Abstract
The effects of water deficit on the activity of antioxidant enzymes including superoxide dismutase (SOD), catalase (CAT), ascorbate peroxidase (APX) and peroxidase (POX), and also on total antioxidant capacity, ion leakage, proline content, chlorophyll fluorescence, gas exchange, chlorophyll and carotenoid content and plant dry mass of potato cultivars (Solanum tuberosum L.) were investigated. For this purpose, the response of ten potato cultivars (Agria, Arinda, Marfona, Banba, Born, Santé, Milva, Satina, Jelly and Spirit) was studied under two irrigation regimes (70 and 20% of soil available water). Water deficit increased the activity of antioxidant enzymes, ion leakage and proline content but decreased total antioxidant capacity, Fv/Fm, gas exchanges, chlorophyll, carotenoid and plant dry mass in all potato cultivars. Based on stress tolerance index (STI), Agria and Born were the most tolerant and sensitive cultivars to water stress, respectively. Positive correlations were found between total antioxidant capacity and the activities of CAT, APX and POX under control irrigation. Proline content positively correlated with plant dry mass and negatively with photosynthesis, transpiration and carbon dioxide concentration in the sub-stomatal chamber under water deficit treatment. The highest increases in ion leakage under water deficit were observed in the most sensitive cultivars. The results of this experiment, therefore, showed that proline accumulation and ion leakage may be related to water stress tolerance of potato cultivars. Also, increased chlorophyll and carotenoid content in resistant cultivars supported potato plants to tolerate water deficit.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
INTRODUCTION
Environmental stresses are major limiting factors for plant growth in many parts of the world [1]. Damages due to water stress, salinity and temperature are more widespread as compared to the other stresses [2]. Under water deficit, the water potential of the soil falls and as a result, the plant cannot absorb water, therefore under these conditions a series of changes in the plant tissues are accrued for plant adaption [3]. Photosynthesis as an important plant production process is affected by drought through inhabitation of RuBisCo activity and disruption of energy balance and distribution [4], and also by the changes in chlorophyll contents and components [5]. The resistance of plants to drought stress is classified into three categories; the first type is escaping from drought. In this case, the period of plant growth ends quickly. The second type is drought avoidance by which some modifications occurs in plants, such as increased root growth and water absorption, decreased transpiration, twisting and reducing the leaf area, reduction in the distance between stomata and the increase of waxy compounds and the third type is resistance to drought stress through the production of some protein substances that increase the plant tolerance to stress [3]. Proline and soluble carbohydrates accumulation and ion leakage (IL) are also affected by water deficit [6]. Besides, environmental stresses with direct and indirect effects on plant water status disrupt the photosynthesis and induce oxidative stress in plants [7]. To avoid destructive effects of oxidative damage caused by the excessive production of reactive oxygen species under stress conditions plants are equipped with an antioxidant defense system designed to silence free radicals, which consists of antioxidant enzymes and non-enzymatic components [7]. Non-enzymatic antioxidants include ascorbate, glutathione, alpha tocopherol and beta-carotene. Among the antioxidant enzymes we can refer to superoxide dismutase (SOD), ascorbate peroxidase (APX), catalase (CAT) and peroxidase (POX). These antioxidants play important roles to modulate the equilibrium between the production and the elimination of free radicals [3, 8]. In areas where potato is grown under water-limited conditions, understanding of plant drought tolerance or adaptation is important. However, selection for drought tolerance is complicated by the fact that the differences in yield reduction cannot be traced back to one or a few major morphological or physiological components while they are needed to develop an efficient screening technique. Moreover, the ability to maintain a high yield under drought is determined by many characteristics and the importance of each factor varies with time and severity of the deficit [9]. In fact, the defense mechanism system often comprises the combination of antioxidant enzymes and non-enzymatic antioxidants [7]. The results of studies have shown that increasing the activity of one or more antioxidant enzymes may increase the resistance to environmental stresses [3, 8]. There are different methods for evaluating total antioxidant capacity. One of these methods is the use of free and stable radical 2-2-D-phenyl-1-picyrilhydrazil (DPPH). DPPH is a lipophilic radical with a maximum absorption at 517 nm [10]. In assessing the DPPH antioxidant capacity, DPPH radicals react with antioxidants and, as a consequence, their amounts are reduced [10]. The reduction of DPPH molecules is occurred when hydroxyl groups donate one hydrogen to DPPH radical and as a result, the color of solution converts from violet-dark to light yellow. Absorption at a wavelength of 517 nm indicates the amount of DPPH remaining. Under water deficit conditions, the cell membrane degrades and therefore, water potential of plant cell decreases which end up increasing IL [10]. In addition, one of the plant’s reactions to drought stress is osmotic regulation in which increasing the accumulation of some amino acids such as betaine and proline content [11]. Regarding the strategic importance of the potato crop, climate changes, declining rainfall and the shortage of water resources, especially in arid and semi-arid regions of the word, this research, therefore, was carried out on ten potato cultivars to study the relationship between the antioxidant potential and plant growth attributes with the biochemical and physiological bases of potato tolerance to water deficit.
