Abstract
Molecular-dynamics simulations are employed to study the glass transition of cyclic polystyrene melts. Gibbs and DiMarzio’s theory predict an increase in glass transition temperature, Tg upon lowering the length of cyclic polymer chains, which is opposite to the well-known trend for linear polymers. Their theory has been confirmed by some experiments; however, others observe a decrease in Tg upon lowering the chain length instead. When volumetric methods are employed to obtain the glass transition temperature in simulated cyclic polystyrene, a slight increase with decreasing cyclic polystyrene chain length is obtained. This increase is more pronounced when glass transition temperatures are obtained from dynamics. Both the glass transition temperature Tg obtained from diffusion data and the ideal glass transition temperature T0 obtained from the decay of the orientational autocorrelation function of the phenyl bond show a clear upturn.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
INTRODUCTION
The molecular-weight dependence of the glass-transition temperature Tg of cyclic polymers is still an open question. A recent article from Floudas group summarizes the experimental results so far [1]. A strong reduction of Tg with decreasing molecular weight (Mn) for linear chains can be fitted by the well-known Flory-Fox relation [2]: \({{T}_{{\text{g}}}} = {{T}_{{{\text{g}},\infty }}} - \frac{K}{{{{M}_{{\text{n}}}}}}.\) This reduction is due to an increase of mobile chain ends for short chains, and a corresponding increase in available free volume. This leads to an increase in overall mobility and hence to a lower glass transition temperature. In cyclic polymers, chain ends are absent, hence any observed dependence of Tg on the molecular weight must originate from other effects. Given the glass-transition phenomenon still lacks a theoretical explanation, knowledge of the molecular-weight dependence of Tg in a cyclic topology may contribute helpful insight.
Unfortunately, the existing literature does not agree on the effect of molecular weight on the glass transition temperature [1–8]. Some groups have reported a decrease of Tg, others an increase of Tg with decreasing chain length. In all cases, the effect is much smaller than that for linear chains and there is no obvious curve that fits the data. Some of the earliest cyclic polystyrene studies in the eighties show a small decrease in Tg with decreasing molecular weight [3]. However, this could be attributed to a larger fraction of linear chains contaminating the lower molecular weight systems. Nevertheless, recent experiments with better systems have confirmed the results. Both Differential Scanning Calorimetry (DSC) [4, 6] and Dielectric Spectroscopy (DS) studies of cyclic polystyrene [1] show that the glass transition temperature drops for short chains.
To the contrary, rather old DSC studies on cyclic poly(dimethylsiloxane) (PDMS) by Clarson et al. show an increase in Tg with decreasing polymerization degree N whose magnitude is about half that of the decrease for linear polymers of the same molecular weight [7]. These results for PDMS have been confirmed more recently in DS measurements by Kirst et al. [8]. The glass transition temperature increases upon a decrease in polymerization degree from 60 to about 10 repeat units. Upon decreasing it further, no additional increase is observed. These authors also performed dynamic Monte Carlo simulations of the same system, which are in agreement with their experimental data.
It is not entirely clear if the qualitative difference of these trends for two polymers results from the difference in chemical structure (PS versus PDMS) or if technicalities of measurement procedures play a role. For instance, the analysis of the DS data requires the choice of a reference frequency.
From a theoretical perspective, the only available theory—that by Gibbs and DiMarzio—predicts an increase of Tg with decreasing N for cyclic polymers independent of chemical detail [9]. The theory is based on the entropic theory of the glass transition [10]. Due to topological constraints, cyclic polymers have a lower entropy than linear ones. This means that the glass-transition temperature of cyclic systems is higher than that of the linear polymers of the same length, since one needs to lower the temperature less to reach the temperature at which the entropy vanishes, which is exactly the definition of the glass transition temperature in entropy-based theories. Gibbs and DiMarzio obtained a detailed expression for the entropy of linear polymers [9]. A calculation of the transition point at which the entropy vanishes agrees with the data for linear polymers. An extension to cyclic polymers predicts a small increase in the transition temperature with decreasing chain length. This approach is strikingly elegant in that there are only two input parameters: the hole energy Eh and the flex energy Δε. Eh characterizes the interaction between chemically non-bonded polymer segments, whereas Δε represents the difference between the higher and the lower minima of the rotational potential. Eh and Δε can be obtained from a fit of the Tg data for linear polymers. The values for cyclic analogues are then essentially based on a non-parametric prediction. This prediction matches the poly(dimethylsiloxane) data of Clarson et al. very well [7]. However, the theory also predicts an increase in Tg with decreasing N for polystyrene, which has not been observed experimentally [1].
