Abstract
Mutant strains of the filamentous cyanobacterium Anabaena strain PCC 7120 ΔHup (dc-Q193S and dc-R284H) with amino acid substitutions located in the vicinity of the FeMo cofactor of nitrogenase possess nitrogenase activity with a hydrogen production rate of approximately 18 μmol Н2/(mg h), which is ~30% lower than that of the parental strain ΔHup. The photosynthetic activity of mutants is also reduced. Unlike the parental strain ΔHup, the dc-Q193S mutant shows a lower temperature optimum for hydrogen photoproduction. This difference is probably due to the lowered filament strength (fragmentation). Hydrogen photoproduction in mutants does not significantly differ from that of the parental strain in relation to the activation energy, light saturation constants (41–62 μmol quanta /(m2 s)), and acetylene-induced inhibition. However, in contrast to the parental strain, hydrogen photoproduction in the mutant strains is not inhibited by molecular nitrogen, i.e., amino acid substitutions cause significant changes in the reaction requiring eight electrons (N2 fixation). The possibility to use nitrogen or atmospheric air instead of argon in the hydrogen production is promising from the practical point of view, though reduced activity and increased fragility of filaments in the studied mutants limit the possibility of their practical use.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
The sustainable development of mankind requires energy. The traditional use of fossil energy sources results in environmental contamination, increase in the carbon dioxide content in the atmosphere, and increase in the average temperature of the Earth surface. This fact determines the orientation toward the use of alternative energy sources, such as solar energy. One of the methods for its accumulation is the bioproduction of hydrogen. Hydrogen represents an ecologically clean energy carrier, since its oxidation product is water.
Photobiological production of hydrogen by cyanobacteria is considered as an ideal process for renewable energy production due to the high availability of water (electron donor) and light (energy source) [1–3]. Heterocystous cyanobacteria represent the most studied group of these bacteria in relation to both physiology and molecular biology. Hydrogen photoproduction in these bacteria is catalyzed by nitrogenase and/or Hox hydrogenase; both enzymes are oxygen-sensitive. In contrast to hydrogenase, nitrogenase catalyzes unidirectional H2 production that is considered to have a greater potential.
Among potential hydrogen producers, Anabaena and Nostoc are the most studied species. In addition to the species-specific characteristics of cyanobacteria, hydrogen photoproduction by nitrogenase can be influenced by other factors, such as the medium composition (especially fixed nitrogen sources), pH, gaseous phase composition, temperature, light intensity, and other process conditions [4–6]. During evaluation of the prospects of hydrogen photoproduction by cyanobacteria, one should take into account such characteristics as the process rate and duration as well as the efficiency of the light energy transformation. A high efficiency of light energy conversion (2.5%) was achieved under low illumination conditions for cyanobacteria immobilized in thin Ca2+-alginate gel films [7]. Long-term (several months) hydrogen photoproduction was demonstrated under aerobic conditions and periodical (outdoor) illumination [8]. However, it is almost impossible to simultaneously achieve the maximum rate, duration, and efficiency of hydrogen photoproduction under the same conditions. In the case of cyanobacteria, it is considered that the hydrogen photoproduction process is already close to the theoretical maximum, and the optimum conditions are already determined [3]. Thus, various mutant strains have been developed and characterized to improve the characteristics of the H2 photoproduction process.
Since the nitrogenase synthesis in heterocystous cyanobacteria is localized in heterocysts, increase in their number should provide an increase in nitrogenase activity. As a rule, the frequency of heterocysts is 5–10% of the number of vegetative cells [9]. To date, mutant strains of Anabaena sp. with the heterocyst frequency reaching 13–18% have been obtained; hydrogen photoproduction at high chlorophyll (Chl) concentrations in these mutants exceeded that of the parental strain by 1.7 times [10].
Since nitrogenase of cyanobacteria is oxygen-sensitive, it requires anaerobic conditions to produce hydrogen [2, 3]. However, along with direct nitrogenase inactivation, there is a possibility of consumption of the evolved hydrogen in the presence of О2 as the electron acceptor that significantly reduces the hydrogen yield [11]. Since hydrogen consumption is catalyzed by uptake Hup hydrogenase, some attempts have been made to obtain mutant strains with the impaired synthesis of this enzyme. To inactivate the gene encoding uptake hydrogenase, different approaches were used for different cyanobacteria (undirected chemical mutagenesis followed by the selection of promising mutants as well as obtaining of ΔhupL, ΔhupS, ΔhupSL, ΔhupW, and ΔxisC mutants). In all cases, a significant increase in the H2 photoproduction was observed [12–17]. In addition, an increased efficiency of the light energy conversion into H2 (4%) was observed in such mutants [17]. At the same time, the lack of Hup hydrogenase may reduce a process' duration, especially in immobilized cultures, since this enzyme is considered to protect a photosynthetic apparatus of vegetative cells from photoinhibition, especially under stress conditions [18].