MATERIALS AND METHODS
Plant material and growing conditions. In this pot research, ten potato cultivars (Solanum tuberosum L.) including Agria, Arinda, Marfona, Banba, Born, Santé, Milva, Satina, Jelly and Spirit were studied at two moisture regimes (70 and 20% of soil available water) under greenhouse condition. An experiment based on a randomized complete block design with four replications was conducted during January, February and March of 2017. The culture medium of the pots contained a mixture of sand, garden soil and rotten animal manure with the ratio of 4 : 1 : 1, respectively. To prevent the spread of diseases (insects, nematods, fungus, etc.), disinfection of wet soil was carried out with the metam sodium (C2H4NNaS2). Before cultivating the potato tubers, they were stored in dark condition at room temperature of 25°C for two weeks to germinate. Then in each pot, one potato tuber (about 50 g) was planted at a depth of approximately 8 cm. From the time of planting till carrying out the drought stress treatment, all pots were irrigated normally and fertilized with a complete fertilizer of 20‑20-20 (a product of CIFO S.P.A., Italy). Water deficit treatment was applied at the stage of forming eight and ten leaves. The instrument for measuring and recording the moisture and soil temperature (IDRG SMS-T1) was used in order to determine the irrigation time. The plants were harvested after four weeks of applying water deficit treatment. Before harvest, the upper fully developed leaves were collected and directly incubated in liquid nitrogen (‒196oC) for analysis of antioxidant enzymes, proline content and other physiological traits.
Enzyme extractions and assays. The amount of 0.1 g of leaf tissue was homogenized using a chilled mortar and pestle and added to it 1 mL of 100 mM potassium phosphate buffer (pH 7.8), containing 0.5% Triton X‑100 and 1% polyvinylpyrrolidone. The extract was centrifuged at 12 000 rpm for 30 min at 4°C. The supernatant was used to assay the following antioxidant enzymes.
The activity of catalase (CAT; EC 1.11.1.6) was measured using the method described by Aebi [12]. The assay buffer contained 3 mL of 50 mM K-phosphate buffer (pH 7.0), 50 µL of enzyme extract and 4.51 µL of H2O2 (30%). The decrease in absorbance at 240 nm was inspected for 1 min, using a spectrophotometer U-1800 (Hitachi, Japan).
The ascorbate peroxidase (APX; EC 1.11.1.11) activity was measured based on the method defined by Nakano and Asada [13]. The assay buffer contained 3 mL of 50 mM K‑phosphate buffer (pH 7.0), containing 100 µL of 5 mM ascorbate, 50 µLof enzyme extract and 4.51 µL of H2O2 (30%). The decrease in absorbance at 290 nm was monitored for 1 min, using a spectrophotometer U-1800 (Hitachi).
The activity of peroxidase (POX; EC 1.11.1.7) was based on the process described by Zaharieva et al. [14]. The assay buffer contained 3 mL of 50 mM K-phosphate buffer (pH 7.0), containing 3.35 µL guiacol, 50 µL of enzyme extract and 4.51 µL of H2O2 (30%). The increase in absorbance at 470 nm was monitored every 30 s for 2 min, using a spectrophotometer U-1800 (Hitachi).
Activity of catalase, ascorbate peroxidase and peroxidase was determined according to the following relationship:
where Δabs—the absorption difference of each enzyme; A—the amount of enzyme extract in the reaction solution (µL); ε—extinction coefficient of the enzyme (39.4, 2.8 and 26.6 mM/cm for CAT, APX and POX, respectively); B is weight of buffer + weight of plant material; P—fresh weight of plant leaves (g).
The activity of superoxide dismutase (SOD; EC 1.15.1.1) was measured using the modified method described by Giannopolitis and Ries [15] and the activity was determined in 1 mL of general phosphate buffer, 33 μL of nitroblue tetrazolium (NBT) and 33 μL of riboflavin. The absorbance at 570 nm was measured using a spectrophotometer U-1800 (Hitachi). Protein content was evaluated for all antioxidant enzymes by using bovine serum albumin as the standard method described by Bradford [16].
Total antioxidant capacity (TAC). The amount of 0.1 g leaf tissue was homogenized using a chilled mortar and pestle in extraction buffer containing 1 mL of alcohol (96%). The extract was centrifuged at 4000 rpm for 5 min at 4oC. The supernatant was used to assay the total antioxidant capacity which was measured using the modified method reported by Bettaieb et al. [17]. The assay buffer contained 2 mL of 2,2-diphenyl-1-picrylhydrazyl solution and 50 μL of extract. The mixture and reaction took place in the dark for half an hour. The absorption wavelength of the 517 nm in spectrometer was set. Then, the inhibition was calculated based on the following equation:
where A0 is the control absorption (non-extract added) and A1 is the sample absorption (extract added).