In this manuscript, we report on computer simulations of thermal properties of the cyclic, atactic polystyrene melt. One reason to use computational tools is that the in-silico made samples contain no contamination with linear polymers, while experimentally synthesizing cyclic polymers of high purity is very challenging. A second reason is the ability to obtain transition temperatures of the same system using a range of volumetric and dynamic measurements. We chose polystyrene since for this polymer a contradiction between experimental results and theory has been reported. The next section describes the polystyrene model and the simulation details. A Results and Discussion section follows this. The simulations show an increase in glass transition temperature with decreasing chain length. The effect is not very pronounced in volumetric measurements. However, the glass transition temperature obtained from dynamic measures, such as diffusion or the phenyl bond orientation relaxation shows a clearly detectable increase in agreement with the theory. The manuscript ends with a Conclusions Section.
MODEL AND SIMULATION METHOD
Molecular-dynamics simulations have been performed for bulk cyclic atactic polystyrene samples. A sample consists of either 32 cyclic polystyrene (cPS) chains of 10 monomers, or 16 cPS chains of 20 monomers, or 16 cPS chains of 40 monomers, or 8 cPS chains of 80 monomers, or 8 cPS chains of 160 monomers. Each monomeric repeat unit contains one benzene ring. The molecular weight of the longest chain is ca. 16.7 kDa, and is below the entanglement value for cyclic PS polymers [11]. Periodic boundary conditions have been applied in all three dimensions.
As in the previous studies, the united-atom model has been applied in the simulations [12–14]. Figure 1 shows the cPS monomeric structure, which consists of two backbone (‒CH‒CH2‒) united atoms and the phenyl ring—the aromatic side group of six united atoms. Hydrogen atoms are collapsed onto the corresponding carbons. The stereochemical configurations of the aromatic groups were generated at random, so that the ratio of meso to racemic dyad ended up near unity. The configurations of the aromatic groups were generated independently for each polymer chain. For these cyclic polymers care has been taken that the internal knots and concatenation of the polymeric cycles are prevented during the preparation step.
Interactions between the united atoms are described by the following potential:
The first term denotes the non-bonded contribution for particles separated by more than two valence bonds, or belonging to different chains, the second and third terms describe bending and stretching, the fourth and fifth terms define the torsional contribution of the backbone and phenyl ring respectively; other terms describe the phenyl ring out-of-plane bending potential, the bending and torsional potential around the Carom–Carom bond and, the improper-torsion potential. The cPS united-atom force field is identical to the one employed in previous studies of the glass transition in linear PS; details can be found there [12–14].
The velocity Verlet algorithm is used to integrate Newton’s equations of motion with a time step of 4 fs [15]. The temperature is controlled using a collisional thermostat [16, 17]. All the simulated systems are initially equilibrated at 600 K, well above the simulated glass transition. The cPS simulations are started from all-trans conformations at very low density (0.012 g/cm3), as shown in Fig. 1. The simulation box is then compressed isotropically with the velocity of 0.1 Å/ps to a target density of 1 g/cm3. At the end of the compression, a short (about 500 ps) NVT run is performed. Subsequently, the melt was further equilibrated in the NpT ensemble at zero pressure using a Berendsen barostat [15]. The total equilibration time is up to 10 μs for the longest chain. At this point statistical quantities like density and radius of gyration do not show systematic trends any longer.