To provide anaerobic conditions during hydrogen photoproduction, one usually uses an argon flow as a gaseous phase, which significantly increases the cost of the process. In this case, the optimum solution would include a realization of the process under nitrogen or air; however, under such conditions, nitrogenase activity would remain at the maximum level for only a short time, since ammonium generated in the course of the N2 fixation would suppress the synthesis of nitrogenase [2]. Due to this reason, some mutants of the Anabaena strain PCC 7120 that did not contain Hup hydrogenase were constructed; these mutants dc‑Q193S and dc-R284H were characterized by amino acid substitutions localized in the vicinity of the FeMo cofactor of nitrogenase [19]. These modifications of nitrogenase resulted in an increased H2 photoproduction during the long-term cultivation of mutants under N2, since H2 production was not reduced in the presence of high concentrations of gaseous nitrogen; at the same time, long-term H2 photoproduction by a parental ΔHup strain required low nitrogen concentrations [19].
The above-described mutants are interesting for biotechnological hydrogen production; therefore, we need to investigate their still unknown properties, including the determination of the maximum rates and constants of light saturation for their photosynthetic and nitrogenase activity, as well as to study the temperature dependence for hydrogen photoproduction. Such information is essential for the arrangement of outdoor experiments with the impossibility to maintain the optimum process conditions. In the theoretical aspect, a comparison of the constants of acetylene inhibition in different mutants may be useful for elucidation of the effect of amino acid replacements on the functioning of the nitrogenase active center.
The purposes of this study were to evaluate the prospects for the use of Anabaena sp. PCC 7120 ΔHup mutants (dc-Q193S and dc-R284H) for outdoor hydrogen production and to compare the effect of light intensity and temperature on their stability and photosynthetic and nitrogenase activity.
MATERIALS AND METHODS
Objects and cultivation conditions. The objects of our study included the parental strain Anabaena sp. PCC 7120 ΔHup with the blocked synthesis of hydrogen-uptake Hup hydrogenase and its dc-Q193S and dc-R284H mutants, kindly provided by Prof. H. Sakurai (Kanagawa University, Japan). The mutants were constructed by Prof. Masukawa and his colleagues [19] and characterized by the substitution of Gln-193 by Ser (dc-Q193S) and Arg-284 by His (dc-R284H); both substitutions were located close to the FeMo cofactor of nitrogenase.
All strains (inoculum) were grown photoautotrophically in eightfold diluted Allen–Arnon liquid medium (AA/8) [19] containing 2.5 mM (NH4)2SO4 (АА/8 + N) and 5 mM TES-KOH buffer (рН 8.2) and supplemented with streptomycin and spectinomycin (2 μg/mL each). The cultivation was carried out in 300-mL Erlenmeyer flasks in air at 27°С under constant stirring (95 rpm); the flasks were illuminated with a light intensity of 30 μmol quanta/(m2 s).
To determine the photosynthetic and nitrogenase (hydrogen production) activities, the cultures were grown on antibiotic-free medium at 27°С under illumination (30–50 μmol quanta/(m2 s)). The inoculum was added into a vial with a glass bubbler in the volume equal to 10% of the working volume of the vial (250–270 mL). The sparging of a vial with air containing 1% СО2 was performed via autoclaved 0.2-μm membrane filters (Pall, United States). We preliminarily selected conditions for centrifugation and stirring of cyanobacteria cultures providing the maximum integrity of filaments. In the further experiments, the centrifugation was carried out at 1200–2300 rpm depending on the volume of a precipitated culture, while the stirring in a cell was carried out at 100–200 rpm. The photosynthetic activity was determined for cultures grown on the medium containing fixed nitrogen (see above).
To study the hydrogen photoproduction process, the parental strain PCC 7120 ΔHup was grown on fixed nitrogen-free medium for five days under purging with air containing 1% СО2. Since dc-Q193S and dc-R284H mutants are characterized by a weak or even missed ability for diazotrophic growth [19] and, like other cyanobacteria, do not form heterocysts in the case of bound nitrogen consumption, they were grown on the АА/8 + N medium up to the complete depletion of bound nitrogen or with the intermediate washing of cells from bound nitrogen. In the second case, after a 3-day biomass accumulation, cells were precipitated by centrifugation (2300 rpm, 3 min), twice washed with a nitrogen-free AA/8 – N medium to remove nitrogen, centrifuged, resuspended in this medium, and cultivated in Erlenmeyer flasks under a constant purging with air as described above. On the fifth day of cultivation (after two nitrogen-free days), medium pH was adjusted to 8.2 by 1 М NaHCO3 (up to a final concentration of ~10 mM). In both cases, nitrogenase activity appeared on the fifth day of cultivation. The majority of experiments were performed on the sixth day using the second approach. The use of the first approach and variations in the cultivation time are indicated in figure captions.