Ion leakage. The measurement of IL was performed according to the method defined by Lutts et al. [18]. From young leaves, 3 discs with a diameter of 1 cm in each replicate were prepared and washed three times with distilled water. The samples were then placed in test tubes and 10 mL of distilled water was added in each tube and placed on a shaker for 24 h at room temperature. Then, the electrical conductivity (Lt) was measured by the electrical conductivity meter (CC-501). Containers containing specimens were placed in an autoclave for 1 h at 120°C, and again the electrical conductivity of the samples was measured (L0). Finally, the percent of IL was calculated from the following equation:
Proline content. The method described by Bates et al. [19] was used to measure proline content. The absorbance of the samples was measured using a spectrophotometer U-1800 (Hitachi) at 520 nm. Proline concentration was determined according to the standard chart and expressed using the following formula in terms of μmol/g of fresh weight of plant leaves:
where Δabs is the absorption of samples.
Chlorophyll, carotenoid content, fluorescence and gas exchange. Chlorophyll and carotenoid content were measured by the method of Lichtenthaler [20] using the acetone extracts of leaves (0.1 g of leaves per 10 mL of 100% acetone cooled to 2–4°C). The contents of chlorophylls a, b, total chlorophyll and carotenoids were determined spectrophotometrically at 661.6, 644.8 and 470 nm after extraction.
Parameter of chlorophyll fluorescence, maximum quantum efficiency of photosystem II (Fv/Fm) was measured applying a fluorometer (Walz, Germany).
Gas exchanges (photosynthesis (CO2 absorbance), stomatal conductance, transpiration, carbon dioxide concentration in the sub-stomatal chamber) were measured by using a portable apparatus model (L.C.I. Software version 1:10 UK), on the youngest adult leaves in plants. All measurements were made between 10 and 13 h.
Plant dry mass. All plant parts (shoots and roots) of each cultivar and in each replicate were dried in an oven at 80°C for 48 h to specify a fill dry weight.
Stress tolerance index (STI). This index used to identify cultivars that produce high plant dry mass under both drought and non-drought conditions and expressed using the following formula [21]:
where Ypi and Ysi are yields of cultivar under control and stress conditions, respectively. \(\overline {Yp} \) is total yield mean of cultivars under control condition.
Data analysis. Data were tested and subjected to analysis of variance (ANOVA) by using SAS and MSTATC programs to determine the difference in both treatments and cultivars and based on a randomized complete block design. Comparison of means was performed by using LSD test (P < 0.05) and the correlation coefficients between the traits were done by using PROC CORR of SAS. In the other hand, principle component analysis (PCA) was performed based on the correlation matrix to reduce the multiple dimensions of data space and the biplot was drawn using Stat Graphics software.
RESULTS
Plant Dry Mass
The interaction effects of cultivars and irrigation levels were significant (P < 0.01) on plant dry mass (PDM) (Table 1). Under normal conditions of irrigation (70% of soil available water), the most and least values of PDM were attained in Agria (25.9 g/plant) and Born (9.37 g/plant). Nevertheless, under drought treatment (20% of soil available water), the maximum and minimum values of PDM were obtained in Marfona (7.95 g/plant) and Born (2.18 g/plant). PDM was significantly reduced in all cultivars at 20 compared to 70% of soil available water. Maximum and minimum reductions were observed (Fig. 1) in Born (76.7%) and Santé (35.2%). Stress tolerance index (STI) was calculated to assess drought tolerance of potato cultivars. The values of STI for cultivars were 0.760 (Agria), 0.617 (Satina), 0.598 (Marfona), 0.351 (Spirit), 0.303 (Sante), 0.277 (Banba), 0.224 (Jelly), 0.207 (Arinda), 0.201 (Milva) and 0.081 (Born).
The Activity of Antioxidant Enzymes
The interaction effects of cultivars and irrigation levels were significant (P < 0.01) on the activities of SOD, CAT, APX and POX (Table 1).
The most and least activities of SOD under control irrigation were observed in cultivars Agria (6.57 U/(min mg protein)) and Born (3.77 U/(min mg protein)) and under drought treatment were achieved in Born (26.8 U/(min mg protein)) and Satina (10.7 U/(min mg protein)). The activity of SOD was increased in all cultivars. Maximum and minimum increases were detected in cultivars (Fig. 2) Born (610%) and Satina (129%).
The most and least activities of CAT under control level of irrigation were observed in cultivars Marfona (12.1 µmol/(min mg protein)) and Jelly (5.02 µmol/(min mg protein)) and under drought treatment were achieved in Born (27.3 µmol/(min mg protein)) and Milva (11.8 µmol/(min mg protein)). CAT activity was increased in all cultivars. Maximum and minimum increases (Fig. 3) were noticed in cultivars Born (410%) and Satina (85.0%).
The most and least activities of APX under control level of irrigation were observed in cultivars Marfona (0.347 µmol/(min mg protein)) and Agria (0.120 µmol/(min mg protein)) and under drought treatment were observed in Born (0.829 µmol/(min mg protein)) and Agria (0.369 µmol/(min mg protein)). APX activity was increased in all cultivars. The highest and the lowest increases were observed in cultivars Born (548%) and Satina (105%) (Fig. 4).
