Abstract
Organisms can produce heat shock proteins (HSPs) in response to elevated temperatures and other abiotic stresses. However, the function of HSPs, including small heat shock proteins (sHSPs), in stress tolerance is not fully explored. To improve our understanding of sHSPs, we isolated a gene of sHSPs from David lily (Lilium davidii Duchartre) called LimHSP16.45. Results reveal that LimHSP16.45 is a cytosolic class II sHSP. The HSP17.6II of Arabidopsis thaliana belongs to the HSP20 chaperone protein and is similar to Lim16.45HSP of David lily in terms of size and structure. Both genes have a small heat shock-like alpha-crystallin domain (ACD) structure. To further study the function of Lim16.45HSP, we overexpress it in Arabidopsis hsp17.6II mutant. Then, we detect the expression of LimHSP16.45 in transgenic plant under abiotic stresses and analyze the heat tolerance of transgenic plant. In addition, we measure the activity of three antioxidant enzymes (peroxidase, catalase and superoxide dismutase) and the content of soluble sugar and proline in transgenic plants under abiotic stresses.We found that transgenic plant is tolerant to heat and oxidative stresses given its increased survival rate relative to the hsp17.6II and wild type. Moreover, the content of soluble sugar and proline considerably increase in the transgenic plant. These results support the positive role of LimHSP16.45 in response to heat stress in plants. We suspect that LimHSP16.45 enhances plant tolerance to abiotic stresses by stimulating the activity of ROS-scavenging enzymes and other protective enzymes and increasing the synthesis of proline.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Plants live in complex and constantly changing environments, which are often adverse or detrimental to growth and development. Harsh environment factors include drought, salinity, high or low temperature, and toxins produced by chemicals [1]. The adverse effects of these abiotic stresses are increasingly aggravated because of climate change. The greenhouse effect is now generally accepted to be the main cause of global warming. High temperatures can cause serious damage, including disturbance of intracellular balance, slowing down or stagnation of plant growth and development, and even plant death [2]. When plants are exposed to rising temperatures, intracellular transcription and translation machinery could be reactivated, initiating a variety of protective mechanisms called heat response [3]. Many small heat shock protein (sHSP) family members are activated by increasing temperatures, and this activation shifts its physical structure from oligomeric forms into dimers, which then readily binds to misfolded proteins that expose parts of hydrophobic plaques [4–6]. The primary function of these sHSPs is to act as a molecular chaperone to prevent the misfolding of proteins and help restore their spatial structure in the cell [7]. Under heat stress, the cytoplasmic level of sHSPs rapidly increases, and heat-activated sHSPs bind to misfolded substrates in an ATP-independent manner to form compounds containing misfolded proteins and sHSPs [8]. In addition, the heat shock protein (HSP) genes of plants can respond to other abiotic stresses, such as cold, salinity, and drought, as well as some signaling molecules, such as abscisic acid, salicylic acid, and hydrogen peroxide (H2O2) [9–11].
sHSPs protecting irreversible protein aggregation are widely distributed in eukaryotes and prokaryotes [6]. According to molecular weight, the HSPs of plants can be divided into six groups: HSP100s, HSP90s, HSP70s, HSP60s, sHSPs (molecular weight MW of 12–40 kD), and HSP40 (DNAJ family) [12, 13]. They mainly play the role of molecular chaperone in the body, helping the normal folding of functional proteins. The number of genes encoding sHSPs is higher in multicellular eukaryotes than in prokaryotes, accounting for 19 in Arabidopsis thaliana [14]. sHSPs belong to the superfamily of chaperones and contain a conserved carboxyl terminus of 90 amino acids called α lens domain [15]. sHSPs are expressed in all three kingdoms and viruses [16, 17]; in vitro, sHSPs bind to partially folded proteins under the action of ATP, preventing their irreversible aggregation. In the presence of sHSPs, the inactive substrate proteins can be refolded, and the substrates can be reactivated under HSP70/DnaK (in some cases involving HSP100/ClpB and GroEL) [18, 19]. When sHSPs function, their oligomers can dissociate into dimers and bind to substrates through their un-conserved amino acid terminal and conserved α lens domains [20, 21]. sHSPs not only act as molecular chaperones but also regulate the fluidity and composition of cell membranes [22]. In different model organisms in related studies, such as Drosophila melanogaster and Caenorhabditis elegans, the loss of several special sHSPs leads to a decrease in life span, whereas the overexpression of sHSPs increase the life span of organisms [23–25]. Therefore, a high level of sHSPs generally has a stabilizing effect on the protein stabilization system.