Polarographic measurement of a photosynthetic activity. The photosynthetic production of oxygen was measured using a Clark electrode. A portion of the culture (4 mL) was added into a 4-mL cell (Hansatech, United Kingdom) at a concentration of 6.0–8.5 mg Chl/L. Oxygen photoproduction was measured after a 2-min adaptation in the dark and the addition of 80 μL of 0.5 М NaHCO3 using different light intensities (10–1700 μmol quanta/(m2 s)) and temperatures (16–35°С). A LETI-60 slide projector (Russia) with the removed slide holder, infrared filter, and installed cuvette with 1% CuSO4 was used as the light source; the total optical path length was 4.5 cm. The photosynthetic rate was calculated as an algebraic difference between the oxygen photoproduction rate under light and oxygen consumption in the dark after a 4-min illumination and was expressed per 1 mg of Chl (μmol О2/(mg h)). The electrode was calibrated at a temperature of the planned experiment by the addition of 4 mL of air-saturated distilled water.
Polarographic measurement of nitrogenase activity. On the day of the experiment, the required volume of a culture was transferred into a flask with a cotton-gauze plug and incubated under light (50 μmol quanta/(m2 s)) on an ELMI S-3L shaker (Sky Line, Latvia) at 27°С and 95 rpm. In the course of the day, the culture was sampled from this flask, concentrated by centrifugation (1200 rpm for 3 min) up to a concentration of 20 mg Chl/L, and placed into 9-mL vials with hermetically twisting plugs followed by the further air replacement with argon. During calculations, we took into account the coefficient of activity reduction in the course of incubation.
Hydrogen photoproduction was measured in a 1-mL cell (Hansatech) under constant stirring (100 rpm) using a Clark electrode. Prior to each measurement, the cell was purged with argon; a culture portion (1 mL) was added into the cell under anaerobic conditions (argon flow) from a vial filled with argon. After a 2-min dark adaptation, illumination was turned on; hydrogen photoproduction was registered, depending on the purpose of the experiment, either at a constant temperature (27°С) and different light intensity (10–2800 μmol quanta/(m2 s)) or at different temperatures (15–45°С) but constant saturating light intensity (500 μmol quanta/(m2 s)). The H2 photoproduction rate was calculated as an algebraic difference between the H2 production rates under light and dark conditions and was expressed per 1 mg of Chl (μmol H2/(mg h)). The electrode was calibrated at a temperature of the planned experiment by the addition of 0.1 mL of H2‑sat-urated distilled water. The H2 content was calculated using tabular data for the temperature dependence of solubility. Results obtained for each temperature (five measurements) were averaged, and calibration coefficients were calculated.
Measurement of nitrogenase activity by gas chromatography. Measurement of hydrogen concentration in the study of the effect of nitrogen and acetylene on the nitrogenase activity was performed using an LXM80 gas chromatograph (Russia) [20]. Portions (3.5 mL or 10 mg Chl/L) of cultures prepared as described above (though not concentrated) were placed into 16-mL Hungate tubes with tightly twisting plugs. The gaseous phase was replaced with argon; then we added 4.8‒100% N2 or 2.3–20% C2H2 that corresponded to 27–570 μM of dissolved N2 and 0.9–7.7 μM of dissolved C2H2, respectively. The tubes were incubated under light (70 μmol quanta/(m2 s)) at 27°С and 95 rpm. After 1.5–2 h of incubation and then every 2 h, the H2 concentration was measured, and the H2 photoproduction rate was calculated per 1 mg of Chl (μmol Н2/(mg h)). In the case of the dc-Q193S mutant, the H2 photoproduction rate was low for the first 2 h but then increased; so the results obtained after 4 and 6 h of incubation were taken into account.
Analytical methods. After the Chl extraction with 85% methanol, its concentration was measured at 664 nm using a V-VIS 1240 Mini spectrophotometer (Shimadzu, Japan). An ammonium concentration in the medium was determined by a microdiffusion method using Nessler’s reagent [21]. A PAR flux density was measured with a QMSW-SS quantometer (Apogee Instruments, United States).
Microscoping. The presence of heterocysts in the filaments of cyanobacteria was determined under a LOMO MIKMED-2 light microscope (LOMO, Russia). The number of heterocysts was determined by the counting of 300–500 cells per each sample. Photographs were made with a Scopetek DCM-900 camera (Scopetek, China) mounted on the eyepiece of the microscope.
Statistical analysis. To perform a quantitative analysis, experimental data on the light intensity effects were approximated according to the Michaelis–Menten equation:
where V and Vmax are the measured and calculated (maximum) oxygen photoproduction rates, Ks is saturation constant, and I is light intensity.
Activation energy was calculated according to the Arrhenius equation:
where k is the reaction rate constant, R is the universal gas constant, and Т is the absolute temperature (in Kelvin).
