Abstract
Solutions of poly(acrylic acid) mixtures with poly(sodium styrenesulfonate) in water and in decimolar hydrochloric acid are studied by potentiometry, viscometry, and rotational rheometry. It is shown that, in a wide range of mixture compositions, thermosensitive polyelectrolyte complexes are formed via ion–dipole interactions of functional groups of polymers. The formation of complexes in the semidilute solution regime is accompanied by a significant increase in viscosity as compared with solutions of the initial polymers, which makes it possible to obtain systems with high viscosity without using large concentrations of polyelectrolytes. The dependence of viscosity on the composition of the mixture passes through a maximum corresponding to the most complete binding of the components of the complex, and the position of the maximum does not depend on the molecular weight of poly(sodium styrenesulfonate) and is determined by the ratio of the concentrations of the functional groups of the polymers. The frequency dependences of the storage modulus and the loss modulus demonstrate that the system experiences strong structuring in the process of complex formation.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
INTRODUCTION
Currently, thermosensitive water-soluble polymers are of great interest owing to the prospect of their use as smart polymeric materials, the rheological properties of which can be tuned by changing not only the solution concentration but also the temperature. The use of polyelectrolytes for this purpose, however, is limited, because of two main reasons. First, the viscosity of semidilute salt-free aqueous solutions of polyelectrolytes is significantly lower than the viscosity of solutions of nonionic polymers of appropriate concentrations. Secondly, for charged macromolecules, water is a good solvent in a wide range of temperatures; therefore, it is extremely difficult to obtain thermosensitive systems based on polyelectrolytes. Both of these problems can be solved by using water-soluble polyelectrolyte complexes (PECs).
As is known [1–4], in polyelectrolyte solutions, there are four concentration regimes: dilute, semidilute without entanglements, semidilute with entanglements, and concentrated. Concentrated solutions are closer in properties to solid polymers plasticized with a solvent; therefore, the focus of both theoretical and experimental research is on the first three regimes. Transition from the dilute to semidilute regime (crossover) occurs under conditions in which the entire volume of the solution is filled with polymer coils, that is, when the average concentration of monomer units in solution becomes equal to the concentration of units inside the coil [5]. The crossover concentration C* can be estimated from the inverse intrinsic viscosity [6]: C* ≈ 1/[η]. In the crossover region, the mechanism of thermal motion of macromolecules changes: if C < C*, the translational diffusion of isolated polymer coils is realized, then at C > C* cooperative diffusion modes appear [7–12], which also allows one to determine C* experimentally. However, the mechanism of solution flow at C > C* remains translational. This is a significant difference between solutions of polyelectrolytes from solutions of nonionic polymers: in the latter case in the region of C*, the interpenetration of polymer coils and the formation of entanglement network begin, with the solution flow mechanism changing from translational to reptational, and the viscosity increases significantly [13, 14]. For formation of the entanglement network in polyelectrolyte solutions, it is necessary to shield electrostatic interactions of like-charged chains, and the concentration of formation of the entanglement network (Ce) in salt-free solutions may exceed C* one to two orders of magnitude [3, 4]. In the presence of low molecular weight salts, shielding occurs not only due to the polyelectrolyte’s own counterions but also due to salt ions; as a result, the difference between C* and Ce decreases. Ce is determined experimentally from a change in the exponent of the concentration dependence of viscosity [3, 4, 15].
The concentration regime of solution significantly affects the reactions of polyelectrolytes with oppositely charged objects: macromolecules [16, 17], colloidal particles [18], and surfactant micelles [19–21]. The products of such reactions (PECs) in dilute solutions, as a rule, are isolated particles of the characteristic composition. While the properties of PECs in dilute solutions are studied in detail by the example of a huge number of various systems (e.g., review [22]), a much smaller number of publications are devoted to the formation of complexes in semidilute solutions. In particular, it was shown [17, 21] that complexation in this concentration regime can lead to the formation of a double network, which contains both entanglement nodes and interchain bonds. Such structuring significantly expands possibilities of the practical application of polyelectrolytes, since it makes it possible to obtain polyelectrolyte systems with high and easily adjustable viscosity.