The temperature increase around an organism causes proteins to collapse, twist, and nonspecifically agglomerate. In addition, other abiotic stresses, such as oxidative stress, heavy metals, ethanol, and toxic materials, can cause the production of nonspecific proteins [17]. In response to oxidative stress, these transcription factors interact with cis-acting elements on the initiation of genes encoding antioxidants and non-enzymatic antioxidants; thus, they perform biological functions. Among the various antioxidant enzymes, ascorbate peroxidase (APX) and catalase (CAT) play the most important roles in scavenging the ROS produced by plants under heat stress. In response to heat stress, the endogenous Ca2+ can regulate multiple signaling pathways [26]. Oxidative stress is the second stress of heat, which is marked by the production of large amounts of reactive oxygen species (ROSs) [11]. The accumulation of hydrogen peroxide in plants is a rapid process [27], and this signal is transmitted by histidine kinases and heat shock transcription factors. HsfA4a is the signal receptor of H2O2 and plays a negative regulatory role in this signaling pathway [28]. When ROS signal attenuation occurs, HsfA5 and HsfA4 form of heterooligomers to inhibit the binding ability of DNA to the activated HasfA4 trimer. Heat shock transcription factors transmit ROS signals to downstream transcription factors through the MAPK signaling pathway. These downstream transcription factors mainly include the Zat family, WRKY transcription factor gene family, multi-protein bridging factor MBF1, and NADH oxidase (Rboh). The Zat family can respond to various stresses, including heat stress, to which Zat7, Zat10, and Zat12 are the most sensitive [29]. Under stress conditions, Rboh enhances an ROS signal by oxidizing NADH and keeps this signal active for a long time. The MAPK signaling pathway can activate the downstream transcription factors related to redox.
As the only sweet lily in China, David lily can adapt to drought and high temperatures in the northwest region of China. Many sHSPs in David lily are assumed to play an important role in the survival of these plants against high temperature stress. The hsp17.6II gene of Arabidopsis thaliana is located in chromosome 5, and the HSP17.6II protein is known as the second family of 17.6 kDa HSP, which belongs to the HSP20 chaperone protein. HSP17.6II is similar to Lim16.45HSP of David lily in terms of size and structure. Both genes also present a small heat shock-like alpha-crystallin domain (ACD) structure. Their amino acid sequences are compared in http://simgene.com/ ClustalW, and the result in GeneDoc software is shown in Fig. 1.
LimHSP16.45 was transformed into A. thaliana mutant hsp17.6II in this study to further understand the effects of heat stress on plant growth and development. Then, the homozygous lines that can be inherited stably were obtained. The direct heat tolerance of the transformed daughter plants was enhanced, and the acquired heat tolerance was observed. Changes in plant cell biology and physiology characteristics under high temperature stress were explored, and the related phenotype and tolerance indexes of heat-tolerant plants were investigated to broaden the understanding of the family of sHSPs. The evolutionary conservatism of sHSPs helped further realize their importance for normal growth, development, and reproduction.
MATERIALS AND METHODS
Ethics statement. David lily (Lilium davidii Duchartre) was planted in the field of Lanzhou University, and permission was obtained from the Lanzhou University for the study.
Generation of constructs and transformation of A-rabidopsis. The cauliflower mosaic virus 35S promoter, the cDNA of LimHSP16.45, and a GFP sequence were inserted into the pBI101.2 binary vector to produce 35S–LimHSP16.45–GFP fusions. The construct was introduced into the Agrobacteriumtum efaciens strain GV3101 and subsequently transformed into hsp17.6II by the floral dip method. As the control, an empty vector was inserted into hsp17.6II. Expression was then monitored in the leaves, roots, stems, and anthers.
Total RNA isolation and RT-PCR analysis. Total RNA was obtained from two-week-old seedling tissues using an RNA isolation kit (TaKaRa). Samples of each tissue type (1 µg) were digested with RNase-free DNase I (TaKaRa) for reverse transcription (RT) with M-MLV reverse transcriptase (Invitrogen, CA, United States). After a 1 : 10 dilution was made, 1 µL of the synthesized cDNA was used for RT-PCR (forward primer 5′‑GGATTCGAAGTTCGAAGTG-3′ and reverse primer 5′-ATCTCAATGGCCTTTGGCTC-3′). Afterward, gels were run to assay for expression by each independent transgenic line, and 18S rRNA was run as control.