The maximum and minimum POX activities under control irrigation were observed in cultivars Marfona (2.933 µmol/(min mg protein)) and Banba (1.277 µmol/(min mg protein)) and under drought treatment were obtained in Marfona (7.787 µmol/(min mg protein)) and Milva (2.617 µmol/(min mg protein)). POX activity was increased in all cultivars. The maximum and minimum significant increases (Fig. 5) were observed in cultivars Born (393%) and Milva (86.5%).
Proline Content
The interaction effects of cultivars and irrigation levels were significant (P < 0.01) on proline content (Table 1). The highest and the lowest contents of proline under control level of irrigation were obtained in cultivars Jelly and Spirit (4.05 and 4.04 µmol/g fr wt, respectively) and Agria (0.51 µmol/g fr wt) and under water stress treatment were achieved in cultivars Marfona (9.74 µmol/g fr wt) and Born (2.04 µmol/g fr wt). The content of proline was increased in all cultivars and the highest and the lowest increases (Fig. 6) were observed in cultivars Agria (1247%) and Born (37.8%).
Total Antioxidant Capacity and Ion Leakage
The effects of cultivars and irrigation levels were significant (P < 0.01) on total antioxidant capacity and IL (Table 1). Water deficit was associated with decreased total antioxidant capacity and increased percent of IL by about 23.3 and 76.7%, respectively (Table 2). However, the interactions between cultivars and irrigation levels were not significant for these two attributes (Table 1). The highest and the lowest total antioxidant capacities (Table 3) were obtained in cultivars Banba (25.03 µg/mL) and Milva (15.66 µg/mL), the most and least values of IL were observed in cultivars Arinda (34.92%) and Satina (19.51%) respectively.
Chlorophyll, Carotenoids Content and Fluorescence
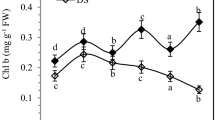
The interaction effects of cultivars and irrigation levels were significant (P < 0.01) on chlorophyll a and the ratio of chlorophyll a/b (Table 1). The highest and the lowest contents (Table 4) of chlorophyll a under control level of irrigation were obtained in cultivars Jelly, Satina (0.500 mg/g fr wt) and Arinda (0.373 mg/g fr wt). The corresponding values for the ratio of chlorophyll a/b were achieved in Milva (2.84) and Agria (1.58). However, under water stress treatment, these values were obtained for Chl a in cultivars Satina (0.466 mg/g fr wt) and Santé (0.263 mg/g fr wt), and for Chl a/b in cultivars Satina (3.14) and Marfona (2.20). The effects of cultivars and irrigation levels were significant (P < 0.01) on Fv/Fm, Chl b, Chl (a + b) and carotenoids (Table 1). Water deficit was associated with decreased Fv/Fm, Chl b, Chl (a + b) and carotenoids by about 25.35, 37.90, 26.68 and 32.68%, respectively (Table 2). However, except Fv/Fm, the differences among cultivars were significant in terms of other measured attributes (Table 1).
Gas Exchange
The effects of cultivars and irrigation levels were significant (P < 0.01) on photosynthesis (CO2 absorbance), stomatal conductance (Gs) and carbon dioxide concentration in the sub-stomatal chamber (Ci) (Table 1). The maximum and the minimum rate (Table 4) of photosynthesis under control level of irrigation were obtained in cultivars Arinda (19.53 µmol CO2/m2 s) and Banba (11.72 µmol CO2/m2 s)), Gs in Jelly (0.125 mol CO2/(m2 s)) and Santé (0.072 mol CO2/(m2 s)) and Ci in Satina (164.5 µmol/mol), Agria (162.8 µmol/mol) and Jelly (44.5 µmol/mol). However, under water stress treatment, these values were obtained for photosynthesis in cultivars Born (2.57 µmol CO2/(m2 s)) and Spirit (0.93 µmol CO2/(m2 s)), Gs in Born (0.027 µmol CO2/(m2 s)), Jelly (0.025 µmol CO2/(m2 s)) and 0.02 µmol CO2/(m2 s)) in other cultivars and Ci in Born (371.3 µmol/mol) and Marfona (187.0 µmol/mol). The effects of cultivars and irrigation levels (Table 1) were significant (P < 0.01) on transpiration. Water deficit was associated with decreased transpiration by about 61.51% (Table 2).
