Abstract
Low temperature (LT) is an important elicitor that triggers anthocyanin biosynthesis. To investigate whether the reactive oxygen species (ROS) produced via RBOH are involved in this process, we analysed the function and mechanism of ROS produced via RBOH during LT-induced anthocyanin biosynthesis in Begonia semperflorens Link & Otto. The results showed that BsRBOHD transcription was upregulated in LT-grown seedlings at the 3rd hour, which was followed by the upregulation of anthocyanin-biosynthesis genes at the 5–9th hour, leading to anthocyanin accumulation on the 2nd day. The LT-induced increases in ROS production, BsRBOHD and anthocyanin-biosynthesis gene transcription, and anthocyanin content were abolished by the pre-treatment of seedlings with DPI [an inhibitor of nicotinamide adenine nucleoside phosphorylase (NADPH) oxidase or DMTU (a H2O2 scavenger)], but were promoted by pre-treatment with NADPH (a substrate of NADPH oxidase). Changes in the chlorophyll fluorescence parameters showed that pre-treatment of DPI or DMTU alleviated the LT-induced decrease in the seedling chlorophyll content and a/b ratio, which subsequently alleviated the LT-induced decreases in the ABS/CSm, TRo/CSm, RC/CSm, ETo/CSm and REo/CSm values. In contrast, NAPDH pre-treatment intensified these changes. Therefore, we suggest that ROS produced via BsRBOHD may be involved in the LT-induced anthocyanin biosynthesis by strengthening the overaccumulation of ROS produced by the overexcitation of PSII reaction centres and overflux from \({\text{Q}}_{{\text{A}}}^{ - }\) to NADP+ in B. semperflorens.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
The accumulation of anthocyanin in plant leaves is a common occurrence that leads the leaves to turn red in autumn or winter [1, 2]. The predominant theory of why anthocyanin biosynthesis occurs during autumn or winter conditions is that anthocyanin production improves the efficiency of amino acid retranslocation to other organs to protect against potential light and cold damage [3]. Because anthocyanin biosynthesis and the onset of low temperature (LT) conditions coincide in autumn, LT conditions are considered to be an important elicitor for anthocyanin biosynthesis in plants during autumn or winter conditions [2, 4].
LT has an effect on many physiological processes in plants that play a role in triggering anthocyanin biosynthesis, such as photosynthesis, carbon metabolism, reactive oxygen species (ROS) production, etc. Photosynthesis is a highly integrated physiological process that involves light energy conversion and carbon assimilation. LT conditions leads to an imbalance in light energy absorption and consumption, which causes excess light energy and ROS production. ROS also function as signal transduction molecules that regulate different pathways in plants that promote the acclimation or resistance of plants to abiotic and biotic stresses [5]. ROS-induced anthocyanin production has been observed in many plants [6, 7]. ROS production occurs in different subcellular compartments, with major ROS-production sites including the chloroplast, plasma membrane and mitochondria [5].
Plant respiratory burst oxidase homologues (RBOHs) are localized on the plasma membrane and can utilize nicotinamide adenine nucleoside phosphorylase (NADPH) or flavin adenine dinucleotide (FAD) as electron donors to catalyse the conversion of O2 into superoxide radicals (\({\text{O}}_{2}^{{\bullet - }}\)), the latter of which is spontaneously or catalytically converted to H2O2 [8]. Plant RBOH proteins have two motifs in their N-terminus that can bind to Ca2+ ions [9], suggesting that NADPH oxidase activity may be activated by Ca2+-dependent stress signals. A core C-terminal region in plant RBOH proteins contains transmembrane domains and a functional oxidase domain responsible for superoxide generation [9], making RBOH proteins important enzymatic sources of ROS [8].
The RBOH gene family is present in most vascular plants and has been isolated from many species. Moreover, different numbers of these genes have been observed in different tissues, which suggest that they have different functions [10]. Genome-wide identification and function analyses have shown that different NADPH oxidase genes are co-expressed with different genes associated with intracellular processes, such as cell wall biosynthesis, defence responses, and signal transduction in wheat [11]. AtRBOHC is involved in the response to mechanical wounding in Arabidopsis thaliana roots [12], whereas SlRBPHB is necessary for drought stress responses in tomato [13]. Additional studies have shown that the level of RBOHD transcription is higher in plants grown under abiotic and biotic stresses [14], and two members of the RBOH gene family, AtRBOHD and AtRBOHF, are involved in the responses of A. thaliana to the majority of stresses [15].
ROS produced via RBOHs play important roles in the acclimation or resistance of plants to abiotic and biotic stresses [14, 16]. Because RBOHs are key enzymes for apoplastic ROS production, ROS produced via RBOHs may permeate through aquaporin into the symplast to regulate target gene expression [17].
