salinity conditions due to the compatibility of a strain of A. brasilense and wheat cultivar. In this experiment, Sp245 and Sp7 work well on both cultivars, but Sp245 showed more positive effects as compared to Sp7. However, not only different strains of bacteria showed different plant responses but also different wheat cultivar showed different responses. Improved growth patterns of inoculated seedlings under salinity conditions are a substantial result of salt tolerance due to inoculation. Indeed, our results indicated that using A. brasilense (at a concentration of 108 CFU/mL) as a natural and eco-friendly substance can be beneficial to improve salt tolerance in wheat cultivars. Previous studies also showed that both strains of A. brasilense (Sp7 and Sp245) caused an increase in physiological parameters of wheat seedlings under non-saline and saline conditions [19]. However, our result originated from different wheat cultivars. It looks like the use of inoculation is an effective strategy to enhanced salinity stress tolerance in plants.
In an inoculation process, the reaction of plant defense enzymes is important in the establishment of a successful association between plant host and bacteria. The application of PGPRs significantly increases some plant defense-related enzymes like PAL and TAL enzymes [4]. These enzymes are involved in the anchoring process and their variation could limit or stimulate their partnership. Zeffa et al. [20] reported that A. brasilense may enhance the defensive mechanism of maize genotypes via changing the activities of these enzymes same as the other PGPRs. The establishment of a symbiosis relationship between plant and bacteria can stimulate the biosynthesis pathway of phenolic compounds as a plant defense mechanism. Therefore, an increase in the activity of TAL and PAL can cause more phenolic compounds in inoculated plants especially with a compatible strain which is an efficient mechanism to deal with the adverse effects of reactive oxygen species during the infection process. As also Singh et al. [2] reported, the above explanation means that the microbial inoculation enhanced polyphenolic accumulation and improved PAL and TAL enzyme activity.
Feduraev et al. [21] reported that PAL and TAL enzymes also have the main role in salinity tolerance in wheat plants through the accumulation of phenylpropanoid compounds. This means inoculation influences the accumulation of polyphenolics and activity of PAL enzyme since polyphenolics compounds are strong antioxidants and PAL is a defense-related enzyme. Therefore, high accumulation of polyphenolics and enhanced PAL enzyme activity in the leaves are supposed to strengthen plants under salinity challenged conditions.
Our result showed that PAL and TAL activity in the inoculated wheat cultivars was higher than the plant exposed to salinity condition, in the dual effect of salinity and inoculation, their activities were much higher than the salinity condition alone. Meanwhile, much higher activities of PAL in inoculated tolerant wheat cultivar than the sensitive one indicated that this positive effect is mostly related to compatibility of wheat cultivar and bacterial strain. However, the dual effect of inoculation and salinity represents the interaction effect on the properties of the association between wheat cultivar with the strain of bacteria. It seems that accumulation of PAL and TAL enzymes is mostly related to compatibly of wheat cultivar and strain of bacteria. However, the effect of environmental factors such as salinity on this process should be considered. It looks like that the higher salinity tolerance could be obtained via the addition of TAL and PAL enzyme activities in a symbiosis process especially between a compatible association of wheat cultivar with A. brasilense strain.
Under salinity stress, the formation of reactive oxygen species in plants damages the plants’ lipids [22]. In this regard, plasma membrane lipids play a crucial role in determining cell structures, regulating membrane fluidity, and transducing signals. Kader [4] reported that plant lipid transfer proteins (LTPs) were thought to participate in membrane biogenesis and regulation the intracellular fatty acid pools. Nevertheless, further isolation and analysis of LTP genes have revealed roles for LTPs including the adaptation of plants to various environmental conditions. LTPs are abundant and involved in various physiological processes in plants and be implicated in abiotic stresses in various species, such as drought, cold, and salt stresses [23]. Almost 49, 52, and 156 members of LTPs have been identified in Arabidopsis, rice, and wheat, respectively [23]. Wang et al. [24] based on gene expression data showed that LTPs are somehow involved in adaptation to salt stress. In this study, we identified a novel LTP gene from wheat cultivar registered as MN052803-LTP, which was dramatically induced and up-regulated rapidly by abiotic and biotic stresses. The result of our study showed that the inoculation of wheat cultivars with A. brasilense (Sp245 and Sp7 strains), and salinity increased the MN052803-LTP expression. However, the MN052803-LTP was expressed at a higher level in the inoculated cultivar especially in the tolerant cultivar under salinity condition. Similar to our results, Benitez et al. [25] reported the up-regulation of oso3go251000-LTP in salt-tolerant cultivars of rice subjected to salt stress for 12 hours. Therefore, it is possible to say that this gene (MN052803-LTP) not only has an important role in the modification of salinity tolerance, the inoculation could positively contribute to this process.