Relationship between the Traits
Correlation coefficients between different traits are presented in Table 5. Under control conditions (70% of soil available water), SOD activity, Chl b, Chl (a + b) and carotenoids content were highly and positively correlated with PDM. Also, APX activity was positively correlated with SOD and CAT activities. Too, POX activity was correlated with CAT and APX activities. Proline content was negatively correlated with IL. Also, transpiration was positively correlated with photosynthesis and proline content. But it was negatively correlated with IL. At the same time under drought condition (20% of soil available water), there were high positive correlations among antioxidant enzymes, chlorophyll, carotenoid contents and also between proline content and PDM. Also, a positive correlation was obtained between transpiration and photosynthesis (CO2 absorbance), STI and PDM. But the negative correlation was achieved proline, photosynthesis, transpiration and Ci. As well, we observed a positive correlation between carotenoid contents and the contents of Chl a, Chl b and Chl (a + b) under both 70 and 20% of soil available water.
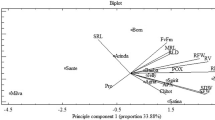
Biplot Analysis
Principle component analysis (PCA) explained that the first and second components revealed more than 55 and 61% of the variation in 70 and 20% of soil available water, respectively (Table 6). Under 70% of soil available water, principle component 1 (PC1) had higher and negative correlation with SOD, chlorophyll, carotenoid contents and PDM. But it had a positive correlation with transpiration. This PC could be named as photosynthetic capacity. Principle component 2 (PC2) had higher and positive correlation with CAT, APX, POX, TAC and IL. But it had higher and negative correlation with proline, photosynthesis, Gs and transpiration. This PC could be named as antioxidative potential. Therefore, higher values of these characters indicated higher amount of PDM varieties. As a result, PC1 could be called «potential biomass of plant dry weight». To categorize the varieties based on PCA, the biplot of PC1 and PC2 was constructed (Fig. 7a). As a result, cultivars Agria, Satina and Marfona were found to have the highest SOD activity, chlorophyll pigments and plant dry mass, but Born, Santé, Arinda and Milva were found to have the lowest plant dry mass (low PC2). In other side, variety Marfona had high potential in terms of CAT, APX and POX enzyme (high PC1) under 70% of soil available water conditions. However, under 20% of soil available water, PC1 had positive correlations with SOD, Fv/Fm, photosynthesis (CO2 absorbance), transpiration and Ci and negative correlations with chlorophyll, carotenoid contents, PDM and STI. Therefore, selection based on higher and negative PC1 values may lead to sensitive cultivars. On the other hand, PC2 was positively correlated with CAT, APX, POX activities, IL and proline content. So, cultivars with high PC2 are not suitable for drought stress conditions. According to the biplot analysis of PC1 and PC2 (Fig. 7b), cultivars Born, Arinda, Santé and Spirit had high PC2 but varieties Agria, Satina and Marfona had high PC1 and were hence identified as preferable tolerant cultivars at the vegetative growth stage under 20% of soil available water conditions.
The biplot displays the traits measured on 10 potato cultivars under 70% (a) and 20% (b) of soil available water. SOD— superoxide dismutase activity, CAT—catalase activity, APX—ascorbate peroxidase activity, POX—peroxidase activity, TAC—total antioxidant capacity, IL—ion leakage, Pro—proline content, Fv/Fm—maximum quantum efficiency of photosystem II, Photo—photosynthesis (CO2 absorbance), Gs—stomatal conductance, E—transpiration, Ci—carbon dioxide concentration in the sub-stomatal chamber, Chl a—chlorophyll a content, Chl b—chlorophyll b content, Chl (a + b)—total chlorophyll a and b content, Chl a/b—ratio chlorophyll a/b, Carot—carotenoids content, PDM—plant dry mass, STI—stress tolerance index.
DISCUSSION
Among different environment stresses such as UV radiation, low or high temperature, mechanical injuries, herbicides, flooding and salinity, drought has the highest rank in limiting crop production in many agricultural areas of the world [1]. Efforts for searching of resistant cultivars to water deficit are thus necessary. Potato is among the plants that are susceptible to water scarcity [22]. The current research was therefore attended to evaluate the response of some potato cultivars to water shortage under greenhouse condition in terms of changes in plant biomass, antioxidant enzymes, chlorophyll pigments, gas exchange, proline content and IL.