In our previous study, the leaves of Begonia s-emperflorens cultivar ‘Super Olympia’, a perennial evergreen plant, showed the potential to accumulate anthocyanin under LT conditions [4]. Furthermore, ROS has been shown to act as an important inducer in this LT‑induced anthocyanin biosynthesis in B. semperflorens [6]. According to our previous transcriptomic data, among the isoforms of BsRBOHDs, only BsRBOHD, a homologue of the Arabidopsis protein RBOHD,was upregulated in B. semperflorens grown under LT conditions of 15/5°C [18, 19]. Therefore, in this study, we used B. semperflorens ‘Super Olympia’ to further investigate the function and mechanism of ROS produced via RBOHs in LT‑induced anthocyanin biosynthesis.
MATERIALS AND METHODS
Plant materials and treatment.Begonia semperflorens ‘Super Olympia’ seeds were germinated in a growth chamber with a 16-h photoperiod, a photosynthetic photon flux density (PPFD) of 100 µmol/(m2 s), 25/15°C day/night temperatures and 100% relative humidity. After germination, the seedlings were watered daily with full-strength Hoagland’s nutrient solution. Seedlings at the four to five leaf stages were used in this study.
Experimental treatments. In the first experiment the seedlings were divided into two groups (approximately 72 seedlings per group) and watered every two days. In the CK group, the seedlings were placed in a growth chamber (day/night temperatures of 25/15°C, a 10-h photoperiod, and a PPFD of 300 µmol/(m2 s)). In the LT group, the seedlings were grown in another separate growth chamber (day/night temperatures of 15/5°C, an 8-h photoperiod, and a PPFD of 300 µmol/(m2 s)). Only the 1st to 3rd leaves were harvested, which were subsequently cut into pieces using scissors. The blended pieces were used as leaf samples, and leaf samples for both groups (0.3 g for each group) were collected at 0, 1, 3, 5, 7 and 9 h and at 1, 2, 3, 4, and 6 d.
In the second experiment eight groups of seedlings with four developed leaves were grown in a growth chamber (day/night temperatures of 25/15°C, a 10-h photoperiod, and a PPFD of 300 µmol/(m2 s)). Four groups of seedlings were pre-treated with H2O, nicotinamide adenine nucleoside phosphorylase (NADPH, 0.05 mM), diphenyleneiodonium chloride (DPI, 0.01 mM) or N,N'-dimethylthiourea (DMTU, 5 mM), for 6 h and then grown under normal temperature conditions (day/night temperatures of 25/15°C, a 10-h photoperiod, and a PPFD of 300 µmol/(m2 s)). Another four groups of seedlings were pre-treated with H2O, NADPH (0.05 mM), DPI (0.01 mM) or DMTU (5 mM) for 6 h and then moved to LT conditions (day/night temperatures of 15/5°C, a 10-h photoperiod, and a PPFD of 300 µmol/(m2 s)). Leaf samples were harvested on the 10th day.
RNA extraction and cDNA synthesis. Total RNA was extracted from mixed leaf samples from three seedlings using the CTAB method according to Liao et al. [20], with minor modifications. A PrimeScriptTM RT reagent kit with gDNA Eraser (TaKaRa, Dalian, China) was used to synthetize cDNA.
Real-time reverse transcription-quantitative PCR (RT-qPCR). The expression of genes encoding NADPH oxidase (BsRBOHD, GenBank Accession No. MH784503), phenylalanine ammonia lyase (BsPAL, GenBank Accession No. KJ930039), chalcone synthase (BsCHS, GenBank Accession No. KJ930037), flavanone 3-hydroxylase (BsF3H, GenBank Accession No. KJ930038) andanthocyanidin synthase (BsANS, GenBank Accession No. KJ930039) was examined by real-time reverse transcription-quantitative PCR (RT-qPCR) analysis. The 18S rRNA gene (GenBank Accession No. KJ959633) was used as an internal reference gene to identify expression differences using each cDNA template. The gene-specific primers used for real-time RT-qPCR analysis were designed using Genescript software (GenScript Biotech Corp. Nanjing, China., Table S1, [6]), and the SYBR® Premix ExTaq™ II (TaKaRa) and StepOne and StepOnePlus Real-Time PCR systems (Applied Biosystems, United States) Kit to conduct the RT-PCR. And then the two-step PCR amplification standard procedure was used for amplification. The thermocycling programme consisted of 40 cycles of 95°C for 30 sec, 95°C for 5 sec and 60°C for 34 sec. The 2–ΔΔCT method was used to perform relative quantization based on the 18S rRNA gene after the cycles were complete.
