Abstract
This article discusses the results of studies carried out in recent years by a team of scientists from the Postovskii Institute of Organic Synthesis, Ural Branch, Russian Academy of Sciences, in cooperation with the First President of Russia Boris Yeltsin Ural Federal University, Ural State Medical University, Volgograd State Medical University, and other scientific and production organizations of the country to create triazavirin (riamilovir) and other direct etiotropic antiviral drugs based on azaheterocyclic derivatives.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
The pandemic of the new coronavirus infection has shown the urgent need to have not only vaccines but also effective chemotherapy drugs in the arsenal of antiviral agents. Their creation is one of the most difficult tasks in medicinal chemistry. This is not only because of the relatively complex structure of the biomolecules that should be selective targets of the action of antiviral substances but also because of the high variability of viruses.
It is well known how risky and time-consuming the development of innovative drugs is: according to statistics, out of several thousand candidate substances, only one demonstrates effectiveness and safety and becomes the basis of the drug brought to registration. Note that the Ural chemo-pharmaceutical school of Academicians I.Ya. Postovskii and O.N. Chupakhin, one of the oldest in the country, has developed about a dozen drugs for various purposes and has implemented industrial technologies for their synthesis. Among these developments is the antiviral drug triazavirin (its international nonproprietary name is riamilovir), which has a wide spectrum of action (Fig. 1). Triazavirin is based on the condensed system of 1,2,4-triazolo[5,1-c]-1,2,4-triazine, a kind of aza analogue of guanine, one of the heterocyclic bases of nucleic acids. It is heterocycles that play the most important role in medicinal chemistry as part of the structure of more than 70% of all drugs.
Returning to the COVID-19 pandemic, let us note that in 2021 the international journal Chemistry of Heterocyclic Compounds devoted a special issue to the fight against viral infections. In particular, it describes the synthesis of triazavirin containing several isotopic labels—with the inclusion of deuterium, carbon-13, and nitrogen-15 atoms in the structure of the drug, which creates unique opportunities for studying the processes of its metabolism and mechanism of action (Fig. 2) [1].
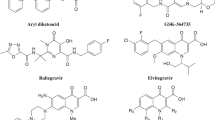
Interest in triazavirin has increased significantly during the pandemic. Recently, a review article was published in a foreign pharmaceutical journal, the authors of which pin certain hopes in the fight against COVID-19 on triazavirin [2]. This review discusses the results of 50 original scientific reports concerning the properties and activity of this drug. In particular, it provides data from an article in the journal Current Molecular Medicine on the interaction of triazavirin with the main protease of coronavirus as a biotarget. According to calculations, the drug has a good affinity for the main protease SARS-CoV-2 (with a binding energy of 9.94 kcal/mol), and its docking to the active center of the main protease is achieved due to the formation of a hydrogen bond with the Asn142 asparagine residue, as well as through electrostatic interactions with amino acid fragments His172, Glu166, Gly138, and Phe140 [3]. However, works [4–6] note significantly lower values of the binding energy of triazavirin with the main protease of the coronavirus. There are also calculated data on the interaction of triazavirin and its structural analogues with RNA-dependent RNA polymerase (RdRp) and the main protease (3CLpro), according to which the drug has a good affinity for these enzymes (Fig. 3).Footnote 1 Triazavirin is often positioned as an inhibitor of the RNA polymerase complex and is associated with favipiravir, molnupiravir, and remdesivir (Fig. 4), as well as with other azaheterocyclic bases and their nucleosides. Recently, great hopes have been associated with molnupiravir, an N-hydroxycytidine-based nucleoside drug, developed by the German biopharmaceutical company Merck and considered to be effective against COVID-19.
Many studies usually mention remdesivir as a reference standard. This antiviral drug is structurally a C-nucleoside of pyrrolo[2,1-f] [1, 2, 4]triazine. It was developed in the United States long before the COVID-19 pandemic, in the mid-2010s, to combat the Ebola virus disease epidemic in West Africa and was later repositioned to combat the coronavirus infection.
The work of Ural chemists on the creation of original drugs of the azoloazine series, which turned out to be active against not only various strains of influenza viruses but also tick-borne encephalitis, West Nile fever, Rift Valley fever, Dengue, and other viral infections, has a deeper history. The antiviral activity of azoloazines is reflected in numerous patents (RU2294936, RU2330036, RU2343154, RU2340614, RU2345080, RU2402552, RU2404182, RU2493158, RU2516936, RU2529487, RU2536874; ЕА 026688 (2017); ЕА 026783 (2017); US 9790277 (2017)) and a number of articles, covering hundreds of substances of the azoloazine series (see review [7]) and their nucleosides (Fig. 5) [8, 9]. The recognition of the high level of the Ural chemistry school is evidenced by the fact that, in 2016, work on the creation of a new generation of antiviral azoloazines was awarded the international Galen Prize.