Arabidopsis growing conditions, stress treatments, and biophysical analysis of transgenic lines.Arabidopsis seedlings were grown on a full-strength Murashige and Skoog (MS; pH 5.8) supplemented with 1% (w/v) Suc and 1 × Gamborg’s vitamins. Seeds were first surface sterilized with a 20% (v/v) bleach solution, washed thoroughly with sterile water, and then placed on MS plates solidified with 1.0% agar. After incubation at 4°C for 2 days in darkness, the seedlings were oriented vertically for growth under a 16 h photo period at 22°C. Approximately 50 surface-sterilized seeds each from wild type (WT) plants and transgenics (T3 generation) were placed on triplicate plates to evaluate the stress tolerance of transgenic Arabidopsis, which constitutively expresses LimHSP16.45–GFP during germination. The MS media were supplemented with or without NaCl, mannitol, or H2O2. The seeds were incubated at 4°C for 2 days before placement at 22°C under a 16 h photo period. Germination rates or the extent of root elongation were scored daily for 7–14 days. Approximately 50 surface-sterilized seeds each from WT or transgenic (T3) plants were incubated at 4°C for 2 days and then exposed to 45°C for 1 to 2 h before placing them on an MS medium to induce high-temperature stress. Germination rates were scored daily for 7 days, and each experiment was performed at least thrice.
Microscopy and image analysis. Fluorescent specimens were observed with a confocal microscope (Zeiss LSM 510 META laser-scanning fluorescence microscope) equipped with an epifluorescence UV light filter set. A 488 nm excitation level and a BP 505-530 filter were used to detect GFP.
Preparation of crude extract and assays of enzymatic activity. Fresh leaves (0.2 g) from Arabidopsis were ground to fine powder in liquid nitrogen and suspended in cold 0.2 M phosphate buffer (pH 8.0) containing 1 mM dithiothreitol and 5 mM ethylenediaminetetraacetic acid (EDTA). After the lysate was centrifuged (16 000 g, 15 min, 4°C), the supernatant was recovered and kept on ice. The assay for the superoxide dismutase (SOD) activity was performed according to the methods of Beyer and Fridovich. A total of 2 mM lactochrome was added to start the reaction using a 3 mL reaction mixture comprising 50 mM phosphate buffer (pH 7.0), 0.1 mM EDTA, 13 mM methionine, 75 mM NBT, and 50 μL of lysate. Absorbance was measured at 560 nm. Activity by ascorbate peroxidase (APX) was determined following the H2O2-dependent oxidation of ascorbic acid (ASC) at 290 nm in a 3 mL reaction mixture comprising 0.3 mM ASC, 0.1 mM H2O2, 50 mM phosphate buffer (pH 7.8), and 50 μL of lysate. The assay for catalase (CAT) activity followed the protocol of Beaumont et al. (1990) and involved monitoring the dismutation of H2O2 at 240 nm in a 3 mL reaction mixture containing 50 mM phosphate buffer (pH 7.0), 10 mM H2O2, and 50 μL of lysate.
RESULTS
LimHSP16.45–GFP Is Constitutively Expressed in Transgenic hsp17.6II Mutant and Is Localized to the Cell Membrane and Endomembrane System
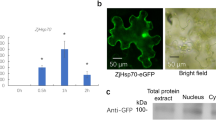
Driven by the 35S promoter (Fig. 2a), a vector for the overexpressed LimHSP16.45–GFP fusion protein was constructed. After the transformation of the construct into the hsp17.6II and following the expression of fusion protein, several independent transgenic lines for LimHSP16.45–GFP overexpression were obtained. The result of RT-PCR showed that the lines 2 and 3 had extremely high expressions of LimHSP16.45 (Fig. 2b). LimHSP16.45–GFP was constitutively expressed in protoplasts or plants. Intracellularly, LimHSP16.45 was localized to the membrane and endomembrane system (Figs. 2c–2e, 2h). Expression was larger in the stomatal guard cells than in other epidermic cells of the leaves from transgenic Arabidopsis (Fig. 2e), suggesting that LimHSP16.45 has a possible role in stomatal regulation.