Measurement of pigments. The anthocyanin contents of plants were measured according to the method of Mita et al. [21], with minor modifications. Each frozen leaf sample (0.3 g) was ground at 4°C with 3 mL of 1% HCl : MeOH (v/v) and then stored under dark conditions at 4°C for 1 d. After centrifugation at 3500 g for 15 min, the supernatant was measured spectrophotometrically at 530 and 657 nm. One unit of anthocyanin equals one absorbance unit (A530 – 0.25 × A657) per millilitre of extraction solution.
The chlorophyll contents of plants were extracted with 80% acetone (v/v) and measured by spectrophotometric absorption at 663 and 645 nm for Chl a and Chl b, respectively [22].
Measurement of ROS. The H2O2 contents of plants were measured according to the method of Brennan and Frenkel [23], with minor modifications. Each frozen leaf sample (0.3 g) was ground with 2 mL of cold acetone. After centrifugation at 12 000 g for 5 min, the supernatant was collected to determine the H2O2 content. The assay mixture contained 0.5 mL of the extract, 50 µL of titanium reagent (20% titanium tetrachloride in concentrated HCl, v/v) and 0.1 mL of NH3 × H2O (25%). After centrifugation at 10 000 g for 5 min, the pellets were washed five times with 1 cm3 of acetone and then dissolved in 3 mL of 2 M H2SO4. The absorbance of the solution was determined at 415 nm.
The \({\text{O}}_{2}^{ - }\) production rate was measured by analysing the formation of nitrite from hydroxylamine in the presence of \({\text{O}}_{2}^{ - }\), according to Elstner and Heupel [24]. Each frozen leaf sample (0.3 g) was ground with 2 mL of phosphate buffer (PBS, 65 mM, pH 7.8) and then centrifuged at 5000 g for 10 min at 4°C. The reaction mixture contained 0.9 mL of phosphate buffer, 0.1 mL of 10 mM hydroxylamine hydrochloride and 1 mL of supernatant. After incubating at 25°C for 20 min, 17 mM sulfanilamide and 7 mM α-naphthylamine were added to the reaction mixture. An equal volume of ethyl ether was added, and the mixture was centrifuged at 1500 g for 5 min. Subsequently, the absorbance of the aqueous solution was read at 530 nm.
Measurement of chlorophyll fluorescence. Chlorophyll fluorescence was measured using an M-PEA Fluorescence Monitoring System (Hansatech Instruments, United Kingdom). Seedlings were dark adapted for at least 30 min prior to measurement. The following PSII behaviour parameters were calculated per excited leaf cross-section (CSm): ABS/CSm (=Fm, light energy absorption), TRo/CSm (amount of excitation energy trapped in PSII reaction centres), DIo/CSm (amount of energy dissipated from PSII), RC/CSm (number of active reaction centres), ETo/CSm (amount of energy used for electron transport), REo/CSm (amount of deoxidized electron in acceptor side of PSI).TRo/ABS (=Fv/Fm, maximum quantum yield of PSII), ETo/TRo (the efficiency of electron moves beyond \({\text{Q}}_{{\text{A}}}^{ - }\)), REo/ETo (the quantum yield for reduction of end electron acceptors at the PSI acceptor side) [25].
Statistical analysis. Each biochemical result is the average of at least three independent replicates. The data were subjected to an analysis of variance using the statistical analysis system SAS 8.0 (SAS Institute, United States). The data were expressed as means ± standard deviation (SD). Differences between treatment means were assessed by the Tukey’s test at P < 0.05.
RESULTS
Time Course Changes in Gene Transcription and Anthocyanin Biosynthesis in B. semperflorens under LT
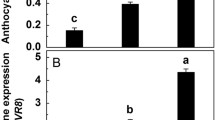
The level of BsRBOHD transcription was significantly increased to 2.02-fold at the 3rd h in the LT‑seedlings compared with that in the CK group (Fig. 1a). The expression of four genes involved in anthocyanin biosynthesis (BsPAL, BsCHS, BsF3H and BsANS) were significantly increased in the LT-seedlings at the 5th, 7th, 7th and 9th h to 2.62-, 6.04-, 4.65- and 2.00-fold compared with that in the CK-seedlings, respectively (Figs. 1b–1e). Subsequently, the anthocyanin content was significantly increased in the LT-seedlings after LT treatment for 2 days, with an increasing trend observed after day 2 (Fig. 1).