The first representative of the azoloazine family, triazavirin, was registered in the register of medicines of the Russian Federation in 2014 (no. LP-002604) as an agent for the treatment of influenza [10]. An important role in the creation of the drug was played by the well-known virologist, Director of the All-Russia Research Institute of Influenza of the Russian Ministry of Health, RAS Academician O.I. Kiselev (St. Petersburg); leader of the Ural chemical school, RAS Academician O.N. Chupakhin (IOS, RAS Ural Branch, and UrFU); teams of the State Research and Testing Institute of Military Medicine of the Russian Ministry of Defense (St. Petersburg) and the Virological Center of the Russian Ministry of Defense (Sergiev Posad, Moscow oblast), as well as the pharmaceutical plant Medsintez (Novoural’sk, Sverdlovsk oblast) and the Ural Center for Biopharmaceutical Technologies (resident of the Skolkovo Innovation Center) [11–14].
Over the past few years, the indications for the use of triazavirin (riamilovir) have been significantly expanded, it has become an over-the-counter drug to treat not only influenza but also other acute respiratory viral infections, and extensive data have been accumulated on its use in the etiotropic therapy of these diseases [15], which has made it possible to conduct a meta-analysis of randomized clinical trials of the drug [16, 17]. Its effectiveness against the new coronavirus infection, as well as its safety in clinical use, was assessed [18–23]. The wide distribution of triazavirin in outpatient practice (during 2020‒2021, 3.6 million packs were sold through the pharmacy network) did not reveal serious side effects.
A number of medical centers in the country, including the Kirov Military Medical Academy (St. Petersburg); Polyclinic no. 3 of the Administration of the President of the Russian Federation; the Research Institute of Pulmonology of the Federal Medical and Biological Agency of Russia; the Burnazyan Federal Medical Biophysical Center of the same agency; and clinical divisions of Krasnoyarsk, Samara, and Ural State Medical Universities, as well as Clinical Hospital no. 14 in Yekaterinburg, have accumulated significant positive experience in the use of triazavirin in the treatment of patients with COVID-19 of varying severity. Importantly, the high efficacy of the drug was confirmed both in the treatment of patients with a PCR-based diagnosis [18–20] and in combination therapy [21, 22], as well as in the prevention of infection in infection foci [23]. The good safety profile of riamilovir (triazavirin) with no side effects made it possible to recommend this drug as the first stage of outpatient therapy for patients with COVID-19, based on the principle of multiple exposures [21]. Also note that experimental data on the effectiveness of triazavirin in experiments on Syrian hamsters infected with the SARS-CoV-2 virus were obtained at the State Research and Testing Institute of Military Medicine (St. Petersburg) [24]. The efficacy and safety of triazavirin was also evaluated in clinical institutions in Harbin [25]. The guidelines of Academician Yang Baofeng (Harbin Medical University, China) note the protective effect of the drug and its ability to inhibit the inflammatory process and reduce the recovery time.
Triazavirin, along with favipiravir, is included in the standard of care for the new coronavirus infection (COVID-19) in military personnel of the Armed Forces of the Russian Federation, in clinical treatment protocols in force in medical institutions in Moscow, and in outpatient conditions at home (according to Order of the Moscow Health Department no. 1131 of October 1, 2020).
The State Research Center for Virology and Biotechnology VECTOR of the Federal Service for Surveillance on Consumer Rights Protection and Human Well-Being (Rospotrebnadzor) (Kol’tsovo, Novosibirsk oblast) is searching for new active molecules based on a cellular test system with account for virtual screening of original compounds of the azoloazine family using both the main protease and RNA polymerase as the targets. UrFU and RAS UB IOS chemists prepared a library of compounds of the azoloazine series (more than 100 substances), some of which have been tested at the SRC VB Vector. In vitro experiments on a cell culture revealed a number of substances active against coronavirus. To search for the leading compounds, computer simulation of the interaction of azoloazines with the two key biotargets was conducted.
Note that it is extremely important today to create not only new antiviral drugs but also means of stopping undesirable post-COVID phenomena such as a cytokine storm, thrombocytopenia, and other complications. In this regard, in our opinion, noteworthy are the results obtained at Volgograd State Medical University under the guidance of Academician A.A. Spasov to inhibit the release of nitric oxide, as well as interleukin IL-6. Among the new derivatives of pyrimidines and triazolopyrimidines, effective cytokine storm inhibitors have been identified, exhibiting activity at the level of the well-known drug dexamethasone, and these substances do not cause immunosuppression (Table 1).