In the present study, under control irrigation (based on 70% of soil available water), among ten tested potato cultivars, the highest biomass production was achieved in Agria (25.9 g/plant) followed by Satina (20.8 g/plant), Marfona (19.43 g/plant) and Spirit (19.4 g/plant), respectively (Fig. 1). These cultivars, therefore, growth better under conditions where water is not limited. Plant dry mass of all cultivars were significantly decreased at 20% of soil available water as compared to the control irrigation. Studies [23, 24] indicate that water deficit affects plant canopy architecture by reducing leaf size and leaf expansion rate and increasing the rate of senescence. Also, stomatal closure occurs as a result of water shortage which in turn limits plant CO2 intake and leads to reduced biomass production. There was significant variation among tested potato cultivars in response to water deficit, as the reduction in biomass production under water stress treatment ranged from 35 (Santé) to 76% (Born). Based on the stress tolerance index, the most drought tolerant cultivar was Agria, followed by Satina, Marfona, Spirit, Santé, Banba, Jelly, Arinda, Milva and Born. Consequently, Agria was the most tolerant and Born was the most sensitive cultivar in terms of reduction in biomass production under water stress imposed during plant vegetative growth stage. Other researchers [25] have also reported the existence of significant variation among potato cultivars in response to water stress. Similar to the findings [5], we detected a decrease in the contents of chlorophyll and carotenoid but an increase in Chl a/b under water deficit (Tables 2, 4). The highest values of Chl b, Chl (a + b) and carotenoids content among cultivars belonged to Agria, Satina, Jelly and the lowest values were observed in Milva and Born, respectively (Table 3). Also, similar to the results of [5, 26], we detected a decrease in the photosynthesis, transpiration and Gs but increases in Ci under water deficit (Tables 2, 4). The maximum values of photosynthesis, Gs and Ci were obtained in Born and the minimum values of these attributes were achieved for photosynthesis in Spirit, Ci in Marfona and with the exception of Gs in Born and Jelly, the lowest values were observed in other cultivars (Table 4). Chlorophyll pigments and gas exchange (photosynthesis (CO2 absorbance), transpiration, Gs and Ci) as an important plant production process are affected by water deficit [4, 5]. In fact, carotenoids are pigments which play a major role in the protection of plants against photo-oxidative processes. They are involved in protecting of the photosynthetic apparatus by quenching single oxygen and other harmful free radicals which are synthesized during photosynthesis [27]. Also, many researchers [26] indicated that under stress conditions photo-damage for photosynthetic system occurred and the higher Ci was directly related to CO2 absorbance and Gs.
The distribution of potato cultivars in the biplot constructed based on PCA, revealed that tolerant cultivars had high chlorophyll, carotenoid, proline contents, PDM and STI (high PC1) but had lower activities of antioxidant enzymes and rates of gas exchange (PC2) (Table 6). In the other hand, susceptible cultivars of potato plants had the lowest chlorophyll, carotenoid, proline contents, PDM and high negative STI value but higher activity of antioxidant enzymes and gas exchange. Accordingly, the resistant cultivars may include Agria, Satina and Marfona. In the contrary, susceptible cultivars are Born, Milva, Arinda and Santé (Fig. 7b).
Water deficit imposed on potato plants in this study was complemented by significantly increase in the activities of antioxidant enzymes and proline content (Figs. 2–6). In fact, water deficit increases the production of reactive oxygen species (ROS) such as superoxide (O–2), hydrogen peroxide (H2O2), and hydroxyl (OH–). Meanwhile, increasing the amount of ROS destroys lipids, cell membranes, macromolecules, DNA and carbohydrates, and thus prevents photosynthetic activities. The mechanism of adaption of plants to water deficit involves the activation of antioxidant enzymes to clean these destructive free radicals under stress [28]. Similar to our findings in the studies of [3, 8] on potato and [15] on corn, the activities of SOD, CAT, APX, POX were increased under drought conditions (Figs. 2–5). Similar to the outcomes of [8], we detected a decrease in the antioxidant scavenging capacity of free radical DPPH under water deficit (Table 2). The highest and the lowest value in total antioxidant capacity between cultivars belonged to Banba (25.03 µg/mL) and Milva (15.66 µg/mL) (Table 3). This reduction occurs during drought stress because of the damage of cell membrane that leads to a decrease in the scavenging ability in plants [8]. However, the interaction between cultivar and drought stress was not significant in this regard (Table 1). In the current study, positive correlations were found between total antioxidant capacity and the activities of CAT, APX and POX under control irrigation. This may indicate that these enzymes have significant contribution in determination of total antioxidant capacity of potato plants under non-stress condition. However, there were no significant correlations between total antioxidant capacity and the activities of measured antioxidant enzymes under stress condition (Table 4). This may imply that under water-limited irrigation other enzymatic, i.e. monodehydroascorbate reductase, dehydroascorbate reductase, glutathione reductase [29] and non-enzymatic antioxidants such as ascorbate, glutathione and alpha tocopherol [8] are involved in the determination of total antioxidant capacity of potato plants. Like other enzymes, SOD activity was increased in all cultivars under water deficit treatment (Fig. 2). However, the extent of increases was smaller in Agria, Satina, Marfona and Spirit that were among the most tolerant cultivars. This means that the increased activity of SOD could not lead to increased tolerance of potato cultivars. Yet, negative correlations were found between the activity of SOD and plant dry mass under water-limited irrigation (Table 5). This may suggest that increased activity of SOD under stress condition was ended up some energy costs for the plants. However, there was positive correlation between the activity of SOD and plant dry mass under control irrigation, indicating the positive role of this enzyme in plant biomass production under none-stress condition. Similar to the findings of Bansal and Nagarajan [11] in potato plants, we observed increasing of proline content in all tested cultivars under water deficit (Fig. 6). Many researchers [11] have reported the genes induced for biosynthesis of proline in potato plants under water deficit conditions. Proline accumulation in plants is one of the common acclimating mechanisms to drought stress. In fact, plants regulate their osmotic potential and adjust to low soil water availability by accumulating osmoregulators such as proline. Moreover, plant stress tolerance is improved by protecting and stabilizing membranes and enzymes during stress by these organic compatible compounds [23]. In the current study, we observed a positive correlation between proline content and plant dry mass under water deficit treatment (Table 5). This indicates that in this study proline had significant role in manipulation of plant biomass production under water stress. However, some studies [30] reported that proline accumulation in potato under water stress was primarily occurred in sensitive cultivars.