As a rule, PECs are formed due to the formation of a system of salt bonds between oppositely charged functional groups. However, if the polyelectrolyte is nonionized, PECs can be formed due to other, weaker types of interactions. These PECs are of particular interest, since weak interactions are easily and reversibly destroyed with a change in temperature, which gives the prospect of obtaining thermosensitive poly-electrolyte systems. For example, poly(acrylic acid) (PAA) and poly(methacrylic acid) complexes with poly(ethylene glycol) formed via hydrogen bonding and hydrophobic interactions are well known [23]. In addition, PAA complexes with both polycations [24, 25] and polyanions [12, 26, 27] formed due to ion–dipole interactions of nonionized carboxyl groups with charged functional groups of polyelectrolytes were discovered. In this paper, our goal was to investigate the effect of complexation on the rheological properties of semidilute solutions of PAA mixtures with poly(sodium styrenesulfonate) (PSS).
EXPERIMENTAL
Objects of Research
PAA was obtained by the hydrolysis of poly(tert-butyl acrylate) synthesized by pseudoliving radical polymerization via the reversible chain transfer mechanism [24]; Mw = 120.5 × 103 and Ð = 1.3.
Two PSS samples (Scientific Polymer Products, United States) were purified of low molecular weight impurities using dialysis and then were freeze dried; Mw = 20.4 × 103 (PSS-1) and Mw = 9.6 × 105 (PSS-2).
Decimolar HCl and NaOH were prepared from Testal fixanals (Germany).
The initial aqueous and hydrochloric acid solutions of PAA and PSS were prepared by dissolving the polymers in water or 0.1 M HCl, respectively, under stirring for 48 h. The concentration of PAA was determined by potentiometric titration in a water–acetone mixture (50 : 50 vol %). The PSS concentration was determined spectrophotometrically at a wavelength of 262 nm; the extinction coefficient was ε = 383 L/(mol cm) [15].
PAA–PSS mixtures were prepared from stock solutions of the same weight concentration taken in different ratios. The composition of the mixtures was characterized by the molar fraction of PAA units (Z = νPAA/(νPSS + νPAA), where νPAA and νPSS are the molar concentrations of PAA and PSS units, respectively.
Analytical Methods
Potentiometric titration. Potentiometric measurements were performed using a pH 211 pH meter (Hanna Instruments, Germany) with a combined glass electrode and thermal compensation. The instrument was calibrated using standard buffer solutions with pH 7.01 and 4.01. A constant pH value was established within 2–4 min (accuracy, ±0.02 pH units). All measurements were carried out at a temperature of 25°C. The degree of ionization of PAA α was calculated as
(in square brackets, the molar concentration of functional groups is indicated).
Capillary viscometry. The relative viscosity of solutions ηrel was determined using Ubbelohde capillary viscometers (solvent efflux times are 18.9 and 74.5 s at 25°C). Semidilute solutions were studied using a viscometer with a shorter efflux time; for dilute solutions, with a longer efflux time. In the transition concentration range, both viscometers were used to verify the correctness of the results. Before measurements, the samples were thermostatted for 15 min, and the temperature in the cell was maintained with an accuracy of ±0.2°C. The reduced viscosity ηred was calculated by the formula \({{\eta }_{{{\text{red}}}}} = \frac{{{{\eta }_{{{\text{rel}}}}} - 1}}{C}\), where C is the solution concentration (g/dL).
Rotational viscometry. The dynamic viscosity was determined on a Rheotest 2.1 rotational viscometer (Germany) with a cylinder–cylinder operation unit in the constant shear stress regime. Prior to measurements, all samples were thermostatted for 15 min; the temperature in the thermostating cell was maintained with an accuracy of ±0.2°C.