Notable differences in the MN052803-LTP expression of the roots and the shoots of both cultivars were observed in the control condition, however, the high expression of MN052803-LTP in the root might be related to the organ-dependent or due to the priority of receiving signals from the rhizosphere. Therefore, this might be an indication of higher activity and/or physiological importance of MN052803-LTP in the root tissues. Wang et al. [26] reported that ThLTPs of Tamarix hispida showed different relative abundance in different tissues. The contribution of MN052803-LTP in the modification of salinity adverse effects in one hand and higher expression of this gene in inoculated condition especially with A. brasilense Sp245 (according to our result) in the other hand, proposed a suggestion that inoculation of a wheat cultivar with a compatible strain can provide a proper tool to improve tolerance of wheat to salinity via regulation of MN052803-LTP expression.
To facilitate endoplasmic reticulum (ER) stress-related research, in most cases, DTT is used as a stress activator [27]. DTT is a redox reagent that can destroy the oxidation conditions required for the formation of disulfide bonds. The important genes involved in ER stress responses were identified and analyzed comprehensively to determine a possible mechanism of stress regulation in plants. However, different gens exhibited a different expression pattern under DTT treatment. Yu et al. [28] reported that DEG genes were significantly up-regulated under DTT (7.5 mM), while GRP94 expression was down-regulated under DTT (7.5 mM) treatment in wheat at 48 h. Probably there might be a relationship between DTT and the expression of the LTPs gene. Our results showed that the concentration of 10 mM DTT significantly decreased the expression of MN052803-LTP, while 5 and 15 mM DTT up-regulated this gene. Importantly, the higher expression of MN052803-LTP in inoculated plants with A. brasilense (Sp245 and Sp7) under 10 mM DTT may indicate the role of A. brasilense in partial removal of the inhibitory effect of DTT. Reduction in the expression of MN052803-LTP in inoculated wheat cultivars under 10 mM of DTT is also induction of the adverse effect of DTT on the symbiotic effect of A. brasilense.
Several studies have shown that LTPs are able to transfer all common lipids and to bind Acyl-CoA like phospholipid [4]. Phosphatidylcholines (PC) are a class of phospholipid that is a major component of biological membrane. In addition, PC also has a regulatory role in most signaling pathways in plants [30]. Under salinity stress and inoculation, the PC content and transcription level of MN052803-LTP was increased, whereas under 10 mM of DTT, the PC content and transcription level of MN052803-LTP was decreased. Deng et al. [30] demonstrated the role of GhLTPG1 in phospholipids transport as well as the relationship between GhLTPG1 expression and phospholipid content. Since LTPs mediate transfer of phospholipids (like PC) to membranes, the similarity of patterns of changes in PC and LTP expression in response to different treatments could be logical. Therefore, it can be said that increase in the expression of MN052803-LTP by A. brasilense, probably can be lead to changes in composition and density of PC content of membrane. In conclusion, although salinity usually increases PAL and TAL enzyme activities in plants, the MN052803-LTP mRNA expression, and phosphatidylcholine content of the wheat cultivars increased in this experiment due to the salinity condition. The higher PAL and TAL enzyme activities, MN052803-LTP mRNA expression, and phosphatidylcholine content in the inoculated plants with A. brasilense under salinity conditions can be considered as a positive role of bacteria for improving salt tolerance. The result of the present study demonstrated that one of the mechanisms of inducing salt tolerance in the wheat cultivar could be through the MN052803-LTP expression, which would be provided by symbiosis with A. brasilense. This gene can be involved in salinity tolerance by supplying the required phospholipids for membranes, such as phosphatidylcholine, activation of the biosynthesis of antioxidant compounds, and the phenylpropanoid pathway.
In conclusion, although salinity usually increases PAL and TAL enzyme activities in plants, the MN052803-LTP mRNA expression, and phosphatidylcholine content of the wheat cultivars increased in this experiment due to the salinity condition. The higher PAL and TAL enzyme activities, MN052803-LTP mRNA expression, and phosphatidylcholine content in the inoculated plants with A. brasilense under salinity conditions can be considered as a positive role of bacteria for improving salt tolerance. However, 10 mM DTT could reduce the effect of A. brasilense on the LTP expression. The result of the present study demonstrated that one of the mechanisms of inducing salt tolerance in the wheat cultivar could be through the MN052803-LTP expression, which would be provided by symbiosis with A. brasilense. This gene can be involved in salinity tolerance by supplying the required phospholipids for membranes, such as phosphatidylcholine, activation of the biosynthesis of antioxidant compounds, and the phenylpropanoid pathway.