Vector construction and localization of overexpressed LimHSP16.45–GFP in hsp17.6II. (a) Structure of overexpressed LimHSP16.45–GFP fusion protein; (b) RT-PCR assay of LimHSP16.45–GFP in wild type, transgenic lines 2 and 3 of Li-mHSP16.45 hsp17.6II, and hsp17.6II; (c, e, and h) 18S rRNA was loaded as control. LimHSP16.45–GFP was localized to the membrane and (d and e) endomembrane system; (f, g) right field of (c) and (d). Scale bars equal to 5 µm for (c, d, f, g) and 20 µm for (e, h).
Expression of LimHSP16.45–GFP Is Induced by Abiotic Stress, and Heat Shock Granules (HSGs) Are Formed under Heat, Salinity, or Osmotic Stress
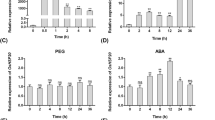
The expression of LimHSP16.45 in David lily significantly increased when the plants were exposed to either low or high temperatures, high salt, or oxidative stress. Compared with that of the untreated controls, the fluorescence intensity of LimHSP16.45–GFP in transgenic Arabidopsis was found to be enhanced when seedlings were treated at 45°C or exposed for 7 days to 150 mM NaCl or 200 mM mannitol (Fig. 3). In addition, HSGs were produced in transgenic lines in response to heat or high salt (Fig. 3, white arrow). We speculated that LimHSP16.45 forms oligomers with physiological functions under temperature, salinity, or osmotic stress.
LimHSP16.45–GFP expression profiles in abiotic-stressed Arabidopsis. Fluorescence intensity was increased over untreated control when seedlings were exposed to the temperature of 45°C for 30 min, 45 min, and 1 h; 150 mM NaCl or 200 mM mannitol for 7 days can also increase the expression of LimHSP16.45–GFP in the leaves of LimHSP16.45 hsp17.6II. HSGs were formed in response to heat, salt, or osmosis (indicated by white arrow).
Heterologous Expression of LimHSP16.45–GFP Improves the Viability of Arabidopsis Cells under High Temperature Stress
In David lily, LimHSP16.45 is highly expressed during the late zygotene to pachytene stages of meiotic prophase I in the pollen mother and tapetal cells, and its expression can be induced by heat or cold. Seed germination for hsp17.6II, WT, and transgenic lines 2 and 3 of LimHSP16.45 hsp17.6II, which overexpressed LimHSP16.45–GFP, was monitored to evaluate possible in vivo functioning in heat-stressed transgenic Arabidopsis. Seeds were treated at 45°C for 45 min (Fig. 4e). The results showed that compared with WT (Fig. 4b), hsp17.6II exhibits a distinct defect in response to high temperature stress (Fig. 4a). Meanwhile, overexpressing LimHSP16.45 can complement this defect in response to high temperatures (Figs. 4c, 4d). These data demonstrated that the transgenic lines had larger cell viability than the control and that the overexpression of LimHSP16.45 exerts an important influence on plant response to high temperature stress.
Overexpression of LimHSP16.45–GFP Recovers the Early Flowering Phenotype of hsp17.6II
The responses of Arabidopsis plants to warm temperature include hypocotyl and petiole elongation, leaf hyponasty, and early flowering. Warm temperature promotes auxin accumulation and activates the gibberellin (GA) and brassinosteroids (BRs) pathway, resulting in hypocotyl elongation. Therefore, early flowering is one of the most typical phenotypes for HSP mutants. In accordance with this conclusion, hsp17.6II had an earlier flowering phenotype (Fig. 5d) relative to WT (Fig. 5a).
Meanwhile, the expression of LimHSP16.45 could complement the function of hsp17.6II similar to that observed in the transgenic lines 2 and 3 of LimHSP16.45 hsp17.6II (Figs. 5b, 5c), which lost the early flowering phenotype. Together with the results of Fig. 4, these data demonstrate that LimHSP16.45 of David lily has a similar function to HSP17.6II of Ar-abidopsis and that both of them belong to the same protein family. This finding was supported by the result of the sequence comparison of LimHSP16.45 and HSP17.6II (Fig. 1).