In the LT-seedlings, the BsRBOHD transcription level first peaked at the 9th h and then again on day 2, and the BsRBOHD transcription level on day 2 was 15.05 times greater that of the first peak and 28.12 times greater than that of the CK-seedlings. In addition, the transcription levels of BsPAL, BsCHS, BsF3H and BsANS in the LT-seedlings began to increase on day 2 and peaked on day 3, and their transcription levels on day 3 were 19.44, 7.52, 16.02 and 14.35 times that of the CK-seedlings, respectively.
Therefore, we suggest that the first peak in BsRBOHD expression may stimulate the expression of anthocyanin-related genes. In other words, the BsRBOHD gene may play a signalling role during the process of LT treatment.
Effects of NADPH, DPI and DMTU on ROS Production and Anthocyanin Biosynthesis in B. semperflorens Grown under Normal and Low Temperature Conditions
To further investigate whether the BsRBOHD gene is involved in LT-induced anthocyanin biosynthesis, we analysed the ROS production, anthocyanin biosynthesis and chlorophyll content in B. semperflorens pre-treated with DPI (an inhibitor of NADPH oxidase), DMTU (an H2O2 scavenger) and NADPH (a substrate of NADPH oxidase) under normal and LT conditions.
The DPI, DMTU and NADPH treatments did not have a significant effect on the H2O2 content, \({\text{O}}_{2}^{ - }\) production rate, transcription of anthocyanin biosynthesis genes (BsPAL, BsCHS, BsF3H and BsANS), and anthocyanin content in Begonia seedlings grown under normal temperature conditions (Fig. 2). In contrast, the production of ROS, gene transcription, and the anthocyanin content were significantly increased in the four groups of seedlings grown under LT conditions compared with the (CK + H2O)-seedlings.
Effects of nicotinamide adenine dinucleotide (NADPH, 0.05 mM), diphenyleneiodonium chloride (DPI, 0.01 mM) and N,N'-dimethylthiourea (DMTU, 5 mM) on hydrogen peroxide (H2O2) contents (a), superoxide anions \({\text{O}}_{2}^{-}\) production rate (b), the relative transcript levels of BsRBOHD (c), BsPAL (d), BsCHS (e), BsF3H (f) and BsANS (g), and anthocyanin contents (h) in leaves of Begonia semperflorens grew under normal (25/15°C) and low temperature (15/5°C). (1) 25/15°C, (2) 15/5°C. Data represent means ± SD of three independent replicates. Different letters indicate significant difference between treatments according to the Tukey’s test at P < 0.05.
Compared with the (LT + H2O)-seedlings, the DPI pre-treatment significantly decreased both the \({\text{O}}_{2}^{ - }\) production rate and H2O2 content to 77.02 and 68.16%, respectively, in the (LT + DPI)-seedlings (Figs. 2a, 2b). The (LT + DMTU)-seedlings showed similar H2O2 contents and \({\text{O}}_{2}^{ - }\) production rates compared with the (LT + DPI)-seedlings (Figs. 2a, 2b). These results suggest that both the DPI and DMTU pre-treatments were effective in our experiment. However, compared with the (LT + H2O)-seedlings, the H2O2 content and \({\text{O}}_{2}^{ - }\) production rate were significantly increased in the (LT + NADPH)-seedlings to 122.84 and 123.86%, respectively (Figs. 2a, 2b), suggesting that NADPH levels, a substrate of NADPH oxidase, may increase NADPH oxidase activity to promote increased ROS production. In addition, the anthocyanin content and the transcription of five genes showed similar changes with ROS production among the four groups of seedlings grown under LT conditions.
Effects of NADPH, DPI and DMTU on the Chlorophyll Content and JIP Test Index in B. semperflorens Grown under Normal and Low Temperature Conditions
As shown in table, the NADPH, DPI and DMTU pre-treatment s did not have a significant effect on the total chlorophyll content (Chl a + b) in B. semperflorens leaves under normal temperature conditions. However, the total chlorophyll content (Chl a + b) was significantly decreased by the LT treatment in the (LT + H2O)-, (LT + NADPH)-, (LT + DPI)-, and (LT + DMTU)-seedlings compared with the (CK + H2O)-seedlings. Moreover, the total chlorophyll (Chl a + b) content was significantly decreased in the (LT + NADPH)-seedlings but significantly increased in the (LT + DPI)- and (LT + DMTU)-seedlings compared with the (LT + H2O)-seedlings. The ratio of Chl a/b was not significantly affected among the four groups of seedling grown under normal temperature conditions, whereas this ratio was significantly decreased in all four groups of seedling grown under LT conditions compared with the (CK + H2O)-seedlings. Compared with the (LT + H2O)-seedlings, the Chl a/b ratio was significantly increased in the (LT + DPI)- and (LT + DMTU)-seedlings, whereas the (LT + NADPH)-seedlings showed significant decreases (Table 1).
