Abstract
Epoxy matrices with a tunable matrix (vitrimers) are synthesized for the first time by curing a cycloaliphatic epoxy resin—3,4-epoxycyclohexylmethyl-3,4-epoxycyclohexane carboxylate—in the presence of 4-methylhexahydrophthalic anhydride as a crosslinking agent and zinc acetylacetonate as an interchain exchange catalyst. It is shown that at room temperature the epoxy vitrimers behave like ordinary thermosets but are capable of reshaping and welding when heated.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
INTRODUCTION
The creation of network materials with a tunable polymer matrix is a new direction in polymer science [1–7]. Products made from such materials can be recycled, repaired (defects in them “self-healed”), and welded together.
This direction was inspired by the team of Prof. L. Leibler in France [1–4], who showed the possibility of making what seemed impossible previously—to obtain thermosets and rubbers that can be processed, like ordinary thermoplastics, while retaining all other valuable properties characteristic of network materials. Processing occurs owing to activation of interchain exchange reactions at high temperatures which lead to neither destruction of the material nor reduction in the total number of chemical bonds in it. Interchain exchange reactions make it possible to recycle the product and give it a new shape. When cooled to the topology freezing transition temperature TV, these reactions slow down, fixing a new topology of the network. In other words, such a polymer can be softened under heating to give it any shape that can be fixed by cooling of the material, as what happens with ordinary glass. Thanks to this analogy, the new material was called a vitrimer, which in French means a polymer with glass properties [1–3]. L. Leibler et al. obtained vitrimers by curing the diepoxide monomer bisphenol A diglycidyl ether (DGEBA) using glutaraldehyde [1] or a mixture of dicarboxylic and tricarboxylic acids [2, 3] in the presence of catalysts (zinc acetate), accelerating the interchain exchange reaction of transesterification. The rate of the transesterification reaction was adjusted by changing the concentration and nature of the catalyst. It was shown [3] that interchain exchange reactions make it possible not only to mold products from network polymeric materials (thermosets and rubbers) but also to “heal” material defects, such as cracks.
Unlike aromatic epoxy resins (DGEBA), cycloaliphatic epoxy resins have low viscosity, are colorless and perfectly transparent, and contain no strong ultraviolet chromophore groups. This makes them more suitable for creating polymeric network nanocomposite materials with a high nanofiller content for use in optics, in particular, for the production of active matrices for liquid-crystal displays [8–11]. As far as we know, cycloaliphatic resin-based vitrimers have not yet been obtained.
The purpose of this work is to obtain network thermosets with a tunable polymer matrix on the basis of cycloaliphatic resin—3,4-epoxycyclohexylmethyl-3,4-epoxycyclohexane carboxylate (EEC)
 ,
,
capable of reshaping and recycling, in particular, by welding.
EXPERIMENTAL
In this study, epoxy vitrimers were prepared using EEC (Aldrich) as a monomer and 4-methylhexahydrophthalic anhydride (MHHPA, 98%, Acros Organics)

as a crosslinking agent. The transesterification reactions were accelerated using zinc acetylacetonate (ZAA) Zn (C5H7O2)2 ⋅ xH2O of chemical grade (Labtekh, Russia):

Synthesis of Thermosets with a Tunable Matrix (Vitrimers)
Samples of epoxy thermosets with a tunable matrix were obtained according to the method developed in [12]. The ZAA powder (0.8093 g) was added to EEC (7.36 g), and the as-obtained mixture was stirred and heated at 150°C for 1 h until the mixture was homogenized. Afterwards, MHHPA (1.162 g) was added, and the resulting mixture was stirred at room temperature and poured into a Teflon mold mounted on a horizontal plate in a Binder drying oven. The temperature was gradually increased (5–6°C/min) to 140°C and the reaction mixture was kept at this temperature for 12 h. The nontunable epoxy polymers were obtained by curing EEC in the presence of MHHPA as described in [8–11]. The conditions for obtaining epoxy networks with tunable and nontunable matrices are given in Table 1.
The gel fraction (%) was evaluated by the results of extraction with boiling acetone in a Soxhlet apparatus for at least 72 h. The curing depth A of epoxy networks was determined using differential scanning calorimetry on a NETZCH DSC 204F1 instrument (Germany) and calculated using the formula [13]
where ∆HT is the amount of heat released during the nonisothermal DSC scanning of the initial reaction mixture and ∆HR is the residual amount of heat released during the DSC scanning of the cured sample.