Heterologous Expression of LimHSP16.45–GFP Decreases the Concentration of H2O2 through the Protection of the Enzymatic Activities of SOD, POD, and CAT
Study has shown that plant damage under heat stress is induced by oxidative stress and that the heterologous expression of LimHSP16.45–GFP enhances the viability of Arabidopsis cells under oxidative stress. Therefore, the concentration of H2O2 of WT, HSP, and transgenic lines 2 and 3 of LimHSP16.45 hsp17.6II were tested. The results showed that the heterologous expression of LimHSP16.45–GFP can decrease the concentration of H2O2 (Fig. 6a). In plants, various abiotic stresses lead to the overproduction of ROSs. These toxic ROSs can damage proteins, lipids, carbohydrates, and DNA, ultimately resulting in oxidative stress. As part of their antioxidant machinery, plants possess an efficient system of enzymes that work jointly to control the cascades of uncontrolled oxidation, scavenge for ROSs, and protect cells from oxidative damage. Herein, enzymatic activities were monitored, and POD, SOD and CAT levels were found to be higher in the transgenic lines 2 and 3 of LimHSP16.45 hsp17.6II than in the HSP after high temperature stress (Figs. 6b–6d). Therefore, some types of relationships were observed between the overexpression of LimHSP16.45 and the stimulation of the activity of ROS-scavenging enzymes.
Overexpression of LimHSP16.45 decreased the concentration of active oxygen and protected the enzyme activities in response to high temperature stress in hsp17.6II. The H2O2 content of hsp17.6II was higher than that of wild type and transgenic lines 2 and 3 of LimHSP16.45 hsp17.6II (a); the SOD (b), POD (c), and CAT (d) enzyme activities were higher in wild type and transgenic lines 2 and 3 of LimHSP16.45 hsp17.6II than in hsp17.6II with or without heat stress application (b). (1) wild type; (2) hsp17.6II; (3) LimHSP16.45hsp17.6II 2; (4) LimHSP16.45 hsp17.6II 3. Data presented are the mean ± SE of three independent experiments. Asterisks indicate statistical significance, *P < 0.05, **P < 0.01 (Student’s t-test).
LimHSP16.45 Increases the Concentration of Soluble Sugar and Proline and Decreases the Concentration of MDA and Relative Electrical Conductivity, Indicating the Expression of LimHSP16.45 under Heat Stress
Under abiotic stress conditions, soluble sugar and proline are the most important osmotic adjustment materials, and their concentrations are increased to avoid the loss of water and the breaking of the metabolic balance of cells under stress conditions. Therefore, the amount of soluble sugar and proline of WT, hsp17.6II, and transgenic lines 2 and 3 of LimHSP16.45 hsp17.6II were tested. The result showed that the heterologous expression of Li-mHSP16.45–GFP can increase the contents of soluble sugar and proline under heat stress (Figs. 7a, 7b). In plant cells, MDA is the marker of the degree of lipid peroxidation. Relative to hsp17.6II, the heterologous expression of LimHSP16.45–GFP can decrease the level of MDA. In this study, the level of MDA in the transgenic lines 2 and 3 of LimHSP16.45 hsp17.6II was even lower than that of WT (Fig. 7c). The increase in conductivity could reflect the damage of lipase under stress, which causes changes in membrane lipid status and leakage of electrolytes. The current results showed that the heterologous expression of LimHSP16.45 can decrease the relative electrical conductivity under stress conditions (Fig. 7d) and, consequently, the leakage rate of electrolytes in plants.
Overexpression of LimHSP16.45 reduced the damage caused by heat stress in hsp17.6II. The contents of proline (a) and soluble sugar (b) were higher in wild type and transgenic lines 2 and 3 of LimHSP16.45 hsp17.6II than in hsp17.6II. The MDA (c) and relative electrical conductivity (d) were lower in wild type and transgenic lines 2 and 3 of LimHSP16.45 hsp17.6II than in hsp17.6II. (1) wild type; (2) hsp17.6II; (3) LimHSP16.45 hsp17.6II 2; (4) LimHSP16.45 hsp17.6II 3. Data presented are the mean ± SE of three independent experiments. Asterisks indicate statistical significance, * P < 0.05, ** P < 0.01 (Student’s t-test).