On a per-unit excited leaf cross-section (CSm), no significant differences were observed in the ABS/CSm, DIo/CSm, TRo/CSm, RC/CSm, ETo/CSm, REo/CSm, TRo/ABS, ETo/TRo and REo/ETo values among the (CK + H2O)-, (CK + NADPH)-, (CK + DPI)- and (CK + DMTU)-seedlings (Fig. 3). Compared with the (CK + H2O)-seedlings, low temperature significantly decreased ABS/CSm, TRo/CSm, RC/CSm, ETo/CSm, REo/CSm, TRo/ABS and ETo/Tro, while significantly increased DIo/ABS and REo/ETo values in all the four groups of seedlings grew under low temperature (Fig. 3).
Effects of nicotinamide adenine dinucleotide (NADPH, 0.05 mM), diphenyleneiodonium chloride (DPI, 0.01 mM) and N,N'-dimethylthiourea (DMTU, 5 mM) on chlorophyll fluorescence parameters in leaves of Begonia semperflorens grew under normal (25/15°C) and low temperature (15/5°C). (1) 25/15°C, (2) 15/5°C. Data represent means ± SD of three independent replicates. Different letters indicate significant difference between treatments according to the Tukey’s test at P < 0.05.
Compared with the (LT + H2O)-seedlings, the (LT + NADPH)-seedlings showed significantly lower ABS/CSm, TRo/CSm, RC/CSm, ETo/CSm, REo/CSm and TRo/ABS values, similar DIo/CSm and ETo/TRo values, and higher REo/ETo values (Fig. 3). However, the (LT + DPI)- and (LT + DMTU)-seedlings showed higher ABS/CSm, TRo/CSm, RC/CSm, ETo/CSm, REo/CSm and TRo/ABS values, similar DIo/CSm and ETo/TRo values, and lower REo/ETo values (Fig. 3).
DISCUSSION
The results of our previous studies showed that LT can induce anthocyanin biosynthesis in B. semperflorens [4, 18]. In our present study, LT conditions significantly upregulated the transcription of four genes involved in anthocyanin biosynthesis within 5–9 h (Figs. 1b–1e). Moreover, these genes were upregulated by LT conditions in the sequence of the anthocyanin biosynthetic pathway and led to a significant accumulation of anthocyanin in B. semperflorens on the 2nd day after treatment (Fig. 1f). Remarkably, BsRBOHD transcription was significantly upregulated in B. semperflorens at the 3rd h after the LT treatment (Fig. 1a), 2 to 5 h earlier than the observed upregulation in the four anthocyanin-biosynthesis genes. The upregulation of RBOH is responsible for ROS production in plants under many biotic and abiotic stresses [14, 16]. ROS acts as a metabolic and biosynthetic signal [26] and is an important inducer in LT-induced anthocyanin biosynthesis in B. semperflorens [6]. Therefore, we inferred that B-sRBOHD may play a role in LT-induced anthocyanin biosynthesis in B. semperflorens.
We also investigated whether this LT-induced anthocyanin biosynthesis is dependent (fully or partly) on ROS produced via BsRBOHD. As shown in Figs. 2a, and 2b, under normal temperature, NADPH, DPI and DMTU pre-treatments did not have a significant effect on ROS production in the B. semperflorens at the 10th day. However, LT significantly increased BsRBOHD transcription (Figs. 1a, 2c) and ROS production (Figs. 2a, 2b) at the 10th day, whether with pre-treatment (NADPH, DPI or DMTU) or not. LT conditions may upregulate RBOH transcription in many plants, and this upregulation of RBOH transcription may be reversed, at least partially, by the application of DPI or DMTU [27]. In our present study, the upregulation of BsRBOHD transcription in the (LT + H2O)-seedlings was partially reversed by the DMTU and DPI pre-treatments (Fig. 2c), which directly led to a reversal of the increase of ROS levels (both H2O2 content and \({\text{O}}_{2}^{ - }\) production rate) in the (LT + DPI)- and (LT + DMTU)-seedlings (Figs. 2a, 2b). In contrast, as a substrate of NAPH oxidase, the 0.05 mM NADPH treatment further promoted ROS production in the (LT + NADPH)-seedlings compared with the (CK + H2O)-seedlings (Figs. 2a, 2b). ROS are important signals that induce anthocyanin accumulation by upregulating late biosynthesis genes and the corresponding regulatory genes [6, 7]. Subsequently, the increases in both transcription levels of four anthocyanin-biosynthesis genes and anthocyanin contents in the (LT + H2O)-seedlings were abolished in (LT + DMTU)- and (LT + DPI)-seedlings, while were strengthened further in the (LT + NADPH)-seedlings compared to the (LT + H2O)-seedlings (Figs. 2d–2h). The above results confirmed our hypothesis that ROS produced via BsRBOHD are involved in LT-induced anthocyanin biosynthesis in B. semperflorens.
