Abstract
In this work, the study of three transition metal mixtures: cobalt-molybdenum (CoMo), nickel-molybdenum (NiMo), and nickel-cobalt-molybdenum (NiCoMo) with phosphorus supported on a γ-Al2O3 were studied for the hydroprocessing of heavy crude oil. The different metallic compositions were incorporated on gamma-alumina support by incipient wetness impregnation. The materials obtained were dried at 110°C and calcined to 450°C (4 h). The catalysts were evaluated using a Parr stainless steel batch reactor at 10.6 MPa and 380°C, for one hour. Mexican heavy crude oil named Ku-Ma-Loob Zaap was used and characterized according to its chemical composition: saturates, asphaltenes, resins, and aromatics (SARA). Sulfur and nitrogen were also determined by chemiluminescence techniques. The physical measurements for qualifying the transport properties were API gravity and kinematic viscosity. Among the tested catalysts, NiCoMoP/γ-Al2O3 presented the highest activity, increasing the API gravity from 12.6 to 24.5°API and decreasing the kinematic viscosity from 9.896 to 45 cSt at 25°C. The increasing activity was strongly related to the reducibility of the metals and weakly to the metals content. The surface area and pore volume did not change with the amount of metal, so no effect related to these properties was observed. Phosphorus presence was not discussed, since approximately the same amount was used in the three samples. However, it is known that phosphorus increased the hydrotreating activity due to the increased acidity of the catalyst, making trimetallic catalysts more active than bimetallic ones. In terms of the chemical composition of the upgraded crude oil, it was evident that the asphaltenes, sulfur, and nitrogen contents decreased sharply.

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
INTRODUCTION
The increasing demand for crude oil combined with the depletion of conventional oil fields has drawn the attention of the oil industry towards the processing of heavier crude oils [1]. The production of heavy or extra-heavy crude oils presents several problems, beginning with transportation to storage tanks up to refining [2]. These factors have been impacting the design of process engineering and the dimensions of the pipes. This type of crude oil does not flow easily within pipelines compared to light crude, due to the presence of polyaromatic structures that contain heteroatoms such as sulfur, nitrogen, and metals (vanadium, iron, and nickel) [3]. Frequently, these oils are characterized into four main families of hydrocarbons: saturated, aromatic, resins, and asphaltene-type compounds (SARA) and usually, these crudes present high viscosity values [4].
Some of the topics that have been studied related to heavy oil hydroprocessing are the presence of asphaltenes in the conversion yields, coke production [5, 6] and the effect of the catalyst properties such as acidity-basicity on the diffusion-adsorption processes [7, 8].
To improve heavy oil quality by decreasing the average molecular weight through hydrogenation and hydrocracking reactions, a metal oxide catalyst is frequently required [9]. The effect of the pore structure, acidity, and metal load has been studied for heavy crude oil hydroprocessing by several authors [4].
The challenging processing of heavy and extra heavy crude oil has been a source of new technologies and catalysts. The catalyst usually consists of metals supported on alumina with different surface characteristics that depend on the preparation methods. Heavy oil upgrading involves eliminating contaminants and reducing viscosity; therefore, research must be directed toward more efficient catalysts [9].
The use of nickel, cobalt, and molybdenum using gamma-alumina as support (NiCoMo/γ-Al2O3) as trimetallic catalyst, has been developed for hydroprocessing purposes for all types of feedstocks by many researchers [10–18]. Hydrodesulfurization (HDS) studies have been carried out using model molecules [10–13] and real feedstocks like atmospheric residual oil [14], lubricant oil [15], vacuum gas oil [16], and heavy gas oil [17, 18]. Regarding the effect of phosphorus (P) presence in the catalyst, it has been published that the number of active sites on the surface of NiMoW/γ-Al2O3 sulfide increases with increasing P concentration reaching a maximum at 1.6 wt % of P [19]. Additionally, according to the same authors, the hydrodesulfurization (HDS) and hydrodenitrogenation (HDN) processes of coker light gas oil derived from Athabasca bitumen in a trickle bed reactor at industrial conditions showed that P doping has a stronger promotional effect on HDN than HDS; and this enhancement in HDN activity could be attributed more to the effect of acidity than to the improvement in dispersion. The NiMoWP/γ-Al2O3 trimetallic catalyst with P loading of 1.6 wt % showed superior hydrotreating activity than the bimetallic NiMoP/γ-Al2O3, and NiWP/γ-Al2O3 catalysts. An explanation of such differences was not given [19]. Finally, as far as it is known, this type of study has not been carried out yet for the direct hydroprocessing of heavy crude oil.