REFERENCES
Askary, M., Mostajeran, A., Amooaghaei, R., and Mostajeran, M., Influence of the co-inoculation Azospirillum brasilense and Rhizobium meliloti plus 2, 4-D on grain yield and N, P, K content of Triticum aestivum (cv. Baccros and Mahdavi), Am.-Eurasian J. Agric. Environ. Sci., 2009, vol. 3, p. 296.
Singh, D.P., Singh, V., Gupta, V.K., Shukla, R., Prabha, R., Sarma, B.K., and Patel, J.S., Microbial inoculation in rice regulates antioxidative reactions and defense related genes to mitigate drought stress, Sci. Rep., 2020, vol. 10, p. 1.
Benizri, E., Baudoin, E., and Guckert, A., Root colonization by inoculated plant growth-promoting rhizobacteria, Biol. Sci. Technol., 2001, vol. 5, p. 557.
Kader, J.-C., Lipid-transfer proteins in plants, Annu. Rev. Plant Biol., 1996, vol. 11, p. 627.
Gonzalez, L.E., Keller, K., Chan, K.X., Gessel, M.M., and Thines, B.C., Transcriptome analysis uncovers Arabidopsis f-box stress induced 1 as a regulator of jasmonic acid and abscisic acid stress gene expression, BMC Genomic, 2017, vol. 18, p. 533.
Xu, Yang, Zheng, X., Song, Y., Zhu, L., Yu, Z., Gan, L., Zhou, S., Liu, H., and Wen, F., NtLTP4, a lipid transfer protein that enhances salt and drought stresses tolerance in Nicotiana tabacum, Sci. Rep., 2018, vol. 8, p. 1.
Blilou, I., Ocampo, J.A., and García-Garrido, J.M., Induction of Ltp (lipid transfer protein) and Pal (phenylalanine ammonia-lyase) gene expression in rice roots colonized by the arbuscular mycorrhizal fungus Glomus mosseae, J. Exp. Bot., 2000, vol. 353, p. 1969.
Lei, L., Chen, L., Shi, X., Li, Y., Wang, J., Chen, D., Xie, F., and Li, Y., A nodule-specific lipid transfer protein AsE246 participates in transport of plant-synthesized lipids to symbiosome membrane and is essential for nodule organogenesis in Chinese milk vetch, Plant Physiol., 2014, vol. 164, p. 1045.
Grosbois, M., Guerbette, F., Jolliot, A., Quintin, F., and Kader, J.-C., Control of maize lipid transfer protein activity by oxido-reducing conditions, Biochim. Biophys. Acta, Lipids Lipid Metab., 1993, vol. 1170, p. 197.
Haji Nia, S., Zarea, M.J., Rejali, F., and Varma, A., Yield and yield components of wheat as affected by salinity and inoculation with Azospirillum strains from saline or non-saline soil, J. Saudi Soc. Agric. Sci., 2012, vol. 11, p. 113.
Döbereiner, J., Forage grasses and grain crops, in Methods for Evaluating Biological Nitrogen Fixation, Bergersen, F.J., Ed., Chichester: Wiley, 1980, p. 535.
Hoagland, D.R. and Arnon, D.I., The Water-Culture Method for Growing Plants Without Soil, Univ. Calif. Circular, vol. 347, Berkeley, CA: Univ. of Calif., 1950, p. 1.
Beaudoin-Eagan, L.D. and Thorpe, T.A., Tyrosine and phenylalanine ammonia lyase activities during shoot initiation in tobacco callus cultures, Plant Physiol., 1985, vol. 78, p. 438.
Xu, H., Jiang, X., Zhan, K., Cheng, X., Chen, X., Pardo, J.M., and Cui, D., Functional characterization of a wheat plasma membrane Na+/H+ antiporter in yeast, Arch. Biochem. Biophys., 2008, vol. 473, p. 8.
Pfaffl, M.W., A new mathematical model for relative quantification in real-time RT–PCR, Nucleic Acids Res., 2001, vol. 29, p. e45.
Livak, K. and Schmittgen, T., Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method, Methods, 2001, vol. 25, p. 402.
Miroliaei, M., Ranjbar, B., Naderi-Manesh, H., and Nemat-Gorgani, M., Thermal denaturation of yeast alcohol dehydrogenase and protection of secondary and tertiary structural changes by sugars: CD and fluorescence studies, Enzyme Microb. Technol., 2007, vol. 40, p. 896.
Folch, J., Lees, M., and Stanley, G.S., A simple method for the isolation and purification of total lipids from animal tissues, J. Biol. Chem., 1957, vol. 226, p. 497.
Baldani, V.L., Baldani, J.I., and Dobereiner, J., Effects of Azospirillum inoculation on root infection and nitrogen incorporation in wheat, Can. J. Microbiol., 1983, vol. 29, p. 924.