Although no significant correlations between IL and plant dry mass were found within the two environments, e.g. normal and water-limited conditions (Table 5), the highest and the lowest value in IL between cultivars belonged to Arinda (34.92%) and Satina (19.51%) that according to stress tolerance index were among the most sensitive and tolerant cultivars, respectively (Table 3). This may indicate that cell membrane damage due to increased ROS and enhanced IL under water stress was more prominent in sensitive cultivars. According to Lu et al. [6] it was also reported increased IL in potato cultivars under drought condition. This may, therefore, verify the use of this trait as a precise index for cultivar selection under drought stress.
In conclusion, in this study we have been able to gather evidence that water deficit increased IL, proline content and the activity of antioxidant enzymes SOD, CAT, APX and POX while decreased total antioxidant capacity, Fv/Fm, gas exchange, chlorophyll, carotenoid contents and plant biomass in potato cultivars. However, the increased activities of antioxidant enzymes did not induce tolerance in potato cultivars under water stress. Accordingly, it seems that under circumstances of this experiment other enzymatic and non-enzymatic antioxidants are involved in the tolerance mechanism of the tested cultivars. Yet, results indicated that these antioxidant enzymes had some contributions in plant growth under non-stress condition. Significant correlations between proline content and low IL with plant biomass revealed the important role of these two attributes in biomass production of potato plants under water stress. Also, increases carotenoids content in resistant cultivars supported potato plants to tolerate water deficit.
REFERENCES
Flexas, J., Bota, J., Galmes, J., Medrano, H., and Ribas-Carbo, M., Keeping a positive carbon balance under adverse conditions: responses of photosynthesis and respiration to water stress, Physiol. Plant., 2006, vol. 127, pp. 343–352.
Greenway, H., Plant response to saline substrates. I: Growth and ion uptake of several varieties of Hordeum during and after NaCl treatment, Aust. J. Biol. Sci., 1962, vol. 5, pp. 16–36.
Placide, R., Shimelis, H., Laing, M., and Gahakwa, D., Physiological mechanisms and conventional breeding of sweet potato (Ipomoea batatas (L.) Lam.) to drought-tolerance, Afr. J. Agr. Res., 2013, vol. 8, pp. 1837–1846.
Rapacz, M., Kosćielniak, J., Jurczyk, B., Adamska, A., and Wojćik, M., Different patterns of physiological and molecular response to drought in seedlings of malt- and feed-type barleys (Hordeum vulgare), J. Agron. Crop Sci., 2010, vol. 196, pp. 9–19.
Afshari, M., Shekari, F., Azemkhani, R., Habibi, H., and Fotokian, M.H., Effects of foliar application of salicylic acid on growth and physiological attributes of cowpea under water stress conditions, Iran Agric. Res., 2013, vol. 32, pp. 56–70.
Lu, Y.Y., Deng, X.P., and Kwak, S.S., Overexpression of CuZn superoxide dismutase (CuZnSOD) and ascorbate peroxidase (APX) in transgenic sweet potato enhances tolerance and recovery from drought stress, Afr. J. Biotech., 2010, vol. 9, pp. 8378–8391.
Mittler, R., Oxidative stress, antioxidants and stress tolerance, Trends Plant Sci., 2002, vol. 7, pp. 405–410.
Lin, K.H., Chao, P.Y., Yang, C.M., Cheng, W.C., Lo, H.F., and Chang, T.R., The effects of flooding and drought stresses on the antioxidant constituents in sweet potato leaves, Bot. Stud., 2006, vol. 47, pp. 417–426.
Spitters, C.J.T. and Schapendonk, A.H.C.M., Evaluation of breeding strategies for drought tolerance in potato by means of crop growth simulation, Plant Soil, 1990, vol. 123, pp. 193–203.
Pokorny, J., Yanishlieva, N., and Gordan, M., Antioxidants in Food Practical Application, Cambridge: Woodhead, 2001.
Bansal, K.C. and Nagarajan, S., Leaf water content, stomatal conductance and proline accumulation in leaves of potato (Solanum tuberosum L.) in response to water stress, Ind. J. Plant Physiol., 1986, vol. 29, pp. 397–404.
Aebi, H., Catalase in vitro, Methods Enzymol., 1984, vol. 105, pp.121–126.
Nakano, Y. and Asada, K., Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts, Plant Cell Physiol., 1981, vol. 22, pp. 867–880.
Zaharieva, T., Yamashita, K., and Matsumoto, H., Iron deficiency induced changes in ascorbate content and enzyme activities related to ascorbate metabolism in cucumber root, Plant Cell Physiol., 1999, vol. 40, pp. 273–280.