The temperature coefficient was calculated using the following formula:
where V is the reaction rate and Т is the absolute temperature (in Kelvin).
The statistical treatment of data was performed using the SigmaPlot 11.0 software package. All experiments were performed in 3–5 biological replications (2–3 analytical replications). Figures containing one curve show the averaged results at a 95% confidence interval. Figures containing 2–3 curves do not indicate a confidence interval, since it did not exceed 20% of the mean value.
RESULTS
Effect of Light Intensity on the Photosynthetic Rate in the Parental (ΔHup) and Mutant (dc-Q193S and dc-R284H) Strains at Different Temperatures
In both parental and mutant strains, the O2 photoproduction rate increased as the light intensity increased (Fig. 1). The character of this dependence was similar at different temperatures (16, 20, 27, and 35°С), excepting the case of a cultivation of the parental strain ΔHup at 16°С, in which a photoinhibition probably occurred. For all strains, the maximum O2 photoproduction rate was observed at 35°С, though the optimum temperature for their growth was 27–30°С. Under the same temperature and light intensity conditions, the value of this parameter for the parental strain exceeded those for the mutants (Fig. 1).
Photosynthetic oxygen production in the parental ΔHup (a) and mutant dc-Q193S (b) and dc-R284H (c) strains depending on the light intensity at different temperatures ((1) 16°С; (2) 20°С; (3) 27°С; (4) 35°С). Experimental data are indicated with dots, while curves (excepting (a); 1—16оС) describe approximation results.
To perform a quantitative analysis, the obtained experimental data were approximated using the Michaelis–Menten equation (Table 1). All values were satisfactorily described by this equation with the correlation coefficient R > 0.91. For all strains, the maximum calculated photosynthesis rate (as well as the obtained experimental values) was higher at 35°С; for all examined temperatures, the highest value of this parameter was observed in the parental strain ΔHup.
At all temperatures included into the study, light saturation constants were lower in mutant strains dc-Q193S and dc-R284H, i.e., they reached the photosynthetic saturation at a lower density of the PAR flux than the parental strain.
Hydrogen Photoproduction by Parental (ΔHup) and Mutant (dc-Q193S) Strains at Different Growth Phases
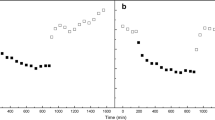
In this assay, the level of H2 photoproduction was measured polarographically using a Clark electrode. No H2 photoproduction activity was observed on the third to fourth days of cultivation, though the microscoping indicated the formation of a small number of heterocysts. The target activity was registered at the fifth day of growth in both parental ΔHup (Fig. 2a) and mutant dc-Q193S (Fig. 2b) strains. On this day, the dc-Q193S culture was in a nitrogen starvation state since the ammonium content in the medium did not exceed 0.4 μg/mL. At the same time, the number of heterocysts in the culture increased. On the sixth day, the activity reached the maximum (that coincided with the beginning or the middle of the stationary phase) and then decreased.
Biomass accumulation ((1) Chl; (2) OD700, 0.1-cm cuvette) and hydrogen photoproduction rate (3) in the course of the growth of parental ΔHup (a) and mutant dc‑Q193S (b) strains. The ΔHup strain was grown under nitrogen-fixing conditions, while the dc-Q193S strain was cultivated on the bound nitrogen-containing medium up to its depletion (without an additional washing).
According to Fig. 2, the growth rate of the mutant strain exceeded that of the parental strain (0.04 and 0.02 h–1, respectively). Note that the parental strain was grown under nitrogen fixation conditions in this case, whereas the mutant strain was cultivated in the medium containing bound nitrogen. If the parental strain was grown in the presence of bound nitrogen, the growth rate increased to 0.04 h–1 (data not shown). The lower final concentration of the mutant strain biomass is explained by ammonium depletion and the impossibility of using molecular nitrogen.
Effect of Light Intensity on Hydrogen Photoproduction by the Parental ΔHup and Mutant dc-Q193S Strains
In this series of experiments, the level of H2 photoproduction was measured by polarography. The H2 photoproduction rate in the parental ΔHup and mutant dc-Q193S strains increased as the light intensity increased from 10 to ~500 μmol quanta/(m2 s) and did not change in the case of the further increase of this parameter to 2820 μmol quanta/(m2 s) (Fig. 3). The higher rate was observed in the parental strain. Approximation of experimental data showed that the calculated maximum H2 photoproduction rate in the parental strain was almost seven times higher than in the mutant strain dc-Q193S, while the saturation constant was 1.5 times lower (Table 2). In our further experiments, we used a light intensity equal to 500 μmol quanta/(m2 s) for all strains.
