Abstract
Changes in invertase activities were studied upon hardening of chilling-sensitive (Nicotiana tabacum L.) and cold-resistant (Arabidopsis thaliana Heynh. (L.)) plants to hypothermia. In tobacco plants, the activity of cytoplasmic and vacuolar invertases was found to decrease by 20% during hardening, while the activity of cell wall invertase increased almost twofold. In arabidopsis, the activity of all three types of invertases increased more than twofold during hardening. The hardened tobacco plants showed a 20% increase in sugar content, while the relative content of sucrose and hexoses (fructose and glucose) in sugars did not change after hardening and equaled approximately 50% each. During hardening of arabidopsis plants, the sugar content increased 2.5 fold, whereas the proportion of sucrose in the total sugar content decreased (from 44 to 24%) and the proportion of hexoses increased (from 56 to 76%). It was proposed that the increase in activity of cell wall invertase in both plant species suppressed the outflow of assimilates and facilitated the accumulation of photosynthates in mesophyll cells of tobacco and arabidopsis leaves. The decrease in activity of cytoplasmic and vacuolar invertases in N. tabacum restricted the formation of hexoses in cells and reduced the efficiency of cold hardening in tobacco plants. More than a twofold increase in the content of soluble carbohydrates in arabidopsis was mainly caused by hexose accumulation, which was due to the increased activity of cytoplasmic and vacuolar invertases and ensured high efficiency of A. thaliana hardening to hypothermia.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Hypothermia is one of the most common environmental factors affecting plant metabolism and determining the productivity and geographical distribution of plants on the planet. Plants are able to significantly increase their tolerance to damaging effects of cold and frost by means of hardening that occurs when plants are exposed to low, noninjuring temperatures [1].
The accumulation of sugars during hypothermia is one of the best studied plant defense responses that occurs during hardening [2]. Sucrose, glucose, and fructose act as the main energy source and as precursors for synthesis of other protective substances; these carbohydrates perform osmoregulatory, cryoprotective, antioxidant, and signaling functions [3].
Implementing the protective properties of sugars upon hypothermia depends largely on the activity of a hydrolytic enzyme invertase (β-fructofuranosidase, EC 3.2.1.26) that splits the main transport sugar species sucrose into glucose and fructose [4]. There are three types of plant invertases that differ in subcellular localization, solubility, and pH optima. The invertase located in cell walls and bound to the wall by ionic bonds, as well as the soluble vacuolar invertase, exhibit the highest activity at pH 4.5–5.0. The soluble invertase located in the plant cell cytoplasm has a pH optimum in the range of 7.0–7.8 [4].
There are numerous and diverse data in the literature concerning activity changes of invertases at low temperatures. In most cases, the role of invertases during plant hardening to hypothermia is ascribed to mobilization of soluble carbohydrates required for the formation of cold resistance. For example, Deryabin et al. [5] showed that a twofold increase in the activity of cell wall invertase during hardening of Solanum tuberosum L. significantly elevated the content of sugars in cells and the apoplast and improved the cold resistance of potato plants. Arabidopsis plants transformed with the CsINV5 gene of vacuolar invertase from Camellia sinensis L. differed from the control plants by altered sucrose/hexose ratio, an elevated hexose content, and increased resistance to hypothermia [6].
Vacuolar invertase was found to be involved in stabilizing photosynthesis in A. thaliana plants at low temperatures [7]. The authors of that study found that arabidopsis inv4 mutants deficient in the activity of vacuolar invertase differed from the wild-type plants by lower values of the maximum quantum yield of photosystem II (PSII) and by lower rates of CO2 assimilation during hypothermia. Remarkably, an increased activity of cytoplasmic invertase could not compensate for the deficiency in vacuolar invertase activity [7].
There is evidence that invertases are involved in plant protection from oxidative stress. For example, A. thaliana plants with mutations in genes encoding cytoplasmic invertase were characterized by an increased expression of genes involved in protection against oxidative stress [8]. Another study revealed that S. tuberosum plants transformed with the gene of apoplastic yeast invertase featured increased sugar content and a significantly lower lipid peroxidation rate under hypothermia as compared to nontransformed plants [9].