A well-known method by which LT triggers anthocyanin biosynthesis is to inhibit photosynthesis and subsequently alter the distribution of light energy [4, 28]. Analyses of chlorophyll fluorescence and pigment contents represent powerful and widely used tools to analyse photosynthesis [25]. Therefore, we investigated changes in chlorophyll fluorescence in Begonia seedlings pre-treated with chemicals that scavenge H2O2 (DMTU), inhibit H2O2 production (DPI), and promoting H2O2 production (NADPH) to elucidate the mechanism underlying the production of ROS via the LT-induced anthocyanin biosynthesis mediated by BsRBOHD.
As shown in the table, the total chlorophyll content (Chl a + b) was significantly decreased in the (LT + H2O)-seedlings compared with the (CK + H2O)-seedlings, which caused a decrease in the ABS/CSm value (Fig. 3a). Because chlorophyll a is more sensitive to ROS than chlorophyll b, a significant decrease in ratio of chlorophyll a/b was observed in the (LT + H2O)-seedlings compared with the (CK + H2O)-seedlings (Table 1). Furthermore, since only some chlorophyll a acts as reaction centres, significant decreases in RC/CSm and TRo/CSm values, which depend on light-harvesting chlorophylls [29], were observed in the (LT + H2O)-seedlings compared with the (CK + H2O)-seedlings (Figs. 3c–3d). Based on the lower ABS/CSm value (Fig. 3a), the (LT + H2O)-seedlings showed higher DIo/CSm values than the (CK + H2O)-seedlings (Fig. 3b), suggesting that relative excess light energy was being produced in the (LT + H2O)-seedlings. Subsequently, the ETo/CSm and REo/CSm values decreased in the (LT + H2O)-seedlings compared with the (CK + H2O)-seedlings (Figs. 3e–3f). All of the above results suggested that LT conditions decreased the chlorophyll contents of seedlings to reduce the efficiency of light absorption, transmission and conversion for electron transport and photosynthesis while increasing the proportion of energy used for dissipation, resulting in overexcited light energy and a subsequent accumulation of ROS. Meanwhile, the (LT + H2O)-seedlings showed significantly lower TRo/ABS and ETo/TRo values than the (CK + H2O)-seedlings, but significantly higher than REo/ETo values (Figs. 3g–3i). Lower TRo/ABS values reflect the occurrence of photoinhibition and a reduction in the efficiency of trapped energy in PSII [25]. Lower ETo/TRo values indicate a reduction in the electron transfer capacity in the acceptor side of PSII [30], suggesting the overexcitation of PSII reaction centres, which may be a production site of ROS. Higher REo/ETo values suggests overflux in the electron transfer efficiency from \({\text{Q}}_{{\text{A}}}^{ - }\) to NADP+ [30], which may be another production site of ROS. Taken together, these results suggest that the combination of LT and moderate light results in an overaccumulation of ROS due to an overexcitation of PSII reaction centres and overflux from \({\text{Q}}_{{\text{A}}}^{ - }\) to NADP+, with the overaccumulation of ROS finally inducing anthocyanin biosynthesis in Begonia seedlings [6].
Compared with the (LT + H2O)-seedlings, ABS/CSm, TRo/CSm, RC/CSm, ETo/CSm and REo/CSm values were significantly decreased in the (LT + NADPH)-seedlings, whereas the (LT + DPI)- and (LT + DMTU)-seedlings showed significant increases (Figs. 3a, 3c–3f). These results suggest that the absorption, transmission and conversion of light energy were deteriorated by the NADPH pre-treatment but were alleviated by pre-treatment with DPI or DMTU. Similar results were also observed with respect to the TRo/ABS value among the (LT + H2O)-, (LT + NADPH)-, (LT + DPI)- and (LT + DMTU)-seedlings (Fig. 3g). Moreover, NADPH pre-treatment further promoted increased REo/ETo values, whereas decreased values were observed in seedlings pre-treated with DPI or DMTU (Fig. 3i). These results suggest that more ROS were produced in the (LT + NADPH)-seedlings than in (LT + H2O)-seedlings, whereas less ROS was produced in the (LT + DPI)- and (LT + DMTU)-seedlings (Figs. 2a–2b). Correspondingly, the (LT + NADPH)-seedlings showed increased anthocyanin-biosynthesis gene transcription and anthocyanin accumulation compared with the (LT + H2O)-seedlings, whereas the (LT + DPI)- and (LT + DMTU)-seedlings showed less (Figs. 2d–2h).