The temperature dependences of the highest Newtonian viscosity were analyzed using the Arrhenius–Frenkel–Eyring equation in logarithmic form:
where A = 10-4 Pa s is the pre-exponential factor, which has the physical meaning of viscosity, corresponding to the thermal oscillation of atoms, and ΔS and ΔH are the entropy and enthalpy of the activation of viscous flow, respectively [28].
Rotational rheometry. The frequency dependences of the storage modulus and the loss modulus were determined on a RheoStress 600 rotary rheometer in a plane–plane cell with a plane diameter of 35 mm and a gap of 0.1 mm at a temperature of 25°C. The measurements were performed in the mode in which the device sets the stain at a constant amplitude of 0.002 mm and registers the shear stress in the frequency range of 0.01–100 Hz. Moduli were calculated in the program Rheo Win Data Manager.
RESULTS AND DISCUSSION
Determining the Boundaries of Concentration Regimes
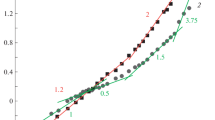
For determining C* and Ce in solutions of the initial polyelectrolytes and their mixtures of different composition, the method of capillary viscometry was used. The solutions of polymers and their mixtures were prepared in 0.1 M HCl. Under these conditions, the carboxyl groups of PAA are not ionized, it behaves like an uncharged polymer, and the effect of polyelectrolyte swelling is suppressed in PSS solutions. Figure 1 shows the dependences of the specific viscosity ηsp on concentration in logarithmic coordinates for solutions of PSS-1, PAA, PSS-2, and PSS-2–PAA mixtures of different composition.
Dependences of logarithm of specific viscosity on logarithm of concentration for (a) (1) PSS-1 and (2) PAA; (b) PSS-2; (c) PSS-2–PAA mixtures of composition Z = (1) 0.42, (2) 0.66, and (3) 0.81; and (d) the initial regions of dependences for (1) PSS-1 and mixtures of composition Z = (2) 0.42, (3) 0.66, and (4) 0.81. T = 25°C.
The results were analyzed using the scaling model of polyelectrolyte solutions [1, 3, 15, 29]. For all studied solutions in the low-concentration range, ηsp ∝ C1.1–1.22. According to the scaling model, this corresponds to a dilute high-salt regime (dynamic viscosity η ∝ C1) [1, 15]. On the basis of deviation from the linear dependence, the crossover concentration C* can be determined. The concentration of formation of the entanglement network Ce was determined from achievement of the second linear section by dependences; the results are presented in Table 1. Since the dependence for short-chain PSS-1 is linear over the entire concentration range studied, Table 1 gives the value of C* obtained by light scattering [12]. Estimation from the magnitude of the inverse intrinsic viscosity gave an obviously overestimated result (4.3%).
In the high-concentration range, ηsp ∝ C3.75 for PAA and all solutions of PAA–PSS mixtures, which corresponds to the semidilute entangled regime for both uncharged polymers (PAA) and polyelectrolytes with a high content of low molecular weight electrolyte [1, 29]. In the case of PSS-2 (Fig. 1b) at a concentration above 3%, ηsp ∝ C1.5, which corresponds to the semidilute entangled low-salt regime [1, 15]. Since the study was carried out at a fixed concentration of a low molecular weight electrolyte (0.1 M HCl), as the concentration increases, the PSS-2 solution changes from the unentangled high-salt regime to the entangled low-salt one.
For short-chain PSS-1 (Fig. 1a), the dependence is linear over the entire concentration range. This means that PSS-1 does not form an entanglement network, which is probably connected with an overly short chain length (about 100 units). The transition from the dilute to semidilute entanglement regime, which is accompanied by a change in the flow mechanism, occurs when each polymer chain forms from 5 to 10 entanglement nodes with adjacent chains [30, 31]. This condition is hardly achievable for short-chain polymers.
The values of C* and Ce for PAK–PSS mixtures, within the error, do not depend on the ratio of components and amount to C* = 1.7 ± 0.1 and Ce = 3.8 ± 0.5 wt % for systems containing PSS-1 and C* = 0.7 ± 0.2 and Ce = 1.7 ± 0.2 wt % for systems containing PSS-2. It is important to note that the concentrations at which the structuring of the solution of mixtures begins are significantly lower than the concentration of the entanglement network formation in the PAA solution (8%). This means that, in the solutions of mixtures, the network is formed not only due to the formation of entanglements but also as a result of interchain interactions PAA–PSS.