Apart from the reports on the significant role of invertases to plant hardening under hypothermia, there are publications denying the involvement of invertases in cold hardening. For example, a significant decrease in the activity of vacuolar invertase was observed during hardening of Brassica oleracea L. At the same time, the content of sugars, especially of glucose and fructose in cabbage leaves showed a three- to fourfold increase. These data led the authors to conclude that vacuolar invertase does not play a significant role in the accumulation of sugars in cabbage plants and in the formation of cold tolerance [10].
Thus, the literature data on the participation of invertases in plant hardening to hypothermia differ greatly depending on species-specific properties of studied plants and experimental conditions. In many publications, the data on the activity of different types of intracellular invertases are missing, which additionally complicates the analysis of the role of these enzymes in the formation of resistance to hypothermia.
The aim of this work was to examine changes in activities of vacuolar, cytoplasmic, and cell-wall invertases in chilling-sensitive (Nicotiana tabacum L.) and cold-resistant (Arabidopsis thaliana Heynh. (L.)) plants in relation to the accumulation of soluble carbohydrates during hardening of these plants to hypothermia.
MATERIALS AND METHODS
Plant materials. The objects of this study were chilling-sensitive tobacco plants (Nicotiana tabacum L., cv. Samsun) and cold-resistant arabidopsis plants (Arabidopsis thaliana Heynh. (L.), ecotype Columbia). The plants were grown in pots with soil in phytotron cabinets of Timiryazev Institute of Plant Physiology (Russian Academy of Sciences) under the following conditions: tobacco was grown at 22°C and a 16-h photoperiod under irradiance of 100 μmol/(m2 s); arabidopsis plants were raised at 22°C and a 8-h photoperiod under irradiance of 100 μmol/(m2 s). The 8-h photoperiod for arabidopsis was chosen because this species belongs to long-day plants. When arabidopsis is cultivated under short-day conditions, it accumulates sufficient biomass of the rosette without the transition to the flowering stage. Experiments were performed with 6-week-old plants. Plants were cold hardened in a KBW-240 climatic chamber (Binder, Germany) for 5 days at 8°C for tobacco and 2°C for arabidopsis; other growth conditions were left unchanged. These hardening protocols were selected in the course of preliminary experiments. Plants not exposed to hardening temperatures were used as the control.
Plant resistance to hypothermia. To assess the resistance of N. tabacum and A. thaliana to low temperatures, unhardened and hardened plants of both species were transferred to a MIR-153 climatic chamber (Sanyo, Japan) and exposed for 1 day at temperatures from –1 to –4°С for tobacco and from –1 to –8°C for arabidopsis. After freezing, the plants were transferred to optimal growth conditions and cultivated for 1 week. Plant survival was calculated as the number of surviving plants (in %) with respect to the total number of plants tested at each temperature.
Determining sugar content. Weighed leaf samples of N. tabacum and A. thaliana (~500 mg) were fixed with 96% boiling ethanol. The leaf tissues were ground in a porcelain mortar, and sugars were extracted three times with 80% ethanol. The content of glucose in the extracts was determined by the glucose oxidase method, and sucrose and fructose were assayed by the Roe method [11]. The results were expressed in milligram per gram dry weight.
CO2 exchange of plants. The CO2 exchange in N. tabacum and A. thaliana plants was studied in an open-type setup with a URAS 2T infrared gas analyzer (Germany) at 22°C (control), 8°C (hardened tobacco plants), and 2°C (hardened arabidopsis plants), i.e., at temperatures identical to temperatures during the growth period and cold hardening. During gas exchange measurements, the rates of net CO2 assimilation and dark respiration were determined and expressed in mg CO2/(g dry wt h). Based on these parameters, the ratio of net photosynthesis to dark respiration was calculated [12].