Thermal Properties
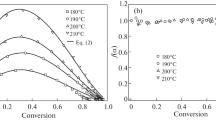
The thermomechanical analysis of epoxy thermosets was performed on a TMA Q400 instrument (TA Industries, United States). Thermomechanical curves were recorded at a constant load of 100 g in the penetration mode during heating in air from room temperature to 350°C at a constant rate of 5°C/min. The testing conditions were as follows: penetration of the probe (diameter, 2.54 mm) into the sample was conducted at a constant load. The middle of the temperature range of the transition of the sample from the glassy to viscous-flow state was taken as Tg. The coefficient of thermal expansion α was measured in the expansion mode. To avoid the influence of the thermal and mechanical history of epoxy thermosets on their thermal properties, the samples were first heated to 250°C and kept at this temperature for 10 min, then cooled to 25°C, and again heated to 250°C at a rate of 5°C/min. The values of Tg and α were determined from the second heating cycle [14]. The value of α was calculated from slopes of the TMA curve before and after Tg. The thermal properties (Tg and α) are presented in Table 1 and Figs. 1 and 2.
(Color online) (a) Thermomechanical curves and strain derivatives with respect to temperature in the range of (b) 40–300 and (c) 290–400°C for samples obtained by curing EEC with MHHPA at [EEC] : [MHHPA] = 1.5 : 1.0 (1) without the addition of ZAA and (2–4) in the presence of ZAA. [ZAA] = (2), 0.4, (3) 2.9, and (4) 3.5 wt %.
Investigation of the Ability of Epoxy Vitrimers for Reshaping and Welding
The ability of epoxy vitrimers for reshaping was studied as follows. A sample (20 × 5 × 1.8 mm) was kept in an oven at 200°C for 30 min, deformed with pliers, and allowed to cool at room temperature.
Tensile testing of the welded joints of the samples was carried out on a Lloid test facility. Rectangular samples (1.8 × 4 × 37 mm) were superimposed on each other. To ensure a good contact between the samples, they were placed in a special clamping device, compressed by ~25%, and left in a drying cabinet at a temperature of 160–300°C for 1–6 h. The overlap area of the rectangular samples was 5 × 5 mm = 25 mm2. Tensile testing of the welded samples was performed at a speed of 5 mm/min. The curves of machine elongation versus load are shown in Fig. 3. The elastic modulus is determined from the slope of the initial linear section of elastic deformation which the material undergoes under loading: \(E = \frac{\sigma }{\varepsilon }\). The test results are collected in Table 2.
RESULTS AND DISCUSSION
Synthesis of Epoxy Thermosets with a Tunable Matrix (Vitrimers) on the Basis of Cycloaliphatic Epoxy Resin
Epoxy vitrimers were synthesized by the thermal curing of EEC in the presence of crosslinking agent MHHPA. To obtain a tunable polymer matrix, the ZAA catalyst accelerating the transesterification reaction was added to the system.
In order to find the optimal conditions for the synthesis of vitrimers, the molar ratio of epoxy monomer and crosslinking agent ([EEC] : [MHHPA] = 1.0 : 1.0 and 1.5 : 1.0) and the concentration of the interchain exchange catalyst ([ZAA] = 0.4–3.5 wt %) were varied. Conditions for obtaining epoxy networks, the content of the gel fraction, and the depth of curing are presented in Table 1.
The thermal properties (Tg, α, and degradation temperature Td) of the samples with the tunable structure were compared with the properties of the nontunable epoxy network obtained by curing EEC in the presence of MHHPA under similar conditions but without the interchain exchange catalyst. It should be noted that, during the synthesis of both tunable and nontunable networks, no curing catalyst was used. The curing of EEC in the absence of the curing catalyst is initiated by the dicarboxylic acid resulting from the hydrolysis of MHHPA in the presence of water contained in the atmosphere [11]. The esterification of EEC by the carboxyl groups of dicarboxylic acids leads to the formation of diesters, the hydroxyl groups of which interact with MHHPA molecules. As a result of two reactions (the addition of the epoxy group to the carboxyl group and the interaction of the hydroxyl group with the anhydride), a three-dimensional network is formed. The mechanism of the first of these reactions (i.e., the reaction of epoxy and carboxyl groups) involves two possible ways: the epoxy group can react either directly with an acid [15, 16] (“the isolated path”) or through a transition state in which it forms a hydrogen bond with the second carboxyl group [17, 18] (“self-initiation”). According to the density functional theory, the second method is dominant [17], since complexation of the epoxy group with the second carboxyl group stabilizes the transition state, thereby reducing the energy barrier of the reaction. Thus, in this reaction, dicarboxylic acid not only reacts with the epoxy resin but also plays the role of the initiator.