The results show that, at a concentration of above 2%, all the systems studied occur in the semidilute concentration regime. Thus, the properties of PAA–PSS mixtures in dilute solutions were studied at a concentration of about 0.1%, and the structure formation in the solutions of mixtures was investigated in a concentration range of 2–15%, which includes both the semidilute unentangled regime and the region of semidilute solutions with entanglements.
Potentiometry of Dilute Solutions
Since only nonionized functional groups of PAA can participate in ion-dipole interactions, complex formation with PSS should shift the equilibrium in the dissociation reaction of carboxyl groups. Figure 2 presents the results of the potentiometric titration of 40 mL of PAA solution with PSS-2 solution (curve 1) together with the data obtained upon dilution of the initial solution with water (curve 2).
When a PAA solution is diluted with water, pH increases slightly, which is associated with a decrease in the concentration of carboxyl groups. In this case, the degree of ionization of PAA remains almost unchanged. At the same time, the pH of the system during titration with the aqueous solution of PSS increases noticeably. The degree of ionization of the carboxyl groups of PAA decreases during titration (Fig. 3).
It should be noted that a similar experiment was carried out in [27] with a total polymer concentration of 4%, that is, in the regime of semidilute solutions. It was found that the pH of the mixture decreases compared with solutions of the starting components. The authors associate the result with the penetration of PSS chains into the PAA entanglement network; as a result, the polyelectrolyte contributes to the dissociation of carboxyl groups like the addition of low molecular weight salts. In our experiment, the concentration of PAA (0.144%) is lower than C*. Under these conditions, no entanglement network is formed. Our results suggest that, in dilute solutions, the nonionized carboxyl groups of PAA are involved in interactions with charged PSS groups. In such a system, either interchain hydrogen bonds or ion-dipole interactions can be realized. Since, as was shown by FTIR spectroscopy [27], the proportion of hydrogen bonds in the PAA–PSS system is much smaller than that in the absence of PSS; therefore, it is the ion-dipole interactions that make the main contribution to complexation.
The data presented in Fig. 3 indicate that, even in the twofold excess of the PSS, a part of the carboxyl groups of PAA remains in the ionized state. The degree of ionization α = 0.0125 at a degree of polymerization of 1680 corresponds to about 20 charges per polymer chain. Electrostatic repulsion of like-charged chains of PAA and PSS can reduce the proportion of groups bound by ion-dipole interactions. This effect can be eliminated using the dependence of the degree of dissociation on pH characteristic of weak polyelectrolytes. With the introduction of strong low molecular weight acids into the solution, the ionization of the carboxyl groups of PAA is completely suppressed; therefore, a part of subsequent experiments were carried out in the presence of 0.1 M HCl.
PAA–PSS Mixtures in Semidilute Solutions
The viscosity of a mixture of two polymers in a common solvent can be described using the logarithmic additivity rule [32]. The dependence of the logarithm of viscosity on the composition of the mixture is linear in the absence of interaction. In the case of interaction of polymers, there is a positive deviation from linearity; in the case of incompatibility, a negative one.
Figure 4 shows the logarithm of dynamic viscosity as a function of composition for PAA–PSS mixtures in 0.1 M HCl (for PAA the degree of ionization α = 0), in water (α = 0.01), and under conditions corresponding to the ionization of half of the carboxyl groups of PAA (the degree of ionization α = 0.5 created by adding the calculated amount of NaOH). For systems shown in Fig. 4a, there is a significant positive deviation from additivity, which indicates the interaction of two polymers in the system. Maximum binding occurs in the range of mixture compositions Z = 0.5–0.7. It is important to note that this composition range corresponds to the region of maximum binding of components in dilute solutions, as was shown in [12] for the PAA–short-chain PSS system.