Electron microscopic examination of chloroplast ultrastructure. To obtain preparations for microscopy, segments from the middle part of well-developed leaves of N. tabacum and A. thaliana were used. The plant material was fixed for 4 h with 2.5% glutaraldehyde in 0.1 M phosphate buffer (pH 7.4) at 4°C. After fourfold washing with phosphate buffer, the material was fixed with 1% OsO4 solution, dehydrated successively with a series of increasing ethanol concentrations and with acetone, and embedded into Epon-812. Ultrathin sections of plant leaves were obtained with an LKB3 ultramicrotome (LKB, Sweden). Sections were viewed using a LIBRA 120 electron microscope (Zeiss, Germany) at a magnification of ×4000. The section area of starch grains in chloroplasts was measured using the built-in software of an electron microscope LIBRA 120, and at least 100 chloroplasts per treatment were examined [13].
Invertase activity assay. Weighed samples of leaves of N. tabacum and A. thaliana (1 g each) were ground in a cold porcelain mortar in a phosphate–citrate buffer, pH 7.0, consisting of 0.1 M citric acid and 0.2 M Na2HPO4·2H2O. The resulting homogenate was dialyzed for 20 h at 4°C against a tenfold diluted phosphate–citrate buffer to remove sugars contained in tissues. The homogenate was then centrifuged for 20 min at 18 600g using a K24D centrifuge (MLW, Germany). The supernatant was used to determine the activity of soluble vacuolar and cytoplasmic invertases. The pellet was washed three times with a diluted buffer; this was performed by centrifuging the suspension at 200g each time to obtain the cell wall fraction, which was used to determine the cell wall invertase activity. The incubation mixture with a total volume of 0.5 mL contained 0.2 mL of the enzyme fraction and 0.3 mL of buffered sucrose, the final concentration of which in the mixture was 150 mM. Acetate buffer (1 M, pH 4.7) was used to determine the activity of acidic invertases (vacuolar invertase and cell wall invertase), while the phosphate–citrate buffer mixture (1 M, pH 7.5) was employed for the assay of alkaline (cytoplasmic) invertase. The incubation time was 1 h and the temperature was 30°C. The enzyme activity was estimated from the amount of glucose formed in the incubation medium, which was determined by the glucose oxidase method [14].
All experiments were performed in six replicates with 3–4 assays per replicate. Each experiment was repeated at least three to four times. The experimental results were processed statistically using SigmaPlot 12.3 software. The histograms show means values and their standard errors. Significant differences between the mean values were revealed by the Student’s t-test at a 95% significance level (P < 0.05). Values that differ significantly from each other are marked with different superscript letters.
RESULTS
Resistance of N. tabacum and A. thaliana Plants to Hypothermia
Resistance of studied plants to hypothermia was estimated by the survival rates after freezing at different temperatures. As can be seen from data in Table 1, the unhardened tobacco plants fully retained their viability after freezing at –1°C. The survival rate of N. tabacum decreased to 57% after the temperature dropped to ‒2°C, and the freezing at –3°C killed all unhardened plants. The hardened tobacco plants successfully withstood temperatures of –2°C (100% survival rate). After freezing at –3°C, 65% of tobacco plants survived, and all plants died after freezing at –4°C.
All unhardened arabidopsis plants survived at freezing temperatures down to –3°C. The survival rate decreased almost twofold (to 55%) after freezing at ‒4°C, and it dropped to zero after freezing at –5°C. The hardened arabidopsis plants remained 100% viable at temperatures down to –6°C. The survival rate of hardened A. thaliana reduced to 45% after freezing at –7°С and dropped to zero after freezing at –8°С.
Sugar Content in N. tabacum and A. thaliana Plants
Table 2 presents data on sugar content in leaves of tobacco and arabidopsis plants before and after cold hardening. During hardening of tobacco plants, the sugar content increased by ~20% (from 15.5 to 19.6 mg/g dry wt), which was largely due to a uniform increase in the content of fructose, glucose, and sucrose. Consequently, the proportions of individual sugar forms remained nearly unchanged during low-temperature hardening (Table 3). The proportions of sucrose and hexoses (fructose and glucose) were equal both before and after hardening and amounted to approximately 50%.