Subsequently, the cPS samples are cooled at a velocity of 0.01 K/ps starting from T = 600 K, keeping the pressure fixed at zero. This simulated cooling is extremely fast compared to the cooling rates used in experiments. We have discussed the consequences of this fast simulated cooling in a recent publication [18]. The cooling is performed in a temperature range between 600 K (well above the glass-transition temperature) and 250 K (well below). Finally, for each chain length and temperature a production run is performed, starting from the initial configurations obtained during the cooling runs. All the production runs are performed in the NpT ensemble at zero pressure. During the production runs, the polymer configurations are stored every 4 ps for further analysis.
RESULTS AND DISCUSSION
Figure 2 shows the specific volume as a function of temperature. For clarity, data for three of the five chain lengths studied are shown. The specific volume is obtained from the average size of the simulation box, which fluctuates during the run. These fluctuations are used to calculate the error in the data, whose order of magnitude is about the size of the symbols. Linear fits to the highest five and lowest four data points are shown. The temperature at which these lines cross is the simulated glass transition temperature Tg [19, 20]. The calculated Tg value for all chain lengths are given in Table 1. The error listed in brackets is obtained from the errors in the fits. There is a slight tendency for the glass transition temperature to increase with decreasing chain length. As was addressed in detail in previous work the simulated glass transition temperatures are higher than in experiments, due to faster cooling rates [18, 21].
The simulated specific volume versus temperature dependences for cPS melts composed of chains with different N = (1) 10, (2) 40, (3) 160. Lines show the linear fits used in the high-T and low-T regions separately. For each simulated sample, the crossover temperature here and on Fig. 3 determines the glass transition.
All transition temperatures are higher than those observed in simulations of linear PS, which is due to the absence of chain ends [21]. More importantly, the simulations of linear chains show a significant decrease of Tg with decreasing chain length, in agreement with experiments and the Flory-Fox relation. This decrease is caused by a decrease in specific volume of shorter chains in the glassy state below Tg. In our simulations of cyclic chains this trend is not observed. However, in the melt above Tg the specific volume is slightly smaller for shorter cPS chains, i.e. the density is larger, which is the reason for the slight dependence of the transition temperature on N. Although the reason for this increase in density is not known, Bannister and Semlyen reported a similar trend for experimental studies of cyclic PDMS [22]; the density of cyclic PDMS slightly increases upon a decrease in polymerization degree from about 200 to 10 repeat units. Upon decreasing it further, a sharp decrease is observed—this latter regime is below the ones we consider for this study.
Subsequently, we look at the translational diffusion of the center of mass (CM) of each cPS chain. As in our previous work [20] and that of others [23], the glass transition temperature can be obtained by plotting the mean-squared displacement (MSD) obtained after a certain time interval. Figure 3 shows this plot for a time interval of 400 ps, the same interval as used in the references above. We verified that the results are not very sensitive to the interval chosen. The glass-transition temperatures are again obtained from fits to data at low and high temperatures [20, 23]. Table 1 also lists the glass transition temperatures obtained from the translational displacements (not shown here) of the individual united atoms obtained using the same method. In both cases, a systematic increase of Tg with decreasing N is observed, this trend is more pronounced than that observed in the volumetric data.
Notice that the Tg values produced by the translational mobility measurements are up to 70 K higher than those obtained from the volumetric measurements. Our previous studies on the glass transition of PS films showed likewise that higher transition temperatures are obtained from dynamic methods than from static ones [20].
As a fourth measure for the glass transition temperature, we use the orientational mobility of the phenyl bonds [20]. The orientational autocorrelation function is given by the second order Legendre polynomial for the reorientation of the side bond, the one connecting the phenyl ring C-atom to the backbone CH united atom (see Fig. 1). It is defined as
where b is the vector representing the bond between the phenyl ring and backbone. Figure 4 shows a sample of this autocorrelation function at T = 510 K. The data are averaged over all cPS chains of a given length and shown for N = 10, 40, 160.