Effect of Temperature on Hydrogen Photoproduction by the Parental and Mutant Strains
In this series of experiments, the level of H2 photoproduction was also measured by polarography. The H2 photoproduction rate in the parental strain ΔHup increased as the temperature grew up to 45°С; however, this dependence was nonlinear at the temperatures exceeding 34°С (Fig. 4a). The H2 photoproduction by dc-Q193S increased as the temperature grew up to 30–34°С then began to decrease (Fig. 4b). The activation energy for the parental and mutant strains was 43.3 and 61.3 kJ/mol, respectively. To quantify temperature effects, the temperature coefficient (Q10) was also calculated; for parental and mutant strains, it was equal to 1.9 and 2.4, respectively.
To find an explanation of the reduced activity of dc-Q193S at temperatures exceeding 30°С, we examined the physiological state (morphology) of the studied cyanobacteria under such conditions. In the case of a temperature increase from 27 to 45°С, as well as decrease to 15°С (accompanied by stirring), the integrity of filaments in the studied mutant strain was significantly impaired compared to the parental strain: we observed filament ruptures and free-swimming heterocysts (Figs. 5b, 5c; no picture for the parental strain is shown). The fraction of free heterocysts in the total number of cells was 1.2, 11.4, and 8.6% at 27, 45, and 15°С, respectively. In the case of the parental strain, the fraction of free heterocysts at these temperatures were 0.4, 1, and 2%, respectively.
Effect of Molecular Nitrogen on Hydrogen Photoproduction by Parental and Mutant Strains
In this series of experiments, the level of H2 photoproduction was measured by gas chromatography. This method allows a researcher to use a wider concentration range of N2 added to a gaseous phase, but not directly into the culture (as in the case of the polarographic method). After the addition of >57 μM of N2, the H2 photoproduction rate in the parental strain significantly decreased; a 50% inhibition was observed at the N2 concentration of 300 μM. Nevertheless, even at the addition of 570 μM of N2, the target activity made 40% of the initial level. At the same time, no inhibition of the H2 photoproduction was observed in mutant strains even at the addition of 570 μM N2 (Fig. 6).
Effect of Acetylene on Hydrogen Photoproduction by Parental and Mutant Strains
In this series of experiments, the level of H2 photoproduction was measured by gas chromatography. In the presence of 0.9 μM acetylene, the observed H2 photoproduction in all three studied strains was reduced 2–5 times and continued to decrease as the acetylene concentration increased to 7.7 μM (Fig. 7). In the presence of 0.9 μM acetylene, the maximum activity reduction was observed in the strain dc‑Q193S, while the minimum activity was observed in the parental strain ΔHup in the presence of 7.7 μM acetylene (Fig. 7). However, taking into account an increased data variation in these experiments, the revealed differences are rather doubtful. The average acetylene concentration, which caused a 50% inhibition of the target activity in all studied strains, was 0.4–1.1 μM.
DISCUSSION
The use of cyanobacteria for hydrogen bioproduction primarily requires high H2 photoproduction rates. The use of genetic engineering makes it possible to develop bacterial strains with an improved H2 photoproduction rate, including strains of nitrogen-fixing heterocystous cyanobacteria with the lack of H2-uptake hydrogenase [22]. According to the existing publications [13, 15], the most promising strains include Anabaena variabilis ATCC 29413 ΔHupSL and Nostoc PCC 7422 ΔHupL, which are able to produce >100 μmol Н2/(mg h). To reach such productivity, the cultures were limited with bound nitrogen and used under anaerobic conditions (100% Ar or Ar + 5% CO2). The maintenance of anaerobic conditions in photobioreactors is expensive, so the use of air or molecular nitrogen as a gaseous phase would be preferable. Unlike bound nitrogen, molecular nitrogen does not inhibit the biosynthesis of nitrogenase; however, in the case of a diazotrophic growth, the nitrogenase activity of cyanobacteria measured under argon is maintained at a high level for only 1–2 days and then rapidly drops because of the accumulation of ammonium representing a nitrogen fixation product [9]. Therefore, a high rate of H2 photoproduction is maintained under such conditions only for a short time. On the other hand, an argon atmosphere also does not provide a prolonged H2 photoproduction, since nitrogen starvation inhibits the biosynthesis of enzymes. Thus, a periodic addition of air as the source of nitrogen into the gaseous phase can be useful in some cases, including immobilized cultures [18]. Carbon deficiency also negatively influences on the H2 photoproduction. At the same time, combination of the excess СО2 and nitrogen shortage may result in the violation of the C/N ratio and the corresponding inhibition of a photosynthetic activity of vegetative cells that, in turn, affects Н2 production in heterocysts [18].
Another approach is based on the obtaining of mutants able to maintain the target activity for a long time in the presence of N2. Masukawa et al. [19] constructed several Hup hydrogenase-deficient mutants of the Anabaena PCC 7120 strain characterized by the dc-Q193S and dc-R284H amino acid substitutions localized in the vicinity of the FeMo cofactor of nitrogenase. These modifications resulted in an increase in the total H2 photoproduction during a long-term cultivation of the mutants under N2, since the H2 evolution did not drop so sharply as in the parental strain. To determine the possibility of using these strains for outdoor H2 production, we performed a detailed study of the effect of external factors, such as light and temperature.