When epoxy vitrimers are heated above Tg in the presence of the interchain exchange catalyst (ZAA), the transesterification reaction begins, which is likely provided by the interaction of carbonyl groups –C = O of the ester with hydroxyl groups of the diester

Under cooling, the interchain exchange of carbonyl and hydroxyl groups slows down, the network topology is frozen, and the system is vitrified:

Figure 1 shows thermomechanical curves and strain derivatives with respect to temperature for the samples obtained by curing EEC and MHHPA at [EEC] : [MHHPA] = 1.5 : 1.0 without addition of ZAA and in the presence of ZAA at [ZAA] = 0.4, 2.9, and 3.5 wt %. The representation of the thermomechanical curves in differential form (Figs. 1b, 1c), which is determined by the first derivative of strain with respect to temperature, makes it possible to clearly trace the formation of relaxation transitions in epoxy networks in a wide temperature range. The first temperature transition is observed in all samples and is associated with devitrification of the material. The second temperature transition appears only in the vitrimers and reflects freezing of the topology of polymer networks when the sample is cooled. It should be noted that a third transition associated with the thermal degradation of the samples is observed at a high temperature. For clarity, this transition is shown separately in Fig. 1c.
A comparative study of the thermal properties of the epoxy thermosets showed that the addition of ZAA at a concentration of 0.4 wt % deteriorates the thermal properties of the epoxy matrix: Tg and Td decrease from 120 to 80°C and from 354 to 340°C, respectively. With increasing concentration of ZAA from 0.4 to 3.5 wt %, the value of Tg rises from 80 to 100°C and Td decreases from 340 to 316°C. A lower value of Tg for the samples with the tunable matrix as a whole can be explained by the plasticizing effect of the catalyst which leads to an increase in the segmental mobility. A gradual decrease in the degradation temperature with an increase in the concentration of the catalyst indicates that the active interchain exchange reactions facilitate the degradation of the material at high temperatures.
For the samples with the tunable matrix, TV was determined from appearance of the second transition on the TMA curves and from a sharp increase in α upon cooling of the sample. It is seen that TV increases from 164 to 246°С with an increase in the concentration of ZAA from 0.4 to 3.5 wt %. This behavior is different from the results obtained by the team of L. Leibler in the thermal study of vitrimers synthesized by curing DGEBA in the presence of a mixture dicarboxylic and tricarboxylic acids and the interchain exchange catalyst zinc acetate [3]. L. Leibler et al. showed that an increase in the concentration of zinc acetate in the epoxy polymer reduces TV (i.e., the sample needs to be cooled further to freeze the topological structure) owing to more active interchain exchange reactions. This discrepancy can be explained as follows. Unlike DGEBA, there are no hydroxyl groups involved in the transesterification reaction in the EEC molecule. The formation of hydroxyl groups occurs as a result of the interaction of epoxy monomer with dicarboxylic acid after the hydrolysis of MHHPA [11]. With an increase in ZAA, which also catalyzes the curing reaction of epoxy monomers, the hydrolysis of MHHPA can be suppressed by accelerating the curing reaction. This will lead to a decrease in the concentration of hydroxyl groups and, as a result, to less active interchain exchange reactions and to an increase in TV. With increasing concentration of MHHPA (change in molar ratio [EEC] : [MHHPA] from 1.5 : 1.0 to 1.0 : 1.0), the concentration of hydroxyl groups probably increases, and a higher activity of exchange processes helps to reduce TV from 246 to 219°C (Table 1). A high TV observed for our vitrimer samples expands the temperature range of their operation under conditions of unchanged structure, which is important for practical application.
Investigation of the Ability of Epoxy Vitrimers for Reshaping and Welding
The presence of tunable links between the chains makes possible such processes as reshaping and welding of two vitrimer samples to each other. We investigated the effect of the concentration of catalyst ZAA and the molar ratio [EEC] : [MHHPA] on these processes.
Figure 4 shows photographs demonstrating the ability of the epoxy thermoset (sample 340) to be reshaped. The reshaping ability shown by the vitrimers obtained is extremely important, since it makes possible the multiple reuse of the products based on thermosets by changing their shape, which earlier (before the creation of vitrimers) seemed impossible. In this paper, we show that reshaping is also possible in the case of vitrimers based on the cycloaliphatic epoxy resin.
To characterize the strength of samples subjected to welding, the stress at break was determined. The stress-strain curves are shown in Fig. 3 The test results are summarized in Table 2.
It is established that the strength of the welded joint depends on the concentration of epoxy monomer EEC. At a molar ratio of [EEC] : [MHHPA] = 1.0 : 1.0, destruction of the welded joint of two strips cut from sample 289 and welded at 160°С (above Tg) occurs very quickly within 6 h at a low load (5 N). When [EEC] : [MHHPA] = 1.5 : 1.0, sample 274 welded under the same conditions can withstand a load of 68 N, and the rupture occurs outside the welded joint (Fig. 3a, Table 2). This result confirms a higher strength of the polymer matrix at the welding site.