Taken together, the above results suggest that the ROS produced via BsRBOHD may be involved in LT‑induced anthocyanin biosynthesis in B. semperflorens by strengthening the overaccumulation of ROS produced by the overexcitation of PSII reaction centres and the overflux from \({\text{Q}}_{{\text{A}}}^{ - }\) to NADP+.
REFERENCES
Hughes, N.M., Winter leaf reddening in 'evergreen' species, New Phytol., 2011, vol. 190, p. 573.
Schaberg, P.G., Murakami, P.F., Butnor, J.R., and Hawley, G.J., Experimental branch cooling increases foliar sugar and anthocyanin concentrations in sugar maple at the end of the growing season, Can. J. Forest Res., 2017, vol. 47, p. 696.
Ensminger, I., Busch, F., and Huner, N.P.A., Photostasis and cold acclimation: sensing low temperature through photosynthesis, Physiol. Plant., 2010, vol. 126, p. 28.
Zhang, K.M., Li, Z., Li, Y., Li, Y.H., Kong, D.Z., and Wu, R.H., Carbohydrate accumulation may be the proximate trigger of anthocyanin biosynthesis under autumn conditions in Begonia semperflorens,Plant Biol., 2013, vol. 15, p. 991.
Choudhury, F.K., Rivero, R.M., Blumwald, E., and Mittler, R., Reactive oxygen species, abiotic stress and stress combination, Plant J., 2017, vol. 90, p. 856.
Qu, Y., Bai, X., Zhu, Y., Qi, R., Tian, G., Wang, Y., Li, Y., and Zhang, K., Reactive oxygen species acts as an important inducer in low-temperature-induced anthocyanin biosynthesis in Begonia semperflorens,J. Am. Soc. Hortic. Sci., 2018, vol. 143, p. 486.
Xu, Z. and Rothstein, S.J., ROS-induced anthocyanin production provides feedback protection by scavenging ROS and maintaining photosynthetic capacity in Arabidopsis, Plant Signal. Behav., 2018, vol. 13: e1451708.
Brandes, R.P., Weissmann, N., and Schröder, K., Nox family NADPH oxidases: molecular mechanisms of activation, Free Radic. Biol. Med., 2014, vol. 76, p. 208.
Suzuki, N., Miller, G., Morales, J., Shulaev, V., Torres, M.A., and Mittler, R., Respiratory burst oxidases: the engines of ros signaling, Curr. Opin. Plant Biol., 2011, vol. 14, p. 691.
Zhou, J., Xiao, X.J., Zhou, Y.H., Shi, K., Chen, Z., and Yu, J.Q., RBOH1-dependent H2O2 production and subsequent activation of MPK1/2 play an important role in acclimation-induced cross-tolerance in tomato, J. Exp. Bot., 2015, vol. 65, p. 595.
Hu, C.H., Wei, X.Y., Yuan, B., Yao, L.B., Ma, T.T., Zhang, P.P., Wang, X., Wang, P.Q., Liu, W.T., Li, W.Q., Meng, L.S., and Chen, K.M., Genome-wide identification and functional analysis of NADPH oxidase family genes in wheat during development and environmental stress responses, Front. Plant Sci., 2018, vol. 9: 906.
Monshausen, G.B., Bibikova, T.N., Weisenseel, M.H., and Gilroy, S., Ca2+ regulates reactive oxygen species production and pH during mechanosensing in Arabido-psis roots, Plant Cell, 2009, vol. 21, p. 2341.
Li, X.H., Zhang, H.J., Tian, L.M., Huang, L., Liu, S.X., Li, D.Y., and Song, F.M., Tomato SLRbohB, a member of the NADPH oxidase family, is required for disease resistance against Botrytis cinerea and tolerance to drought stress, Front. Plant Sci., 2015, vol. 6: 463.
Kadota, Y., Shirasu, K., and Zipfel, C., Regulation of the NADPH oxidase RBOHD during plant immunity, Plant Cell Physiol., 2015, vol. 56, p. 1472.
Noctor, G., Reichheld, J.P., and Foyer, C.H., ROS-related redox regulation and signaling in plants, Semin. Cell Dev. Biol., 2018, vol. 80, p. 3.
Zhou, J., Xu, X.C., Cao, J.J., Yin, L.L., Xia, X.J., Shi, K., Zhou, Y.H., Chen, Z., and Yu, J.Q., Heat shock factor HsfA1a is essential for R gene-mediated nematode resistance and triggers H2O2 production, Plant Physiol., 2018, vol. 176, p. 2456.