The dependences for solutions in water and hydrochloric acid coincide within the limits of error; hence, the residual ionization of PAA hardly affects complex formation. At the same time, when PAA is strongly charged, electrostatic repulsion causes the PAA–PSS complex to collapse, with a slight negative deviation from linearity (Fig. 4b). An increase in the molecular weight of PSS does not change the position of the maximum of viscosity dependence on composition (Fig. 4a). The close location of the viscosity maxima for systems containing long- and short-chain PSS indicates that the maximum binding is achieved at approximately the same concentration ratio of sulfo and carboxyl groups, regardless of the molecular weight of PSS. This fact further confirms that it is precisely the interaction of functional groups that underlies complexation in the PAA–PSS system. A significant increase in the viscosity of the mixtures as compared with the viscosity of the solutions of the initial polymers is observed in the semidilute solution regime; there is no such effect in dilute solutions (compare the initial sections in Fig. 1d). Consequently, an increase in viscosity during complexation is due to the different structure of the networks of semidilute solutions of the complexes and initial polyelectrolytes.
Rheological Properties of Semidilute Solutions of PAA–PSS Mixtures
The effect of complexation on the properties of networks formed in semidilute solutions was studied by rotational viscometry. Figure 5 shows the flow curves of semidilute aqueous solutions of PAA, PSS‑2, and their mixtures. It can be seen that the dynamic viscosity of solutions of the complexes exceeds the viscosity of solutions of individual polyelectrolytes by more than an order of magnitude. In addition, the solutions of the complexes are characterized by anomalous viscous properties: the flow curves at low shear rates reach a plateau corresponding to the highest Newtonian viscosity. At high rates, the viscosity decreases with increasing shear rate, which is caused by the destruction of the structure of the solution under the action of shear stress.
The study of the effect of temperature on the rheological properties of semidilute solutions of PAA–PSS mixtures provides an opportunity to obtain additional information about structuring of the system in the process of complexation.
Since the complex is stabilized by weak ion-dipole interactions, it can be expected that it will possess thermal sensitivity. This assumption is confirmed by the dependence of the logarithm of the highest Newtonian viscosity on the molar fraction of the PAA units obtained at different temperatures (Fig. 6). For example, if for the solutions of initial polyelectrolytes the highest Newtonian viscosity decreases by a factor of ~3 with an increase in temperature from 30 to 60°C, then for the solution of the complex with composition Z = 0.74 the viscosity decreases by more than an order of magnitude in the same temperature range. This fact indicates that the degree of binding decreases markedly with increasing temperature; that is, equilibrium in the reaction between PAA and PSS shifts toward the initial polyelectrolytes. This fundamentally distinguishes this system from complexes formed with participation of salt bonds that do not exhibit thermal sensitivity.
At the same time, the data presented indicate that, even at a temperature of 60°C, the type of dependence corresponds to a system with interacting components. In other words, although the degree of binding decreases with increasing temperature, the complexes in semidilute solutions exist over the entire temperature range studied. This means that the activation parameters of their viscous flow can be determined.
The dependences of the logarithm of the highest Newtonian viscosity on the reciprocal temperature for semidilute solutions of PAA, PSS-2, and the complexes of all investigated compositions are linear; the examples of the corresponding dependences are shown in Fig. 7.
From the results of linear approximation, the entropy and enthalpy of activation of the viscous flow were calculated (Experimental); the results are presented in Table 2.
The sign and magnitude of the entropy of viscous flow activation reflect the process of structure change during the flow of semidilute solution. For all samples, except for the PSS-2 solution, the change in entropy is positive. Consequently, the main contribution is provided by the process of structure disordering, that is, network destruction. For the PSS-2 solution, the change in entropy is negative, which indicates that the processes of unfolding and orientation of macromolecules predominate.
The enthalpy of viscous flow activation for the PSS‑2 solution is lower than for the PAA solution. This may be due to a significantly higher charge of the PSS chains compared to the PAA macromolecules. The maxima of enthalpy and entropy of viscous flow activation are in the range of compositions corresponding to the maximum binding of the complexes (Fig. 4).