DISCUSSION
Bioinformatics analysis showed that the amino acid sequence similarity of David lily sHSP LimHS-P16.45 and Arabidopsis sHSP HSP17.6II reached over 60%, indicating their homology (Fig. 1). In previous studies, we cloned a gene for a sHSP from the David lily named LimHSP16.45 based on its protein molecular weight. Its expression was induced by many kinds of abiotic stresses in both the lily and transgenic plants of Arabidopsis. Heterologous expression enhanced cell viability of the latter under high temperatures, high salt, and oxidative stress, and heat shock granules (HSGs) formed under heat or salinity treatment [30]. To further explore the role of LimHSP16.45 in stress response, we constructed overexpressed David lily LimHSP16.45 transgenic lines in the background of hsp17.6II.
Gene hsp17.6 encodes a cytosolic small heat shock protein with chaperone activity that is induced by heat and osmotic stress. Through heat resistance experiments under heat treatment conditions, the seedlings of hsp17.6II mutant were less tolerant to heat than the WT plant. However, the transgenic of LimHSP16.45 can reverse the weakness of hsp17.6II and show enhanced acquired heat tolerance function (Fig. 4). In addition, under the same growth conditions, the phenotype of the early flowering of transgenic lines relative to hsp17.6II disappeared. These results indicated that the heterogeneous expression of the David lily LimHSP16.45 gene could restore the missing function of the hsp17.6II. The results also completely proved the functional similarity of the two homologous proteins.
sHSPs mainly act as molecular chaperones to perform biological functions; their expression levels rapidly increase in the presence of abiotic stress, and their resistance to stress in various plants has been reported [6]. In this research, David lily sHSP LimHSP16.45 was determined to be mainly located in the cytoplasm and belonged to an sHSP of the cytoplasmic family. The changes in its expression and localization under heat stress and other abiotic stresses were further analyzed. With the increasing high-temperature treatment time and concentration, the fluorescence intensity in the cytoplasm increased, and the localization range expanded. The heat shock particles accumulated in the cytoplasm under high temperature, sodium chloride, mannitol, and hydrogen peroxide possibly due to the increased expression of HSPs under stress. All these results demonstrated that LimHSP16.45 plays an important role in plant responses to various abiotic stresses.
Heat stress produces excessive ROSs, which oxidize proteins, lipids, and nucleic acids, thus damaging cell growth and ultimately affecting plant growth and development. The transgenic plant showed lower concentration of ROSs under heat stress than hsp17.6II and WT. Three protective enzyme (SOD, POD, and CAT) activities were detected and showed an increase in the transgenic plant (Fig. 6). This finding indicates that LimHSP16.45 could enhance the ability of plants to resist oxidative stress by regulating the balance of ROSs in vivo and the activity of oxidase scavengers. Interestingly, the activity of antioxidant enzymes and the content of proline are increasing with or without heat stress in transgenic plants. The most possible reason is that the transgenic lines used 35S promoter instead of the promoter of LimHSP16.45 itself. This phenomenon also reflects the homology of sHSPs in plants. Next, we will consider to verify this phenomenon with its own promoter. The heat tolerance of plants was achieved by increasing the synthesis of proline in the body, and the increase in the content of soluble sugar and proline was found to be involved in the osmotic regulation in the body (Fig. 7). In addition, the transgenic plant showed low MDA content and electrical leakage rate under heat stress, indicating that LimHSP16.45 protein can improve plant resistance to stress by protecting the integrity of the cell membrane under heat stress.
In conclusion, the overexpression of LimHSP16.45 in the background of hsp17.6II can endow plants with high tolerance to heat and antioxidant stress. This research can help us understand the molecular mechanism of heat stress. The discovery of HSPs in plants of different species indicates the universality of HSPs, and the complementary effects of the genetic transformation between different species indicate the similarity in their functions. The significance of the current study is to reveal the stress resistance mechanism of David lily at the molecular level by understanding the biological function of sHSPs in the resistance to stress of David lily and by establishing a theoretical foundation for improving the quality of David lily through the genetic transformation of sHSPs.
REFERENCES
Zhu, J.K., Salt and drought stress signal transduction in plants, Annu. Rev. Plant Biol., 2002, vol. 53, p. 247.
Kotak, S., Larkindale, J., Lee, U., von Koskull-Doring, P., Vierling, E., and Scharf, K.D., Complexity of the heat stress response in plants, Curr. Opin. Plant B-iol., 2007, vol. 10, p. 310.