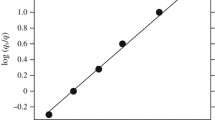
Subsequently, for every chain length at each temperature the P2 data are fitted to stretched exponentials: \({{P}_{2}} = a{{e}^{{ - {{{\left( {\frac{t}{\tau }} \right)}}^{\beta }}}}}.~\) From these fits inverse relaxation times (τ–1) are obtained. These are plotted in Fig. 5 as a function of inverse temperature. The Vogel-Fulcher Tammann (VFT) relation is fitted to obtain the ideal transition temperature T0:
The logarithm of the inverse relaxation times (in ps–1) obtained from fits of P2 relaxation data to stretched exponentials, as a function of inverse temperature (symbols). T0 is obtained from fits of the data to Eq. (3) (lines). N = (1) 10, (2) 40, (3) 160.
Only three representative chain lengths are plotted in Fig. 5; the results for all N are given in Table 1. In agreement with the other results, the glass transition temperature—here called T0—increases with decreasing chain length.
According to the Adam-Gibbs (AG) theory, the longer relaxation time at lower temperatures is a manifestation of a decrease in configurational entropy due to structural changes in the system [24]. The ideal glass transition temperature T0 is the temperature at which the configurational entropy extrapolates to zero. Clearly, this T0 temperature is lower than an empirical crossover temperature Tg, which separates two thermal regimes with qualitatively different dependencies of either density or diffusivity. Experimentally [24], the ratio Tg/T0 is roughly 1.3, which is in good agreement with our results. The slower relaxation of the side phenyl bond for shorter cPS as observed in Fig. 4 implies a smaller configurational entropy. As a result, the shorter cPS have to be cooled less than the longer cPS in order for the configurational entropy to vanish. This causes the increase in T0 upon lowering chain length. Note that the slower local relaxation of phenyl bonds is most likely caused by the fact that at temperatures above the transition one shorter cyclic chains pack denser than longer ones (see Fig. 2).
CONCLUSIONS
The simulations above find that the glass-transition temperature of cyclic PS polymers increases with decreasing chain length. If obtained from specific volume, the effect is extremely small. On the other hand, data analyzed using dynamic methods, like CM diffusion, united atom diffusion, and the orientational relaxation of the second Legendre polynomial, show a distinct increase with decreasing N.
It is well known that the glass transition can be characterized by a range of temperatures [24, 25]. In a recent paper by Lipson et al. on the connection between the glass transition and free volume, two temperatures are emphasized [26]. The first transition at Tg signals a qualitatively different dependence of volume on temperature as a result of structural reorganization. It resembles the glass transition in an associating polymer system at which there is a steep increase in intermolecular connections [27]. This transition can be obtained from quasi-static experiments. The second transition at T0 is defined as the temperature at which all volume is “occupied” and is connected to the Gibbs and DiMarzio ideal glass transition temperature at which the configurational entropy vanishes [28, 29]. At this temperature T0 the relaxation time or viscosity approaches infinity and the system behaves as a solid. The data presented in this study indicate that both temperatures, Tg and T0, increase with decreasing chain length. This is in agreement with the Gibbs-DiMarzio’s theory [9], however some DSC and DS experiments on cPS detected an opposite trend [1].
Ultimately, the increase in the glass transition temperature with decreasing cPS chain length we obtained from their dynamics might be due to the observed increase in density with decreasing cPS chain length for temperatures above the transition. The shortest cyclic polymers (N = 10) have a higher density, hence a slower decaying orientational autocorrelation function, which in turn leads to a larger transition temperature. Given this increase in cPS’s glass transition temperature with decreasing N is predicted by Gibbs-DiMarzio’s theory [9], we speculate that the observed increase in density of cyclic polymers with decreasing chain length could be due to configurational entropy. We hope that the results reported in this paper shed some light on the puzzle of the glass transition of cyclic polymers.