In arabidopsis plants, the total sugar content increased during hardening by a factor of 2.5 (from 23.7 to 60.3 mg/g dry wt). This gain resulted from the increase in sucrose content by almost 40% and from the 3.5-fold increase in fructose and glucose content. After hardening of A. thaliana, the proportions of different sugar forms changed significantly toward the reduction in relative amount of sucrose in total sugar content (from 44 to 24%) and toward the rise in proportion of hexoses (from 56 to 76%).
CO2 Exchange in N. tabacum and A. thaliana Plants
In our experiments, the hardening to hypothermia caused nearly identical changes in parameters of CO2 exchange in both plant species examined (Figs. 1a, 1b). The rate of net photosynthesis decreased twofold: from 6.5 to 3.1 mg CO2/g dry wt per hour for tobacco, and from 9.4 to 4.7 mg CO2/g dry wt per hour for arabidopsis. The rate of dark respiration decreased approximately threefold: from 4.8 to 1.5 mg CO2/g dry wt per hour for tobacco and from 6.3 to 2.1 CO2/g dry wt per hour for arabidopsis. Such changes in CO2 exchange led to a 1.5-fold increase in the net photosynthesis to dark respiration ratio in both species.
Changes in parameters of CO2 exchange in (a) tobacco and (b) arabidopsis plants during hardening to hypothermia ((1) net photosynthesis; (2) dark respiration; (3) net photosynthesis to dark respiration ratio). Different letters above the bars denote significant differences of mean values at P < 0.05.
Starch Grain Area in Chloroplasts of N. tabacum and A. thaliana
Electron microscopic observations revealed significant changes in both the section area of starch grains and their frequency in chloroplasts of N. tabacum and A. thaliana during cold hardening (Figs. 2, 3). In tobacco plants, the section area of one starch grain and the total area of starch grains per chloroplast increased by more than twofold (Table 4). As a result of these changes, the fractional area of starch grains per chloroplast increased from 24 to 46%.
In arabidopsis, unlike tobacco, the section area of a starch grain did not change during hardening, but the number of starch grains increased (Fig. 3). Therefore, the total area of starch grains in the chloroplast enlarged by almost 25% (Table 4). Consequently, the fractional area of starch grains, expressed in percent from the chloroplast section area, increased from 12 to 16%.
Invertase Activities in N. tabacum and A. thaliana Plants
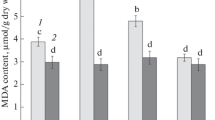
Figure 4 shows changes in the activity of invertases in leaves of tobacco and arabidopsis plants during low-temperature hardening. During hardening of tobacco plants, the activities of cytoplasmic and vacuolar invertases reduced by ~20%, whereas the activity of cell-wall invertase increased almost twofold (Fig. 4a).
In arabidopsis leaves, the activities of all three types of invertases increased more than twofold during hardening to hypothermia (Fig. 4b).
DISCUSSION
Cold hardening of plants improves their tolerance to hypothermia, which allows the plants to withstand lower damaging temperatures compared to unhardened plants [15]. One reliable and frequently used method to assess the effectiveness of hardening consists in subjecting whole plants to freezing with the subsequent estimation of plant survival rate [16]. Our data showed that the resistance of N. tabacum and A. thaliana to freezing temperatures increased after cold hardening, although to different extents. Judging from the lowest temperature sufficient for survival of all tested plants (survival rate 100%), we can state that the resistance of tobacco plants increased after hardening by 1°C (change from –1 to –2°C). The efficiency of cold hardening of A. thaliana was significantly higher. The hardened arabidopsis plants increased their frost resistance by 3°C (from –3 to –6°C).
The damage and death of plants at freezing temperatures is known to result from ice formation in their tissues. The formation of intracellular ice crystals always leads to cell death. Extracellular ice formation prevents the intracellular mechanical damage and preserves the plant cell viability [17]. Therefore, the strategy of plant adaptation to freezing is based on the formation of ice in intercellular spaces and avoiding ice formation inside the cell [2].