Increasing the welding temperature for the same duration reduces the strength of the welding joint. Strips of sample 340 welded at 200°C for 6 h withstood only 19.8 N (Fig. 3b, Table 2). Practically the same strength of the welding joint (20.4 N) can be achieved under a shorter welding of the samples (1 h) at a temperature of 250°С (sample 338, Fig. 3c, Table 2).
It is important that in all cases the samples are destroyed outside the welding joint; that is, the welding joint is stronger than the matrix of the initial thermoset. This finding indicates a high welding efficiency.
Thus, the thermosets based on the cycloaliphatic epoxy resin with the tunable matrix which are capable of effective reshaping and welding thanks to the vitrimer matrix are obtained. Optimal conditions (160°С, 6 h) are determined which make it possible to achieve the reaction rate of transesterification optimal for efficient reshaping, self-healing, and welding without deteriorating the thermal properties of the samples.
CONCLUSIONS
For the first time, cycloaliphatic epoxy resin-based thermoset vitrimers that can change their topology when heated above Tg in the presence of interchain exchange catalyst ZAA have been obtained. The topology of the epoxy network is changed as a result of the transesterification reaction via the interaction of carbonyl groups –C=O of ester with hydroxyl groups of products of incomplete curing of the epoxy monomer (see the above scheme).
It is shown that the Tg values of the synthesized vitrimers are slightly lower than those of similar polymers with the nontunable matrix. This may be caused by the plasticizing effect of the catalyst which leads to an increase in segmental mobility. The tensile testing of welding joints of the samples showed that the destruction of the samples occurs outside the welding joint and depends on the temperature and duration of heating of the samples. The optimal temperature and time of heating of the samples were determined at which it is possible to achieve the rate of transesterification optimal for the effective reshaping, self-healing, and welding without a significant decrease in Tg.
REFERENCES
D. Montarnal, M. Capelot, F. Tournilhac, and L. Leibler, Science 334, 965 (2011).
M. Capelot, M. M. Unterlass, F. Tournilhac, and L. Leibler, ACS Macro Lett. 1, 789 (2012).
M. Capelot, D. Montarnal, F. Tournilhac, and L. Leibler, J. Am. Chem. Soc. 134, 7664 (2012).
W. Denissen, G. Rivero, R. Nicolaÿ, L. Leibler, J. M. Winne, and F. E. Du Prez, Adv. Funct. Mater. 25, 2451 (2015).
W. Denissen, J. M. Winne, and F. E. Du Prez, Chem. Sci. 7, 30 (2016).
W. Denissen, M. Droesbeke, R. Nicolaÿ, L. Leibler, J. M. Winne, and F. E. Du Prez, Nat. Commun. 8, article 14857 (2017).
M. Guerre, C. Taplan, R. Nicolaÿ, J. M. Winne, and F. E. Du Prez, J. Am. Chem. Soc. 140, 13272 (2018).
A. I. Barabanova, P. L. Shevnin, T. A. Pryakhina, K. A. Bychko, V. V. Kazantseva, B. G. Zavin, Ya. S. Vygodskii, A. A. Askadskii, O. E. Philippova, and A. R. Khokhlov, Polym. Sci., Ser. A 50, 808 (2008).
A. I. Barabanova, A. A. Askadskii, O. E. Philippova, and A. R. Khokhlov, Procedia Chem. 4, 352 (2012).
A. A. Askadskii, E. S. Afanasyev, M. D. Petunova, A. I. Barabanova, L. M. Goleneva, V. I. Kondrashchenko, and O. E. Philippova, Polym. Sci., Ser. A 56, 318 (2014).
A. I. Barabanova, B. V. Lokshin, E. P. Kharitonova, I. V. Karandi, E. S. Afanasyev, A. A. Askadskii, and O. E. Philippova, Colloid Polym. Sci. 297, 409 (2019).
O. E. Philippova and A. I. Barabanova, Patent RU 2638169 C2 (2017).
Thermal Characterization of Polymeric Materials, Ed. by E. A. Turi (Acad. Press, New York, 1981).
H. Yi, Thermochim. Acta 433, 98 (2005).
P.-J. Madec and E. Maréchal, Adv. Polym. Sci. 71, 153 (1985).
M. F. Sorokin and E. L. Gershanova, Kinet. Katal. 8, 512 (1967).
U. Q. Ly, M.-P. Pham, M. J. Marks, and T. N. Truong, J. Comput. Chem. 38, 1093 (2017).
E. Rokaszewski, Pol. J. Chem. 52, 1487 (1978).
Funding
This work was supported by the Russian Foundation for Basic Research, project code 18-53-76007 ERA_a.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Barabanova, A.I., Afanasyev, E.S., Askadskii, A.A. et al. Synthesis and Properties of Epoxy Networks with a Tunable Matrix. Polym. Sci. Ser. A 61, 375–381 (2019). https://doi.org/10.1134/S0965545X19030039
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0965545X19030039