Zeffa, D.M., Perini, L.J., Barbosa, M., Silva, B., Sousa, N.V., Scapim, C.A., Oliveira, A.L.M., Junior, A.T.A., and Goncalves, L.S.A., Azospirillum brasilense promotes increases in growth and nitrogen use efficiency of maize genotypes, PLoS One, 2019, vol. 14, p. e0215332.
Feduraev, P., Skrypnik, L., Riabova, A., Pungin, A., Tokupova, E., Maslennikov, P., and Chupakhina, G., Phenylalanine and tyrosine as exogenous precursors of wheat (Triticum aestivum L.) secondary metabolism through PAL-associated pathways, Plants, 2020, vol. 9, p. 476.
Santi, C., Molesini, B., Guzzo, F., Pii, Y., Vitulo, N., and Pandolfini, T., Genome-wide transcriptional changes and lipid profile modifications induced by Medicago truncatula N5 overexpression at an early stage of the symbiotic interaction with Sinorhizobium meliloti, Genes, 2017, vol. 8, p. 396.
Li, G., Hou, M., Liu, Y., Pei, Y., Ye, M., Zhou, Y., Huang, C., Zhao, Y., and Ma, H., Genome-wide identification, characterization and expression analysis of the non-specific lipid transfer proteins in potato, BMC Genomics, 2019, vol. 20, p. 375.
Wang, H., Kwon, H., Yim, W., Lim, S., Moon, J., Lee, B., Seo, Y., Kim, W., and Jang, C., Expressional diversity of wheat nsLTP genes: evidence of sub functionalization via cis-regulatory divergence, Genetica, 2010, vol. 138, p. 852.
Benitez, L.C., da Maia, L.C., Ribeiro, M.V., Pegoraro, C., Peters, J.A., de Oliveira, A.C., and Braga, E.J., Salt induced change of gene expression in salt sensitive and tolerant rice species, J. Agric. Sci., 2013, vol. 53, p. 251.
Wang, C., Yang, C., Gao, C., and Wang, Y., Cloning and expression analysis of 14 lipid transfer protein genes from Tamarix hispida responding to different abiotic stresses, Tree Physiol., 2009, vol. 29, p. 1607.
Yang, X., Srivastava, R., Howell, S.H., and Basham, D.C., Activation of autophagy by unfolded proteins during endoplasmic reticulum stress, Plant J., 2016, vol. 85, p 95.
Yu, X., Wang, T., Zhu, M., Zhang, L., Zhang, L., Jing, E., Ren, Y., Wang, Z., Xin, Z., and Lin, T., Transcriptome and physiological analyses for revealing genes involved in wheat response to endoplasmic reticulum stress, Plant Biol., 2019, vol. 19, p. 193.
Singh, A., Kanwar, P., Pandey, A., Tyagi, A.K., Sopory, S.K., Kapoor, S., and Pandey, G.K., Comprehensive genomic analysis and expression profiling of phospholipase C gene family during abiotic stresses and development in rice, PLoS One, 2013, vol. 30, p. e62494.
Deng, T., Yao, H., Wang, J., Wang, J., Xue, H., and Zuo, K., GhLTPG1, a cotton GPI-anchored lipid transfer protein, regulates the transport of phosphatidylinositol monophosphates and cotton fiber elongation, Sci. Rep., 2016, vol. 6, p. 1.
ACKNOWLEDGMENTS
The authors wish to thank Dr. Michael Rothballer from AMP Research Unit Microbe Plant Interactions for providing the bacterial strains.
Funding
Financial support of this study was provided by the University of Isfahan.
Author information
Authors and Affiliations
Contributions
Dr. A. Mostajeran contribution: the idea, design and conducting the project, and manuscript editing. M. Riahi: layout and performing the experiment, preparing the manuscript draft. Dr. M. Miroliaei: adviser in the LTPs subject.
Corresponding author
Ethics declarations
Conflict of interests. The authors declare that they have no conflicts of interest.
Statement on the welfare of humans or animals. This article does not contain any studies involving animals performed by any of the authors.
Additional information
Abbreviations: DTT—dithiothreitol; NFB—nitrogen-fixing bacteria; PC—phosphatidylcholine; PGPRs—plant growth-promoting rhizobacteria.
Supplementary Information
Rights and permissions
About this article
Cite this article
Riahi, M., Mostajeran, A. & Miroliaei, M. Azospirillum brasilense Can Modulate Salt Stress in Triticum aestivum via MN052803-LTP Regulation and Phosphatidylcholines Content. Russ J Plant Physiol 68, 890–900 (2021). https://doi.org/10.1134/S1021443721050150
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1021443721050150