This strategy relies largely on soluble carbohydrates that accumulate in plants during low-temperature hardening [18]. Our experiments revealed substantial differences between N. tabacum and A. thaliana in their ability to accumulate sugars at hardening temperatures. In tobacco plants, the leaf sugar content increased by only 20% with respect to that of unhardened plants, whereas a 2.5-fold increase was noted in arabidopsis. These results are fully consistent with the data on resistance of tobacco and arabidopsis plants to freezing temperatures (Table 1), and they help to explain large differences in the efficiency of hardening between N. tabacum and A. thaliana.
The degree of sugar accumulation in the cell at hardening temperatures depends largely on relative rates of photosynthesis (a source of assimilates) and respiration (an assimilate consumer) [12]. In our experiments, the net photosynthetic rate in both plant species reduced during hardening to a lesser extent than the rate of dark respiration, which led to a 1.5-fold increase in the ratio of net photosynthesis to dark respiration. The parameters of CO2 exchange underwent comparable changes in both species; hence, the large difference in the content of soluble sugars between N. tabacum and A. thaliana cannot be interpreted in terms of photosynthesis to respiration ratio.
There is evidence in the literature that the degradation of starch grains in chloroplasts provides an additional source of sugars during cold hardening [19]. For testing this assumption, we determined the section area of starch grains in chloroplasts using electron microscopy. We found that hardening was followed by the increase, rather than the decrease, in the area of starch grains both in N. tabacum and A. thaliana. In arabidopsis chloroplasts, the gain in total area of starch grains did not exceed 25%, and the fractional area of starch grains was ≤16% of the chloroplast section area. In tobacco, starch synthesis was so intense at hardening temperatures that the area of starch grains occupied almost half of the chloroplast section area (Table 4). Thus, N. tabacum and A. thaliana differed significantly in the patterns of photoassimilate accumulation during hardening to hypothermia. It is reasonable to assume that tobacco plants accumulated the major part of photosynthates in the form of starch, which limited their ability to accumulate soluble carbohydrates in leaves and reduced the efficiency of hardening. In arabidopsis plants, unlike in tobacco, the major part of assimilates apparently accumulated in leaves in the form of sugars, which promoted the development of plant resistance to freezing temperatures.
The accumulation of sugars in leaves of the studied plants upon hardening to hypothermia was accompanied by changes in proportions of different forms of sugars. In tobacco, the ratio of sucrose and hexose content was approximately 50 : 50% both before and after hardening. By contrast, the proportion of hexoses (mainly glucose) in the total sugar content in arabidopsis increased significantly due to an almost twofold reduction in the relative content of sucrose (Table 3). Since changes in the sucrose to hexose ratio depend on the activity of invertases known to hydrolyze sucrose in cells [20], we carried out a series of experiments to determine the activity of invertases before and after hardening.
Our studies with tobacco and arabidopsis plants revealed both common and quite distinct changes in the activity of invertases during hardening. One feature common to both species was a twofold increase in the activity of cell wall invertase during hardening. The cell wall invertase is involved in regulation of phloem loading with assimilates [21]. Depending on how connections between mesophyll and phloem cells are organized, the symplast and apoplast pathways of phloem loading are distinguished. In plant species with the symplastic transport, the mesophyll and phloem cells are connected into a continuous symplast where assimilates move along the intercellular network of plasmodesmata. Species with disconnected mesophyll and phloem symplastic domains use the apoplast as an intermediate place for loading assimilates before their entry into the phloem. Such plants are referred to as the apoplastic transport type species [22]. Since N. tabacum and A. thaliana plants employ the apoplastic phloem loading [23], the activity of cell wall invertase is of great importance in regulating the outflow of assimilates from leaves to roots. We suppose that the increased activity of cell wall invertase accelerated sucrose hydrolysis in the apoplast and, thereby, suppressed phloem loading in hardened tobacco and arabidopsis plants. The hexoses formed during sucrose hydrolysis returned to mesophyll cells, thus increasing sugar content in leaves [24].