A photosynthetic activity of the parental strain exceeded that of mutants, and the light saturation constant Ks of ΔHup (153 μmol quanta/(m2 s) was also higher than the corresponding values in mutant strains (52 and 76 μmol quanta/(m2 s); Fig. 1; Table 1). Photoinhibition was observed only at low temperatures (Fig. 1a). In relation to the nitrogenase activity (hydrogen evolution), Ks values in the parental and dc‑Q193S strains were almost the same (41 and 62 μmol quanta/(m2 s), respectively; Fig. 3; Table 2). These values were certainly lower than the maximum outdoor daylight intensity. In the course of our short-term experiments, we did not observe any inhibition of the H2 photoproduction at the light intensity close to the natural daylight.
The temperature dependence of H2 photoproduction within the temperature range of 15–30°С was also similar in both parental and dc-Q193S strains. However, the activity of the dc-Q193S mutant decreased at temperatures exceeding 30°С (Fig. 4), which was probably caused by a reduced strength of its filaments and their fragmentation (Fig. 5).
The rate of H2 photoproduction in the parental strain ΔHup was close to the earlier published data [19], while the value of this parameter in mutants was lower. This fact can also be explained by a high sensitivity of this process to mechanic effects occurring during culture preparation and analysis (centrifugation, re-suspension, and stirring of a culture). The details of these procedures may vary in different laboratories. However, even in the case of the similar preparation procedures, there could be a difference in the initial physiological state of parental and mutant cultures. The parental strain was grown in the absence of fixed nitrogen and at the expense of nitrogen fixation, while mutant strains were cultivated first in the presence of bound nitrogen (up to its depletion or washing of cells) and then under air in the absence of bound nitrogen as described by Masukawa et al. [19]. In contrast to the parental strain, the ability of mutants to fix nitrogen is extremely limited, so they could be in a nitrogen starvation state. According to the existing data, limitation in relation to bound nitrogen promotes the formation of heterocysts. However, severe limitation resulted in a reduction of a number of vegetative cells located between two heterocysts in Anabaena cylindrica filaments; in this case, the photosynthetic activity of vegetative cells decreased, so the provision of heterocysts with metabolites was reduced [23]. Earlier, the fragmentation of filaments (up to their dissociation into single cells) was observed for the case of nitrogen starvation of short-chain Anabaena sp. PCC 7120 mutants [24]. Mechanical strength of filaments can also be reduced by mutations in genes encoding cell wall components [25]. It was found that Fra and SepJ proteins are important for both integrity of filaments and intracellular communications; fraH mutants had a tendency towards the fragmentation and release of heterocysts [26]. In turn, inactivation of the АmiC1 amidase influences the SepJ localization and prevents fragmentation peculiar to sepJ or fraCfraD mutants [27]. As for our experiments, it still remains unclear whether mutations in the vicinity of the active center of nitrogenase could affect the strength of filaments. Most likely, there is a nonspecific effect associated with nitrogen starvation.
Molecular nitrogen inhibited H2 photoproduction only in the parental strain; 50% inhibition was observed in the presence of 300 μM N2 (Fig. 6). This value is comparable with the data obtained for purple bacteria Rhodobacter capsulatus, when 50% inhibition was observed in the presence of 114 μM N2 [28]. However, 40% N2 almost completely inhibited hydrogen production by purple bacteria, whereas Anabaena PCC 7120 strain ΔHup used in our study demonstrated ~40% hydrogen-producing activity even at 100% N2. This fact is probably caused by the lack of hydrogen consumption required for the synthesis of nitrogen-containing compounds in the absence of Hup hydrogenase. The fact that purified nitrogenase from Azotobacter vinelandii still remains active even at 5000 kPa of N2 argues for this assumption [29]. Unlike the parental ΔHup strain, molecular nitrogen did not inhibit H2 photoproduction in the dc-Q193S mutant even at a concentration equal to 100% of a gaseous phase (Fig. 6), which confirms the data obtained by Masukawa et al. [19]. Acetylene inhibition of hydrogen photoproduction was observed in both parental and mutant (dc-Q193S and dc-R284H) strains, though the difference between them is unreliable (Fig. 7).
Along with ATP, both H2 evolution and acetylene reduction to ethylene processes require two electrons from ferredoxin, while reduction of molecular nitrogen requires eight electrons. Since molecular nitrogen does not inhibit H2 evolution and is not used for the growth of the dc-Q193S mutant, one can suppose that this point mutation results in the lack of a nitrogenase ability to accumulate eight electrons, whereas the ability to receive two electrons and to transmit them to a substrate molecule remains almost intact.