Another interesting observation was made when studying the action of triazavirin in conditions of hypercytokinemia. In experiments on intact animals, the antiaggregant activity of the drug was revealed, which increased by 2.2 times under the indicated conditions. This makes it possible to conclude that it exhibits antiaggregant action in in vitro experiments only when macrophages are activated by lipopolysaccharide, and in in vivo experiments it inhibits platelet aggregation without such activation and under conditions of hypercytokinemia. The data obtained suggest that the antiplatelet action of triazavirin may have a positive effect under viral infection (Fig. 6) [26]. In our opinion, the newly revealed properties of the drug will contribute to its broader use in medical practice.
Notes
A full-color version of Fig. 3 is available in the electronic version of the journal Vestnik Rossiskoi Akademii Nauk on the website of the ICC Akademkniga.
REFERENCES
T. S. Shestakova, S. L. Deev, I. A. Khalymbadzha, et al., “Antiviral drug Triazavirin, selectively labeled with 2H, 13C, and 15N stable isotopes: Synthesis and properties,” Chem. Heterocycl. Compd. 57 (4), 479−482 (2021).
I. Malík, J. Čižmárik, G. Kováč, and M. Pecháčová, “Triazavirin might be the new hope to fight Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2),” Čes. Slov. Farm. 70, 18–25 (2021).
S. Shahab and M. Sheikhi, “Triazavirin—Potential inhibitor for 2019-nCoV coronavirus M protease: A DFT study,” Curr. Mol. Med. 21 (8), 645–654 (2021).
M. A. A. Ibrahim, A. H. M. Abdelrahman, K. S. Allemailem, et al., “In silico evaluation of prospective anti-COVID-19 drug candidates as potential SARS-CoV-2 main protease inhibitors,” Protein J. 40 (30), 296–309 (2021).
S. Ercan and E. Çınar, “A molecular docking study of potential inhibitors and repurposed drugs against SARS-CoV-2 main protease enzyme,” J. Indian Chem. Soc. 98 (3), Article no. 100041 (2021).
S. Chtita, A. Belhassan, A. Aouidate, et al., “Discovery of potent SARS-CoV-2 inhibitors from approved antiviral drugs via docking and virtual screening,” Comb. Chem. High Throughput Screen. 24 (3), 441–454 (2021).
V. L. Rusinov, E. N. Ulomskii, O. N. Chupakhin, V. N. Charushin, “Azolo[5,1-c]-1,2,4-triazines as a new class of antiviral compounds,” Russ. Chem. Bull. 57 (5), 985−1014 (2008).
O. N. Chupakhin, S. L. Deev, T. S. Shestakova, et al., “Non-natural nucleosides based on 1,2,4-triazolo[1,5-a]pyrimidin-7-ones,” Heterocycles 80 (2), 1149−1163 (2010).
I. A. Khalymbadzha, T. S. Shestakova, J. O. Subbotina, et al., “Synthesis of acyclic nucleoside analogues based on 1,2,4-trizolo[1,5-a]pyrimidin-7-ones by one-step Vorbruggen glycosylation,” Tetrahedron. 70 (6), 1298−1305 (2014).
E. G. Deeva, V. L. Rusinov, V. N. Charushin, et al., “Antiviral drug Triazavirin®: From screening to clinical trials,” Drug Dev. Registr., No. 2, 144–151 (2014).
O. I. Kiselev, E. G. Deeva, T. I. Mel’nikova, et al., “New antiviral drug Triazavirin: Results of phase II clinical trials,” Probl. Virol. 57 (6), 9−12 (2012).
I. Karpenko, S. Deev, O. Kiselev, et al., “Antiviral properties, metabolism, and pharmacokinetics of a novel Azolo-1,2,4-triazine-derived inhibitor of influenza A and B virus replication,” Antimicrob. Agents Chemother. 54 (5), 2017−2022 (2010).
S. Ya. Loginova, S. V. Borisevich, V. A. Maksimov, et al., “Study of the antiviral activity of triazavirin against the influenza A (H5N1) virus in cell culture,” Antibiot. Chemother. 52 (11–12), 18−20 (2007).
S. Ya. Loginova, S. V. Borisevich, V. L. Rusinov, et al., “Evaluation of the toxicity of a new domestic anti-influenza chemotherapy drug triazavirin,” Antibiot. Chemother. 57 (11–12), 8–10 (2012).