Giannopolitis, C.N. and Ries, S.K., Superoxide dismutase: occurrence in higher plants, Plant Physiol., 1977, vol. 59, pp. 309–314.
Bradford, M.M., A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein–dye binding, Anal. Bi-ochem., 1976, vol. 72, pp. 248–254.
Bettaieb, I., Knioua, S., Hamrouni, I., Limam, F., and Marzouk, B., Water deficit impact on fatty acid and essential oil composition and antioxidant activities of cumin (Cuminum cyminum L.) aerial parts, J. Agric. Food Chem., 2011, vol. 59, pp. 328–334.
Lutts, S., Kinet, J.M., and Bouharmon, J., NaCl induced senescence in leave of rice (Oryza sativa L.) cultivars differing in salinity resistance, Ann. Bot., 1996, vol. 78, pp. 389–398.
Bates, L.S., Waldren, P.R., and Teare, D.I., Rapid determination of free proline for water stress studies, Plant Soil, 1973, vol. 39, pp. 205–208.
Lichtenthaler, H.K., Chlorophylls and carotenoids: pigments of photosynthetic biomembranes, Methods Enzymol., 1987, vol. 148, pp. 350–382.
Fernandez, G.C.J., Effective selection criteria for assessing stress tolerance, Proc. Int. Symp. on Adaptation of Vegetables and Other Food Crops in Temperature and Water Stress, Cuo, C.G., Ed., Taiwan, 1992, pp. 257–270.
Shock, C.C., Feibert, E.B.G., and Saunders, L.D., Potato yield and quality response to deficit irrigation, Hort. Sci., 1998, vol. 33, pp. 655–659.
Rudoplh, A.S., Crowe, J.H., and Crowe, L.M., Effects of three stabilizing agents—proline, betaine, and trehalose on membrane phospholipids, Arch. Biochem. B-iophys., 1986, vol. 245, pp. 134–143.
Fleisher, D.H., Timlin, D.J., and Reddy, V.R., Interactive effects of carbon dioxide and water stress on potato canopy growth and development, Agron. J., 2008, vol. 100, pp. 711–719.
Hassanpanah, D., Evaluation of potato cultivars for resistance against water deficit stress under in vivo conditions, Potato Res., 2010, vol. 53, pp. 383–392.
Cheng, Y.J., Deng, X.P., Kwak, S.S., Chen, W., and Eneji, A.E., Enhanced tolerance of transgenic potato plants expressing choline oxidase in chloroplasts against water stress, Bot. Stud., 2013, vol. 54, pp. 1–9.
Collins, A., Carotenoids and genomic stability, Mutat. Res., 2001, vol. 475, pp. 1–28.
Sofo, A., Tuzio, A.C., Dichio, B., and Xiloyannis, C., Influence of water deficit and re-watering on the components of the ascorbate, glutathione cycle in four interspecific Prunus hybrids, Plant Sci., 2005, vol. 169, pp. 403–412.
Fazeli, F., Ghobanli, M., and Nikman, V., Effect of drought on biomass, protein content, lipid peroxidation and antioxidant enzymes in two sesame cultivars, Biol. Plant., 2007, vol. 51, pp. 98–103.
Schafleitner, R., Gutierrez, R., Espino, R., Gaudin, A., Pérez, J., Martinez, M., Dominguez, A., Tincopa, L., Alvarado, C., Numberto, G., and Bonierbale, M., Field screening for variation of drought tolerance in (Solanum tuberosum L.) by agronomical, physiological and genetic analysis, Potato Res., 2007, vol. 50, pp. 71–85.
ACKNOWLEDGMENTS
This work was supported by the Isfahan University of Technology, Faculty of Agriculture, and Department of Horticulture Science.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflict of interest. This article does not contain any studies involving animals or human participants performed by any of the authors.
Additional information
The article is published in the original.
Abbreviations: APX—ascorbate peroxidase; CAT—catalase concentration in the substomatal chamber; Chl a—chlorophyll a; Chl b—chlorophyll b; Chl a/b—ratio chlorophyll a/b; Ci—carbon dioxide concentration in the substomatal chamber; DPPH—2-2-D-phenyl-1-picyrilhydrazil; Fv/Fm— maximum quantum efficiency of photosystem II; Gs—stomatal conductance; IL—ion leakage; NBT—nitroblue tetrazolium; PDM—plant dry mass; POX—peroxidase; SOD—superoxide dismutase; STI—stress tolerance index; TAC—total antioxidant capacity.
Rights and permissions
About this article
Cite this article
Alhoshan, M., Zahedi, M., Ramin, A.A. et al. Effect of Soil Drought on Biomass Production, Physiological Attributes and Antioxidant Enzymes Activities of Potato Cultivars. Russ J Plant Physiol 66, 265–277 (2019). https://doi.org/10.1134/S1021443719020031
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1021443719020031