The main distinctions between N. tabacum and A. thaliana in terms of invertase activity were manifested at the level of intracellular invertases. During hardening to hypothermia, the activities of cytoplasmic and vacuolar invertases showed opposite changes in cells of tobacco and arabidopsis. Such opposite changes could determine different features of sugar accumulation in leaves of the two studied species. We observed a ~20% decrease in the activity of intracellular invertases in tobacco and a twofold increase in arabidopsis. The reduced activity of intracellular invertases in N. tabacum restricted the formation of hexoses in cells; therefore, the sucrose-to-hexose ratio did not appreciably change during low-temperature hardening of these plants. The elevated activity of cytoplasmic and vacuolar invertases in hardened A. thaliana plants can account for the large increase in relative content of hexoses, which was concurrent with the drop in sucrose proportion. The invertase activity giving rise to the increased proportion of hexoses in the total sugar content is important for the formation of frost resistance during hardening [25]. The formation of two molecules (glucose and fructose) upon the hydrolysis of a sucrose molecule increases the osmotic potential of the cell, which lowers the freezing point of solution and averts the formation of ice crystals [26].
In addition to osmotic and cryoprotective functions, the high intracellular glucose content in hardened A. thaliana plants might also perform a signaling function. Some data in the literature indicate the role of glucose in the transmission of intracellular signals [27]. A possible glucose sensor is hexokinase 1 (HXK1) located in the cytosol, chloroplasts, mitochondria, and the nucleus [28]. According to some reports, the elevated content of hexoses in arabidopsis plant cells at low temperatures activates the expression of COR15B and LEA3 genes [6, 19]. One of the main functions for proteins encoded by cold-responsive genes (COR), as well as by genes for late embryogenesis abundant (LEA) proteins, is to protect cell structures from dehydration [29]. Therefore, it is reasonable to assume that HXK1 might perceive an elevated glucose concentration in arabidopsis cells and participate in glucose signal transduction, thus activating the expression of genes responsible for the formation of cell resistance to dehydration, which is crucially important for the enhancement of freeze tolerance in plants [30].
Thus, the presented results lead us to state that invertases play a significant role in hardening of N. tabacum and A. thaliana plants to hypothermia. We suppose that the increase in the activity of cell wall invertase in both plant species inhibited phloem loading and the outflow of assimilates from leaves to roots. This provided conditions for the accumulation of photosynthetic products in leaf mesophyll cells of tobacco and arabidopsis plants. The decrease in activity of cytoplasmic and vacuolar invertases in tobacco plants suppressed sucrose hydrolysis in cells of this species. The low level of hexose accumulation and the concurrent acceleration of starch synthesis in chloroplasts restrained the cell’s ability to accumulate sugars and reduced the efficiency of cold hardening in tobacco plants. Arabidopsis plants, unlike tobacco, accumulated the major part of assimilates in the form of soluble carbohydrates rather than in starch grains of chloroplasts. More than a twofold increase in sugar content in arabidopsis was mainly due to the accumulation of hexoses owing to the increased activity of cytoplasmic and vacuolar invertases, which ensured high efficiency of A. thaliana hardening to hypothermia.
REFERENCES
Larcher, W., Physiological Plant Ecology: Ecophysiology and Stress Physiology of Functional Groups, Berlin: Springer-Velag, 2003, p. 513.
Trunova, T.I., Rastenie i nizkotemperaturnyi stress. 64‑e Timiryazevskie chteniya (Plants and Low-Temperature Stress: 64th Timiryazev’s Readings), Moscow: Nauka, 2007.