Lindquist, S., The heat-shock response, Annu. Rev. Biochem., 2007, vol. 55, p. 1151.
Cheng, G., Basha, E., Wysocki, V.H., and Vierling, E., Insights into small heat shock protein and substrate structure during chaperone action derived from hydrogen/deuterium exchange and mass spectrometry, J. B-iol. Chem., 2008, vol. 283, p. 26634.
Stengel, F., Baldwin, A.J., Painter, A.J., Jaya, N., Basha, E., Kay, L.E., Vierling, E., Robinson, C.V., and Benesch, J.L., Quaternary dynamics and plasticity underlie small heat shock protein chaperone function, Proc. Natl. Acad. Sci. USA, 2010, vol. 107, p. 2007.
Basha, E., Jones, C., Blackwell, A.E., Cheng, G., Waters, E.R., Samsel, K.A., Siddique, M., Pett, V., Wysocki, V., and Vierling, E., An unusual dimeric small heat shock protein provides insight into the mechanism of this class of chaperones, J. Mol. Biol., 2013, vol. 425, p. 1683.
Charng, Y.Y., Liu, H.C., Liu, N.Y., Hsu, F.C., and Ko, S.S., Arabidopsis Hsa32, a novel heat shock protein, is essential for acquired thermotolerance during long recovery after acclimation, Plant Physiol., 2006, vol. 14, p. 1297.
Zwirowski, S., Klosowska, A., Obuchowski, I., Nillegoda, N.B., Piróg, A., Zieztkiewicz, S., Bukau, B., Mogk, A., and Liberek, K., Hsp70 displaces small heat shock proteins from aggregates to initiate protein refolding, EMBO J., 2017, vol. 36, p. 783.
Rampino, P., Mita, G., Assab, E., de Pascali, M., Giangrande, E., Treglia, A.S., and Perrotta, C., Two sunflower 17.6HSP genes, arranged in tandem and highly homologous, are induced differently by various elicitors, Plant Biol., 2009, vol. 12, p. 13.
Sun, W., Bernard, C., van de Cotte, B., van Montagu, M., and Verbruggen, N., At-HSP17.6A, encoding a small heat-shock protein in Arabidopsis, can enhance osmotolerance upon overexpression, Plant J., 2001, vol. 27, p. 407.
Volkov, R.A., Panchuk, I.I., Mullineaux, P.M., and Schoffl, F., Heat stress-induced H2O2 is required for effective expression of heat shock genes in Arabidopsis,Plant Mol. Biol., 2006, vol. 61, p. 733.
Sanmiya, K., Suzuki, K., Egawa, Y., and Shono, M., Mitochondrial small heat-shock protein enhances thermotolerance in tobacco plants, FEBS Lett., 2004, vol. 557, p. 265.
Qiu, X.B., Shao, Y.M., Miao, S., and Wang, L., The diversity of the DnaJ/Hs40 family, the crucial partners for Hsp70 chaperones, Cell. Mol. Life Sci., 2006, vol. 63, p. 2560.
Haslbeck, M. and Vierling, E., A first line of stress defense: small heat shock proteins and their function in protein homeostasis, J. Mol. Biol., 2015, vol. 427, p. 1537.
Nakamoto, H. and Vígh, L., The small heat shock proteins and their clients, Cell. Mol. Life Sci., 2007, vol. 64, p. 294.
Eyles, S.J. and Gierasch, L.M., Nature’s molecular sponges: small heat shock proteins grow into their chaperone roles, Proc. Natl. Acad. Sci. USA, 2010, vol. 107, p. 2727.
Richter, K., Haslbeck, M., and Buchner, J., The heat shock response: life on the verge of death, Mol. Cell, 2010, vol. 40, p. 253.
Friedrich, K.L., Giese, K.C., Buan, N.R., and Vierling, E., Interactions between small heat shock protein subunits and substrate in small heat shock protein–substrate complexes, J. Biol. Chem., 2004, vol. 279, p. 1080.
Basha, E., Lee, G.J., Breci, L.A., Hausrath, A.C., Buan, N.R., Giese, K.C., and Vierling, E., The identity of proteins associated with a small heat shock protein during heat stress in vivo indicates that these chaperones protect a wide range of cellular functions, J. Biol. Chem., 2004, vol. 279, p. 7566.