Rotational Rheometry of Semidilute Solutions of PAA–PSS Mixtures
For a more detailed study of the rheological properties of the resulting networks, the semidilute solutions of PAA, PSS-2, and their mixtures of composition 0.42 were investigated by rotational rheometry. As a result, the frequency dependences of the storage modulus and the loss modulus were obtained, which made it possible to consider separately the viscous and elastic properties of the systems under study.
For solutions of the starting polymers (Fig. 8), the dependences of both moduli on frequency are linear, while G ' ∝ ω2, G '' ∝ ω1, and G ''> G ', which corresponds to the viscous state of the solutions [33, 34]. In solution of the mixture, the frequency dependences of the moduli change dramatically (Fig. 9).
The dependence of the storage modulus on frequency shows a tendency to reach a plateau at high frequencies, the loss modulus in the same region passes through a maximum, and the storage modulus becomes higher than the loss modulus, indicating a transition to the rubbery state.
The exponents in the dependences of the storage modulus and the loss modulus on frequency in the viscous flow range are reduced compared with the dependences obtained for solutions of individual polymers: G' ∝ ω0.8 and G'' ∝ ω0.6, which corresponds to structuring of the system. In addition, in the low-frequency region, the storage modulus shows the tendency to reach the second plateau. Similar low-frequency plateaus were observed previously [35–38] for polylysine complexes with poly(glutamic acid) [35] and for protein hydrogels [36], but the authors did not give an unambiguous interpretation of this phenomenon. The authors of [37] associate the low-frequency plateau with the yield strength of the system and the formation of a solid three-dimensional structure. In [38], the appearance of a low-frequency plateau for DSMO solutions of a ternary copolymer of acrylonitrile, methyl acrylate, and sodium itaconate was explained by structuring due to the formation of interchain hydrogen bonds with participation of the solvent.
CONCLUSIONS
Thus, using the example of the PAA–PSS system, it was shown that the interaction of carboxyl groups of nonionized PAA and sulfo groups of PSS leads to the formation of thermosensitive complexes. The complexes exist in both dilute and semidilute solutions; however, the concentration regime has a decisive influence on the rheological properties of such systems. It was shown that, in the semidilute regime, complexation causes a significant structuring of solutions due to the interaction of functional groups of polyelectrolytes. The three-dimensional structure formed in semidilute solutions is stabilized by both conventional entanglement nodes and the system of interchain ion-dipole interactions. The introduction of the complexing agent not only makes it possible to obtain systems with high viscosity at a relatively small concentration of initial polyelectrolytes but also enables one to regulate the rheological properties of such systems over a wide range by changing the temperature, concentration, or ratio of polymer components.
REFERENCES
A. V. Dobrynin, M. Rubinstein, and R. H. Colby, Macromolecules 28, 1859 (1996).
M. Doi and S. F. Edwards, The Theory of Polymer Dynamics (Clarendon Press, Oxford, 1988).
M. Rubinstein, R. H. Colby, and A. V. Dobrynin, Phys. Rev. Lett. 73, 2776 (1994).
K. C. Tam and G. Tiu, J. Non-Newtonian Fluid Mech. 46, 275 (1993).
P. Debye, J. Chem. Phys. 14, 636 (1946).
M. Muthukumar, J. Chem. Phys. 107, 2619 (1997).
M. Sedlàk, Langmuir 15, 4045 (1999).
P. Štěpánek and W. Brown, Macromolecules 31, 1889 (1998).
T. Nicolai and W. Brown, in Light Scattering: Principles and Development, Ed. by W. Brown (Clarendon Press, New York; Oxford Univ. Press, Oxford, 1996).
A. N. Semenov, Phys. A (Amsterdam) 166, 263 (1990).
N. Boudenne, S. H. Anastasiadis, G. Fytas, M. Xenidou, N. Hadjichristidis, A. N. Semenov, and G. Fleischer, J. Phys. Rev. Lett. 77, 506 (1996).