In relation to the hydrogen bioproduction in the presence of molecular nitrogen, the use of a directed mutagenesis of nitrogenase in cyanobacteria looks promising. One can specify the criterium for the selection of such mutants. It is known that the highest rate of a long-term H2 production in cyanobacteria is observed in the presence of 5% molecular nitrogen [30]. Therefore, 10–20-fold increase of the inhibition constant (78% : 5%) will make it possible to use the atmospheric concentration of nitrogen as the limiting concentration and to maintain a high nitrogenase activity of cyanobacteria associated with H2 production for a long time. However, large-scale hydrogen production under natural conditions also depends on other properties of cyanobacteria. Concerning the reaction to external environmental factors, no significant difference was observed between three strains studied, i.e., all of them were equally suitable for this purpose. At the same time, decreased activity and (especially) increased fragility of filaments in the nitrogenase-modified mutants limits the possibilities of their use.
REFERENCES
Prince, R.C. and Kheshgi, H.S., The photobiological production of hydrogen: potential efficiency and effectiveness as a renewable fuel, Crit. Rev. Microbiol., 2005, vol. 31, p. 19.
Tamagnini, P., Leitão, E., Oliveira, P., Ferreira, D., Pinto, F., Harris, D.J., and Lindblad, P., Cyanobacterial hydrogenases: diversity, regulation and applications, FEMS Microbiol. Rev., 2007, vol. 31, p. 692.
Bothe, H., Schmitz, O., Yates, M.G., and Newton, W.E., Nitrogen fixation and hydrogen metabolism in cyanobacteria, Microbiol. Mol. Biol. R., 2010, vol. 74, p. 529.
Dutta, D., De, D., Chaudhuri, S., and Bhattacharya, S., Hydrogen production by cyanobacteria, Microb. Cell Fact., 2005, vol. 4, p. 1.
Allahverdiyeva, Y., Leino, H., Saari, L., Fewer, D.P., Shunmugam, S., Sivonen, K., and Aro, E.M., Screening for biohydrogen production by cyanobacteria isolated from the Baltic Sea and Finnish lakes, Int. J. Hydrogen Energ., 2010, vol. 35, p. 1117.
Dasgupta, C.N., Gilbert, J.J., Lindblad, P., Heidorn, T., Borgvang, S.A., Skjanes, K., and Das, D., Recent trends on the development of photobiological processes and photobioreactors for the improvement of hydrogen production, Int. J. Hydrogen Energ., 2010, vol. 35, p. 10218.
Kosourov, S., Murukesan, G., Seibert, M., and Allahverdiyeva, Y., Evaluation of light energy to H2 energy conversion efficiency in thin films of cyanobacteria and green alga under photoautotrophic conditions, Algal Res., 2017, vol. 28, p. 253.
Tsygankov, A.A., Fedorov, A.S., Kosourov, S.N., and Rao, K.K., Hydrogen production by cyanobacteria in an automated outdoor photobioreactor under aerobic conditions, Biotechnol. Bioeng., 2002, vol. 80, p. 777.
Wolk, P., Heterocyst formation, Annu. Rev. Genet., 1996, vol. 30, p. 59.
Masukawa, H., Sakurai, H., Hausinger, R.P., and Inoue, K., Increased heterocyst frequency by patN disruption in Anabaena leads to enhanced photobiological hydrogen production at high light intensity and high cell density, Appl. Microbiol. Biotechnol., 2017, vol. 101, p. 2177.
Tsygankov, A., Serebryakova, L., Rao, K., and Hall, D., Acetylene reduction and hydrogen photoproduction by wild type and mutant strains of Anabaena at different CO2 and O2 concentrations, FEMS Microbiol. Lett., 1998, vol. 167, p. 13.
Mikheeva, L.E., Schmitzh, O., Shestakov, S.V., and Bothe, H., Mutants of the cyanobacterium Anabaena variabilis altered in hydrogenase activities, Z. N-aturforsch. C, 1995, vol. 50, p. 505.
Happe, T., Schutz, K., and Bohme, H., Transcriptional and mutational analysis of the uptake hydrogenase of the filamentous cyanobacterium Anabaena variabilis ATCC 29413, J. Bacteriol., 2000, vol. 182, p. 1624.
Lindberg, P., A hydrogen-producing, hydrogenase-free mutant strain of Nostoc punctiforme ATCC 29133, Int. J. Hydrogen Energ., 2002, vol. 27, p. 1291.
Yoshino, F., Ikeda, H., Masukawa, H., and Sakurai, H., High photobiological hydrogen production activity of a Nostoc sp. PCC 7422 uptake hydrogenase-deficient mutant with high nitrogenase activity, Mar. Biotechnol., 2007, vol. 9, p. 101.