Giese, K.C., Basha, E., Catague, B.Y., and Vierling, E., Evidence for an essential function of the N terminus of a small heat shock protein in vivo, independent of in vitro chaperone activity, Proc. Natl. Acad. Sci. USA, 2005, vol. 102, p. 18896.
Basha, E., Friedrich, K.L., and Vierling, E., The N‑terminal arm of small heat shock proteins is important for both chaperone activity and substrate specificity, J. Biol. Chem., 2006, vol. 281, p. 39943.
Balogi, Z., Török, Z., Balogh, G., Jósvay, K., Shigapova, N., Vierling, E., Vígh, L., and Horváth, I., “Heat shock lipid” in cyanobacteria during heat/light-acclimation, Arch. Biochem. Biophys., 2005, vol. 436, p. 346.
Morrow, G. and Tanguay, R.M., Drosophila melanogaster Hs22: a mitochondrial small heat shock protein influencing the aging process, Front. Genet., 2015, vol. 6: 1026.
Fleckenstein, T., Kastenmuller, A., Stein, M.L., Peters, C., Daake, M., and Krause, M., The chaperone activity of the developmental small heat shock protein Sip1 is regulated by pH-dependent conformational changes, Mol. Cell, 2015, vol. 58, p. 1067.
Vos, M.J., Carra, S., Kanon, B., Bosveld, F., Klauke, K., Sibon, O.C., and Kampinga, H.H., Specific protein homeostatic functions of small heat-shock proteins increase lifespan, Aging Cell, 2015, vol. 15, p. 217.
Reddy, A.S., Ali, G.S., Celesnik, H., and Day, I.S., Coping with stresses: roles of calcium- and calcium/calmodulin-regulated gene expression, Plant Cell, 2011, vol. 23, p. 2010.
Mittler, R., Oxidative stress, antioxidants and stress tolerance, Trends Plant Sci., 2002, vol. 7, p. 1360.
Qu, A.L., Ding, Y.F., Jiang, Q., and Zhu, C., Molecular mechanisms of the plant heat stress response, Biochem. Biophys. Res. Commun., 2013, vol. 432, p. 203.
Rizhsky, L., Davletova, S., Liang, H., and Mittler, R., The zinc finger protein Zat12 is required for cytosolic ascorbate peroxidase 1 expression during oxidative stress in Arabidopsis,J. Biol. Chem., 2004, vol. 279, p. 11736.
Mu, C., Zhang, S., Yu, G., Chen, N., Li, X., and Liu, H., Overexpression of small heat shock protein LimHSP16.45 in Arabidopsis enhances tolerance to abiotic stresses, PLoS One, 2013, vol. 8: e82264. https://doi.org/10.1371/journal.pone.0082264
ACKNOWLEDGMENTS
Thanks to Ministry of Education Key Laboratory of Cell Activities and Stress Adaptations, School of Life Sciences, Lanzhou University.
Funding
This work was financially supported by grants from the National Natural Science Foundation of China (project nos. 31770326 and 31300229), and Fundamental Research Funds for the Central Universities (project no. lzujbky-2017-149).
Author information
Authors and Affiliations
Contributions
Changjun Mu, Ruizhen Yang, and Xiaofeng Li designed the experiments; Ruizhen Yang and Guanzhong Yu performed most of the experiments; Haojie Li interpreted data and generated figures; Changjun Mu and Ruizhen Yang wrote the manuscript.
Corresponding author
Ethics declarations
The authors declare that they have no conflict of interest. This article does not contain any studies involving animals or human participants as objects of research.
Additional information
Abbreviations: ACD—alpha-crystallin domain; APX—ascorbate peroxidase; CAT—catalase; HSGs—heat shock granules; HSP—heat shock protein; POD—peroxidase; ROSs—reactive oxygen species; sHSP—small heat shock protein; SOD—superoxide dismutase; WT—wild type.
Supplementary material
Rights and permissions
About this article
Cite this article
Yang, R., Yu, G., Li, H. et al. Overexpression of Small Heat Shock Protein LimHSP16.45 in Arabidopsis hsp17.6II Mutant Enhances Tolerance to Abiotic Stresses. Russ J Plant Physiol 67, 231–241 (2020). https://doi.org/10.1134/S102144372002017X
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S102144372002017X