REFERENCES
A. Pipertiz, Md. D. Hossain, M. J. Monteiro, and G. Floudas, Macromolecules 51, 1488 (2018).
G. B. McKenna, in Comprehensive Polymer Science, Ed. by G. Allen, J. C. Bevington, C. Booth, and C. Price (Pergamon Press, Oxford, 1989).
J. Roovers, Macromolecules 18, 1359 (1985).
P. G. Santangelo, C. M. Roland, T. Chang, D. Cho, and J. Roovers, Macromolecules 34, 9002 (2001).
D. Huang, S. L. Simon, and G. B. McKenna, J. Chem. Phys. 122, 084907 (2005).
L. Zhang, R. Elupula, S. M. Grayson, and J. M. Torkelson, Macromolecules 50, 1147 (2017).
S. J. Clarson, K. Dogson, and J. A. Semlyan, Polymer 26, 39 (1958).
K. Kirst, F. Kremer, T. Pakula, and J. Hollingshurst, Colloid Polym. Sci. 272, 1420 (1994).
J. H. Gibbs and E. A. Di Marzio, J. Chem. Phys. 28, 373 (1958).
E. A. Di Marzio and C. M. Guttman, Macromolecules 20, 1403 (1987).
E. R. Semlyan, Cyclic Polymers (Springer, New York, 1986).
A. V. Lyulin and M. A. J. Michels, Macromolecules 35, 1463 (2002).
A. V. Lyulin, N. K. Balabaev, and M. A. J. Michels, Macromolecules 35, 9595 (2002).
B. Vorselaars, A. V. Lyulin, and M. A. J. Michels, Macromolecules 40, 6001 (2007).
M. P. Allen and D. J. Tildesley, Computer Simulation of Liquids (Clarendon Press, Oxford, 1987).
A. S. Lemak and N. K. Balabaev, Mol. Simul. 15, 223 (1995).
A. S. Lemak and N. K. Balabaev, J. Comput. Chem. 17, 1685 (1996).
A. V. Lyulin, N. K. Balabaev, A. R. C. Baljon, G. Mendoza, C. W. Frank, and D. Yoon, J. Chem. Phys. 146, 203314 (2017).
A. R. C. Baljon, R. Barber DeGraaff, M. H. M. van Weert, and R. Khare, Macromolecules 38, 2391 (2005).
A. R. C. Baljon, S. Williams, N. K. Balabaev, F. Paans, D. Hudzinskyy, and A. V. Lyulin, J. Polym. Sci., Part B: Polym. Phys. 48, 1160 (2010).
A. V. Lyulin, N. K. Balabaev, and M. A. J. Michels, Macromolecules 36, 8574 (2003).
D. J. Bannister and J. A. Semlyen, Polymer 22, 377 (1979).
Y. Zhou and S. T. Milner, Macromolecules 50, 5599 (2017).
G. Adam and J. H. Gibbs, J. Chem. Phys. 43, 139 (1965).
J. Dudowicz. K. Freed, and J. Douglas, J. Chem. Phys. 109, 21350 (2005).
R. P. White and J. E. G. Lipson, Macromolecules 49, 3987 (2016).
A. R. C. Baljon, D. Flynn, and D. Krawzenek, J. Chem. Phys. 126, 044907 (2007).
T. G. Fox and P. J. Flory, J. Appl. Phys. 21, 581 (1950).
T. G. Fox and P. J. Flory, J. Polym. Sci. 14, 315 (1954).
R. Shima and R. F. Boyer, J. Chem. Phys. 37, 1003 (1962).
ACKNOWLEDGMENTS
This work was partly carried out on the Dutch national e-infrastructure with the support of SURF Cooperative. Simulations were also carried out using supercomputers at SDSU’s Computational Sciences Research Center and the Joint Supercomputer Center of the Russian Academy of Sciences.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Baljon, A.R., Mendoza, G., Balabaev, N.K. et al. Glass-Transition Temperature of Cyclic Polystyrene: A Computational Study. Polym. Sci. Ser. A 63, 356–362 (2021). https://doi.org/10.1134/S0965545X21030019
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0965545X21030019