E. P. Tikhonova, T. Yu. Kuz’mina, N. V. Andronova, et al., “Study of the effectiveness of antiviral drugs (Umifenovir, Triazavirin) against acute respiratory viral infections,” Kazan. Med. Zh. 99 (2), 215−223 (2018).
A. U. Sabitov, O. P. Kovtun, N. A. Batskalevich, et al., “Meta-analysis of randomized clinical trials of the efficacy of riamilovir in the etiotropic therapy of influenza,” Antibiot. Chemother. 66 (5–6), 58−71 (2021).
A. U. Sabitov, O. P. Kovtun, N. A. Batskalevich, et al., “Meta-analysis of randomized clinical trials of the efficacy of riamilovir in the etiotropic therapy of acute respiratory viral infection,” Antibiot. Chemother. 66 (5–6), 48−57 (2021).
A. U. Sabitov, V. V. Belousov, A. S. Edin, et al., “Practical experience with the use of the drug Riamilovir in the treatment of patients with COVID-19 of moderate severity,” Antibiot. Chemother. 65 (7–8), 27−30 (2020).
K. V. Kas’yanenko, O. V. Mal’tsev, K. V. Kozlov, et al., “Clinical efficacy and safety of riamilovir in the treatment of patients with infection caused by SARS-CoV-2,” Antibiot. Chemother. 65 (11–12), 16−21 (2020).
A. U. Sabitov, P. V. Sorokin, and S. Yu. Dashutina, “Efficacy and safety of Riamilovir in the treatment of patients with COVID-19,” Antibiot. Chemother. 66 (1–2), 33−37 (2021).
K. A. Zykov, E. A. Sinirsyn, A. V. Rvacheva, et al., “Substantiation of a new algorithm for outpatient drug therapy of patients with COVID-19 based on the principle of multiple exposures,” Antibiot. Chemother. 66 (3–4), 49−61 (2021).
K. V. Kas’tanenko, K. V. Kozlov, O. V. Mal’tsev, et al., “Evaluation of the effectiveness of riamilovir in the complex therapy of patients with COVID-19,” Ther. Arch. 93 (3), 290−294 (2021).
A. U. Sabitov, P. V. Sorokin, and S. Yu. Dashutina, “Experience of prophylactic use of the drug Riamilovir in the foci of coronavirus infection (COVID-19),” Ther. Arch. 93 (4), 435–439 (2021).
S. V. Chepur, A. V. Smirnova, A. N. Kirienko, et al., “Study of the activity of the drug Riamilovir against SARS-CoV-2 infection in Syrian hamsters,” Antibiot. Chemother. 66 (7–8), 13−19 (2021).
X. Wu, K. Yu, Y. Wang, et al., “Efficacy and safety of triazavirin therapy for coronavirus disease 2019: A pilot randomized controlled trial,” Engineering 10, 1185−1191 (2020).
A. A. Spasov, A. F. Kucheryavenko, V. S. Sirotenko, et al., “Antiplatelet activity of riamilovir under conditions of lipopolysaccharide intoxication,” Bull. Exp. Biol. Med. 173, 41−45 (2022).
Funding
This work was supported by the Russian Foundation for Basic Research and the National Natural Science Foundation of China within research project no. 20-53-55003.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
The authors declare that they have no conflicts of interest.
Additional information
Translated by B. Alekseev
RAS Academician Valerii Nikolaevich Charushin is a Vice President of the RAS, Chair of the RAS Ural Branch, and Chief Researcher at the Laboratory of Heterocyclic Compounds, the Postovskii Institute of Organic Synthesis (IOS), RAS Ural Branch. RAS Corresponding Member Vladimir Leonidovich Rusinov is Head of the Department of Organic and Biomolecular Chemistry, the UrFU Institute of Chemical Engineering, and a Leading Researcher at the IOS Laboratory of Heterocyclic Compounds, RAS Ural Branch. Mikhail Viktorovich Varaksin, Cand. Sci. (Chem.), is Director of the UrFU Institute of Chemical Engineering and a Researcher at the IOS Laboratory of Coordination Compounds, RAS Ural Branch. RAS Academician Oleg Nikolaevich Chupakhin is IOS Research Supervisor, RAS Ural Branch. RAS Academician Ol’ga Petrovna Kovtun is UrSMU Rector. RAS Academician Aleksandr Alekseevich Spasov is Head of the VSMU Department of Pharmacology.
Rights and permissions
About this article
Cite this article
Charushin, V.N., Rusinov, V.L., Varaksin, M.V. et al. Development of Drugs with Direct Antiviral Action Based on Azaheterocyclic Systems. Her. Russ. Acad. Sci. 92, 505–510 (2022). https://doi.org/10.1134/S1019331622040104
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1019331622040104