Tarkowski, L.P. and van den Ende, W., Cold tolerance triggered by soluble sugars: a multifaceted countermeasure, Front. Plant Sci., 2015, vol. 6, art. ID 203. https://doi.org/10.3389/fpls.2015.00203
Sturm, A., Invertases. Primary structures, functions, and roles in plant development and sucrose partitioning, Plant Physiol., 1999, vol. 121, p. 1. https://doi.org/10.1104/pp.121.1.1
Deryabin, A.N., Burakhanova, E.A., and Trunova, T.I., Apoplastic sugars and cell wall invertase are involved in formation of the tolerance of cold-resistant potato plants to hypothermia, Dokl. Biochem. Biophys., 2015, vol. 465, p. 366.
Qian, W., Xiao, B., Wang, L., Hao, X., Yue, C., Cao, H., Wang, Y., Li, N., Yu, Y., Zeng, J., Yang, Y., and Wang, X., CsINV5, a tea vacuolar invertase gene enhances cold tolerance in transgenic Arabidopsis, BMC Plant Biol., 2018, vol. 18, art. ID 228. https://doi.org/10.1186/s12870-018-1456-5
Weiszmann, J., Furtauer, L., Weckwerth, W., and Nagele, T., Vacuolar sucrose cleavage prevents limitation of cytosolic carbohydrate metabolism and stabilizes photosynthesis under abiotic stress, FEBS J., 2018, vol. 285, p. 4082. https://doi.org/10.1111/febs.14656
Xiang, L., Le Roy, K., Bolouri-Moghaddam, M.R., Vanhaecke, M., Lammens, W., Rolland, F., and van den Ende, W., Exploring the neutral invertase—oxidative stress defence connection in Arabidopsis thaliana, J. Exp. Bot., 2011, vol. 62, p. 3849. https://doi.org/10.1093/jxb/err069
Deryabin, A.N., Dubinina, I.M., Burakhanova, E.A., Astakhova, N.V., Sabelnikova, E.P., and Trunova, T.I., Influence of yeast-derived invertase gene expression in potato plants on membrane lipid peroxidation at low temperature, J. Therm. Biol., 2005, vol. 30, p. 73. https://doi.org/10.1016/j.jtherbio.2004.07.002
Sasaki, H., Ichimura, K., Imada, S., and Yamaki, S., Sucrose synthase and sucrose phosphate synthase, but not acid invertase, are regulated by cold acclimation and deacclimation in cabbage seedlings, J. Plant Physiol., 2001, vol. 158, p. 847.
Turkina, M.V. and Sokolova, S.V., Methods for the determination of monosaccharides and oligosaccharides, in Biokhimicheskie metody v fiziologii rastenii (Biochemical Methods in Plant Physiology), Pavlinova, O.A., Ed., Moscow: Nauka, 1971.
Klimov, S.V., Cold hardening of plants is a result of maintenance of an increased photosynthesis/respiration ratio at low temperature, Biol. Bull. (Moscow), 2003, vol. 30, p. 48.
Trunova, T.I., Astakhova, N.V., Deryabin, A.N., and Sabel’nikova, E.P., Ultrastructural organization of chloroplasts of the leaves of potato plants transformed with the yeast invertase gene at normal and low temperature, Dokl. Biol. Sci., 2003, vol. 389, p. 176.
Klimov, S.V., Popov, V.N., Dubinina, I.M., Burakhanova, E.A., and Trunova, T.I., The decreased cold-resistance of chilling-sensitive plants is related to suppressed CO2 assimilation in leaves and sugar accumulation in roots, Russ. J. Plant Physiol., 2002, vol. 49, p. 776.
Xin, Z. and Browse, J., Cold comfort farm: the acclimation of plants to freezing temperatures, Plant Cell Environ., 2000, vol. 23, p. 893.
Zuther, E., Schulz, E., Childs, L.H., and Hincha, D.K., Clinal variation in the non-acclimated and cold–acclimated freezing tolerance of Arabidopsis thaliana accessions, Plant Cell Environ., 2012, vol. 35, p. 1860. https://doi.org/10.1111/j.1365-3040.2012.02522.x
Ashworth, E.N. and Pearce, R.S., Extracellular freezing in leaves of freezing-sensitive species, Planta, 2002, vol. 214, p. 798.