E. A. Litmanovich, E. V. Kotova, and V. V. Efremov, Colloid Polym. Sci. 297, 371 (2019).
P. G. De Gennes, J. Chem. Phys. 55, 572 (1971).
M. Doi and S. F. Edwards, J. Chem. Soc., Faraday Trans. 2 74, 1789 (1978).
D. C. Boris and R. H. Colby, Macromolecules 1, 5746 (1988).
R. C. W. Liu, Y. Morishima, and F. M. Winnik, Polymer 34, 340 (2002).
V. E. Dreval’, G. B. Vasil’ev, E. A. Litmanovich, and V. G. Kulichikhin, Polym. Sci., Ser. A 50, 751 (2008).
J.-P. Chapel and J.-F. Berret, Curr. Opin. Colloid Interface Sci. 17, 97 (2012).
H. Bu, A.-L. Kjoniksen, K. D. Knudsen, and B. Nyström, Colloids Surf., A 293, 105 (2007).
Q. Wu, Y. Shangguan, M. Du, J. Zhou, and Q. Zheng, J. Colloid Interface Sci. 339, 236 (2009).
Q. Wu, M. Du, Y. Shangguan, J. Zhou, and Q. Zheng, Colloids Surf., A 332, 13 (2009).
A. F. Thünemann, M. Müller, H. Dautzenberg, J. F. Joanny, and H. Löwen, Adv. Polym. Sci. 166, 113 (2004).
A. D. Antipina, V. Yu. Baranovskii, I. M. Papisov, and V. A. Kabanov, Vysokomol. Soedin., Ser. A 14, 941 (1972).
E. A. Litmanovich, E. V. Chernikova, G. V. Stoychev, and S. O. Zakharchenko, Macromolecules 43, 6871 (2010).
E. A. Litmanovich, S. O. Zakharchenko, G. V. Stoychev, and A. B. Zezin, Polym. Sci., Ser. A 51, 616 (2009).
K. Bergfeldt, L. Piculell, and F. Tjerneld, Macromolecules 28, 3360 (1995).
C. O. M’Bareck, Q. T. Nguyen, M. Metayer, J. M. Saiter, and M. R. Garda, Polymer 45, 4181 (2004).
A. Tager, Physical Chemistry of Polymers (MIR, Moscow, 1978) [in Russian].
E. Di Cola, T. A. Waigh, and R. H. Colby, J. Polym. Sci., Part B: Polym. Phys. 45, 774 (2007).
T. A. Kavassalis and J. Noolandi, Macromolecules 22, 2709 (1989).
Y. H. Lin, Macromolecules 20, 3080 (1987).
G. M. Bartenev and L. A. Vishnitskaya, Vysokomol. Soedin. 6, 751 (1964).
G. V. Vinogradov and A. Ya. Malkin, Rheology of Polymers (Khimiya, Moscow, 1980) [in Russian].
H. A. Barnes, A Handbook of Elementary Rheology (Univ. Wales Inst. Non-Newtonian Fluid Mech., Aberystwyth, 2000).
A. B. Marciel, S. Srivastava, and M. V. Tirrell, Soft Matter 14, 2454 (2018).
S. Tang, M. Wang, and B. D. Olsen, J. Am. Chem. Soc. 11, 3946 (2015).
S. Onogi and T. Matsumoto, Polym. Eng. Rev. 1, 45 (1981).
V. G. Kulichikhin, S. O. Ilyin, M. V. Mironova, A. K. Berkovich, I. E. Nifant’ev, and A. Ya. Malkin, Adv. Polym. Technol. 37, 1076 (2018).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Litmanovich, E.A., Efremov, V.V. Rheological Properties of Poly(acrylic acid) Complexes with Poly(sodium styrenesulfonate) in Semidilute Aqueous Solutions. Polym. Sci. Ser. A 61, 743–753 (2019). https://doi.org/10.1134/S0965545X19060051
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0965545X19060051