Khetkorn, W., Lindblad, P., and Incharoensakdi, A., Inactivation of uptake hydrogenase leads to enhanced and sustained hydrogen production with high nitrogenase activity under high light exposure in the cyanobacterium Anabaena siamensis TISTR 8012, J. Biol. Eng., 2012, vol. 6, p. 1.
Nyberg, M., Heidorn, T., and Lindblad, P., Hydrogen production by the engineered cyanobacterial strain Nostoc PCC 7120 ΔhupW examined in a flat panel photobioreactor system, J. Biotechnol., 2015, vol. 215, p. 35.
Kosourov, S., Leino, H., Murukesan, G., Lynch, F., Sivonen, K., Tsygankov, A.A., and Allahverdiyeva, Y., Hydrogen photoproduction by immobilized N2-fixing cyanobacteria: understanding the role of the uptake hydrogenase in the long-term process, Appl. Environ. Microb-iol., 2014, vol. 80, p. 5807.
Masukawa, H., Sakurai, H., Hausinger, R.P., and Inoue, K., Sustained photobiological hydrogen production in the presence of N2 by nitrogenase mutants of the heterocyst-forming cyanobacterium Anabaena,Int. J. Hydrogen Energ., 2014, vol. 39, p. 19444.
Laurinavichene, T., Tekucheva, D., Laurinavichius, K., Ghirardi, M., Seibert, M., and Tsygankov, A., Towards the integration of dark and photo fermentative waste treatment. 1. Hydrogen photoproduction by purple bacterium Rhodobacter capsulatus using potential products of starch fermentation, Int. J. Hydrogen Energ., 2008, vol. 33, p. 7020.
Lyubimov, V.I., L’vov, N.P., and Khristin, B.I., Modification of microdiffusion method for ammonium determination, Prikl. Biokhim. Mikrobiol., 1968, vol. 4, p. 120.
Shestakov, S.V. and Mikheeva, L.E., Genetic control of hydrogen metabolism in cyanobacteria, Russ. J. Genet., 2006, vol. 42, p. 1512.
Silverman, S.N., Kopf, S.H., Bebout, B.M., Gordon, R., and Som, S.M., Morphological and isotopic changes of heterocystous cyanobacteria in response to N2 partial pressure, Geobiology, 2018, vol. 17, p. 60.
Bauer, C.C., Buikema, W.J., Black, K., and Haselkorn, R., A short-filament mutant of Anabaena sp. strain PCC 7120 that fragments in nitrogen-deficient medium, J. Bacteriol., 1995, vol. 177, p. 1520.
Burnat, M., Herrero, A., and Flores, E., Compartmentalized cyanophycin metabolism in the diazotrophic filaments of a heterocyst-forming cyanobacterium, Proc. Natl. Acad. Sci. USA, 2014, vol. 111, p. 3823.
Herrero, A., Stavans, J., and Flores, E., The multicellular nature of filamentous heterocyst-forming cyanobacteria, FEMS Microbiol. Rev., 2016, vol. 40, p. 831.
Bornikoel, J., Carrión, A., Fan, Q., Flores, E., Forchhammer, K., Mariscal, V., Mullineaux, C.W., Pezer, R., Silber, N., Wolk, C.P., and Maldener, I., Role of two cell wall amidases in septal junction and nanopore formation in the multicellular cyanobacterium Anabaena sp. PCC 7120, Front. Cell. Infect. Microbiol., 2017, vol. 7, p. 366.
Laurinavichene, T.V., Tsygankov, A.A., and Gogotov, I.N., Similarities and differences between alternative and Mo-containing nitrogenases in Rhodobacter capsulatus cells, Prikl. Biokhim. Mikrobiol., 1994, vol. 30, p. 389.
Liang, J. and Burris, R.H., Interactions among nitrogen, nitrous oxide, and acetylene as substrates and inhibitors of nitrogenase from Azotobacter vinelandii,Biochemistry, 1988, vol. 27, p. 6726.
Lichtl, R.R., Bazin, M.J., and Hall, D.O., The biotechnology of hydrogen production by Nostoc flagellifo-rme grown under chemostat conditions, Appl. Microbiol. Biotechnol., 1997, vol. 47, p. 701.
Funding
The adjustment of methods and obtaining of preliminary experimental data were supported by the Russian Foundation for Basic Research (project no. 15-54-50032). Data clarification, repetition, and analysis, as well as the manuscript preparation, were supported by the Russian Science Foundation (project no. 19-14-00255).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflict of interest. This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Translated by N. Statsyuk
Abbreviations: AA medium—Allen and Arnon medium; Chl—chlorophyll.
Rights and permissions
About this article
Cite this article
Romanova, A.I., Laurinavichene, T.V. & Tsygankov, A.A. Features of Anabaena PCC 7120ΔHUP Mutants with Amino Acid Substitutions in Nitrogenase. Russ J Plant Physiol 67, 386–395 (2020). https://doi.org/10.1134/S1021443720010161
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1021443720010161