Ma, Y., Zhang, Y., Lu, J., and Shao, H., Roles of plant soluble sugars and their responses to plant cold stress, Afr. J. Biotechnol., 2009, vol. 8, p. 2004.
Sicher, R., Carbon partitioning and the impact of starch deficiency on the initial response of Arabidopsis to chilling temperatures, Plant Sci., 2011, vol. 181, p. 167. https://doi.org/10.1016/j.plantsci.2011.05.005
Barau, J., Grandis, A., de Andrade Carvalho, V.M., Teixeira, G.S., Zaparoli, G.H.A., Scatolin do Rio, M.A., Rincones, J., Buckeridge, M.S., and Guimarães Pereira G.A., Apoplastic and intracellular plant sugars regulate developmental transitions in witches broom disease of cacao, J. Exp. Bot., 2015, vol. 66, p. 1325. https://doi.org/10.1093/jxb/eru485
Roitsch, T. and González, M.-C., Function and regulation of plant invertases: sweet sensations, Trends Plant Sci., 2004, vol. 9, p. 606.
Gamalei, Yu.V., Floema lista (Leaf Phloem), St. Petersburg: Nauka, 1990.
Gamalei, Yu.V., Pakhomova, M.V., and Syutkina, A.V., Ecological aspects of assimilate transport. I. Temperature, Fiziol. Rast., 1992, vol. 39, p. 1068.
Chikov, V.I. and Bakirova, G.G., Role of the apoplast in the control of assimilate transport, photosynthesis, and plant productivity, Russ. J. Plant Physiol., 2004, vol. 51, p. 420.
Livingston, D.P. and Henson, C.A., Apoplastic sugars, fructans, fructan exohydrolase, and invertase in winter oat: responses to second-phase cold hardening, Plant Physiol., 1998, vol. 116, p. 403. https://doi.org/10.1104/pp.116.1.403
Reyes-Díaz, M., Ulloa, N., Zúñiga-Feest, A., Gutiérrez, A., Gidekel, M., Alberdi, M., Corcuera, L.J., and Bravo, L.A., Arabidopsis thaliana avoids freezing by supercooling, J. Exp. Bot., 2006, vol. 57, p. 3687.
Gupta, A.K. and Kaur, N., Sugar signaling and gene expression in relation to carbohydrate metabolism under abiotic stresses in plants, J. Biosci., 2005, vol. 30, p. 761.
Moore, B., Zhou, L., Rolland, F., Hall, Q., Cheng, W.H., and Liu, Y.X., Role of the Arabidopsis glucose sensor HXK1 in nutrient, light, and hormonal signaling, Science, 2003, vol. 300, p. 332.
Wilhelm, K.S. and Thomashow, M.F., Arabidopsis thaliana cor15b, an apparent homologue of cor15a, is strongly responsive to cold and ABA, but not drought, Plant Mol. Biol., 1993, vol. 23, p. 1073.
Jang, J.C., Leόn, P., and Sheen, J., Hexokinase as a sugar sensor in higher plants, Plant Cell, 1997, vol. 9, p. 5.
Funding
This work was supported by the Ministry of Education and Science of the Russian Federation (State Assignment no. 121040800153-1 “Mechanisms of Plant Adaptation to the Factors of Aridization of the Global Climate and Anthropogenic Pollution of the Environment”).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interests. The authors declare that they have no conflicts of interest.
Statement on the welfare of humans or animals. This article does not contain any studies involving animals performed by any of the authors.
Additional information
Translated by A. Bulychev
Abbreviations: HXK1—hexokinase 1.
Rights and permissions
About this article
Cite this article
Popov, V.N., Astakhova, N.V. Changes in Invertase Activities during Hardening to Hypothermia of Nicotiana tabacum L. and Arabidopsis thaliana Heynh. (L.). Russ J Plant Physiol 68, 1218–1226 (2021). https://doi.org/10.1134/S1021443721050149
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1021443721050149