Grondin, A., Rodrigues, O., Verdoucq, L., Merlot, S., Leonhardt, N., and Maurel, C., Aquaporins contribute to ABA-triggered stomatal closure through OST1-mediated phosphorylation, Plant Cell, 2015, vol. 27, p. 1945.
Bi, H., Guo, M., Wang, J., Qu, Y., Du, W., and Zhang, K., Transcriptome analysis reveals anthocyanin acts as a protectant in Begonia semperflorens under low temperature, Acta Physiol. Plant., 2018, vol. 40, p. 10.
Wang, J., Guo, M., Li, Y., Wu, R., and Zhang, K., High-throughput transcriptome sequencing reveals the role of anthocyanin metabolism in Begonia semperflorens under high light stress, Photochem. Photobiol., 2018, vol. 94, p. 105.
Liao, Z., Chen, M., Guo, L., Gong, Y., Tang, F., Sun, X., and Tang, K., Rapid isolation of high-quality total RNA from taxus and ginkgo, Prep. Biochem. Biotec-h., 2004, vol. 34, p. 209.
Mita, S., Murano, N., Akaike, M., and Nakamura, K., Mutants of Arabidopsis thaliana with pleiotropic effects on the expression of the gene for β-amylase and on the accumulation of anthocyanin that are inducible by sugars, Plant J., 1997, vol. 11, p. 841.
Arnon, D.I., Copper enzymes in isolated chloroplasts. Polyphenoloxidases in Beta vulgaris,Plant Physiol., 1949, vol. 24, p. 1.
Brennan, T. and Frenkel, C., Involvement of hydrogen peroxide in the regulation of senescence in pear, Plant Physiol., 1977, vol. 59, p. 411.
Elstner, E.F. and Heupel, A., Inhibition of nitrite formation from hydroxylammoniumchloride: a simple assay for superoxide dismutase, Anal. Biochem., 1976, vol. 70, p. 616.
Pan, C., Ahammed, G.J., Li, X., and Shi, K., Elevated CO2 improves photosynthesis under high temperature by attenuating the functional limitations to energy fluxes, electron transport and redox homeostasis in tomato leaves, Front. Plant Sci., 2018, vol. 9: 1739.
Saxena, I., Srikanth, S., and Chen, Z., Cross talk between H2O2 and interacting signal molecules under plant stress response, Front. Plant Sci., 2016, vol. 7: 570.
Mei, Y., Chen, H., Shen, W., Shen, W., and Huang, L., Hydrogen peroxide is involved in hydrogen sulfide-induced lateral root formation in tomato seedlings, BMC Plant Biol., 2017, vol. 17, p. 162.
Tisarum, R., Theerawitaya, C., Samphumphuang, T., and Cha-um, S., Regulation of anthocyanin accumulation in rice (Oryza sativa L. subsp. indica) using MgSO4 spraying and low temperature, Arch. Agron. Soil Sci., 2018, vol. 64, p. 1663.
Parvanova, D., Popova, A., Zaharieva, I., Lambrev, P., Konstantinova, T., Taneva, S., Atanassov, A., Goltsev, V., and Djilianov, D., Low temperature tolerance of tobacco plants transformed to accumulate proline, fructans, or glycine betaine. Variable chlorophyll fluorescence evidence, Photosynthetica, 2004, vol. 42, p. 179.
Li, Y.T., Liang, Y., Li, Y.N., Che, X.K., Zhao, S.J., Zhang, Z.S., and Gao, H.Y., Mechanisms by which Bisphe-nol A affect the photosynthetic apparatus in cucumber (Cucumis sativus L.) leaves, Sci. Rep., 2018, vol. 8: 4253.
Funding
This research was supported by the Natural Science Foundation of Henan Province (project no. 182300410091) and the Key Scientific Research Project of High Education in Henan Province (project no. 18B220004).
Author information
Authors and Affiliations
Contributions
This information is published at the request of the authors to determine the contribution of each author to the study.
Corresponding author
Ethics declarations
This article does not contain any studies involving animals or human participants performed by any of the authors. The authors declare no conflicts of interest.
Additional information
Abbreviations: DMTU—N,N'-dimethylthiourea; DPI—diphenyleneiodonium chloride; LT—low temperature; RBOHs—plant respiratory burst oxidase homologues.
Supplementary material
Rights and permissions
About this article
Cite this article
Zhang, K.M., Tian, G., Li, X.H. et al. ROS Produced via BsRBOHD Plays an Important Role in Low Temperature-Induced Anthocyanin Biosynthesis in Begonia semperflorens. Russ J Plant Physiol 67, 250–258 (2020). https://doi.org/10.1134/S1021443720020181
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1021443720020181