Therefore, the main objectives of this work were the preparation and evaluation of three different catalysts named NiMoP/γ-Al2O3, CoMoP/γ-Al2O3, and NiCoMoP/γ-Al2O3 for hydroprocessing the crude oil to eliminate sulfur and nitrogen contaminants, decrease the viscosity of the crude oil, and facilitate transportation. These catalysts were chosen due to their bifunctional properties for hydrogenation and hydrocracking and their resistance to high levels of contaminants like sulfur and nitrogen [19]. The effect of the type and metal load of each catalyst was studied for heavy crude oil hydroprocessing using a Parr stainless steel batch reactor at 10.6 MPa and 380°C, for one hour.
EXPERIMENTAL
Catalyst synthesis. The catalysts were prepared using commercial γ-Al2O3 support (SBET = 225 m2/g, pore volume = 0.40 cm3/g and pore diameter = 90 Å). Metal and phosphorus solutions were co-impregnated on the support by means of the incipient wetting impregnation method (2 h). Solutions were prepared using the following compounds: 98 wt % Ni(NO3)2·6H2O (Aldrich), 99 wt % (NH4)6Mo7O24 (Aldrich), 98 wt % Co(NO3)2 (Aldrich), and 86 wt % H3PO4 (Baker). The solutions were prepared to give approximate concentrations for obtaining catalysts with an equivalent metal content for their comparison (Table 1). After impregnation, catalysts were dried at 110°C for 12 h and calcined in an air atmosphere at 450°C (heating program: 20°C/h) for 4 h. The catalyst obtained was in its oxide form. The catalyst sulfide form was obtained by a sulfidation procedure with a mixture of H2S/H2 (5/10 (vol/vol)) at a rate of 60 mL/min for 4 h at 3 MPa and 270°C.
Characterization of the catalysts. The metallic composition of the catalysts was obtained by elemental analysis, using a Perkin-Elmer Model 3100 Atomic Absorption Spectrophotometer. Phosphorous was also determined by Atomic Adsorption Spectroscopy using a phosphorous hollow cathode tube. The surface area and pore size distribution were determined by nitrogen physisorption at –195.8°C using a Micromeritics ASAP 2010. The samples were first dried at 178°C and evacuated at 350°C (approximately 2–5 h). The data were treated by the standard BET method to calculate the specific surface area (SBET). The total pore volume (Vp) was calculated from the amount of N2 adsorbed [P/P0 = 0.98]. Temperature programming reduction (TPR) was carried out in an AMI-200 Zeton-Altamira equipment using a mixture of hydrogen in argon (10 vol % H2/Ar, TPR-H2) at temperatures from 30 to 850°C with a heating rate of 10°C/min. The reduction study was performed on both the oxide and sulfide samples. The hydrogen consumption was measured with a thermal conductivity detector (TCD).
Characterization of the heavy crude oil (HCO) and reaction products. The Ku-Ma-Loob Zaap crude oil sample, a heavy crude oil (HCO), was kindly provided by PEMEX. The physical and chemical properties of the feedstock and products were established by the following methods: API gravity was measured by the ASTM-D287 method (Standard Test Method for API Gravity of Crude Petroleum and Petroleum Products (Hydrometer Method)). The kinematic viscosity, ASTM-D445 (Standard Test Method for Kinematic Viscosity of Transparent and Opaque Liquids and Calculation of Dynamic Viscosity), was determined using a rotary viscosimeter. SARA (saturates, aromatics, resins, and asphaltenes) was determined by the ASTM-D4124 method (Standard Test Method for Separation of Asphalt into Four Fractions). The sulfur contents were measured by the ASTM-D4294 method (Standard Test Method for Sulfur in Petroleum and Petroleum Products by Energy Dispersive X-ray Fluorescence Spectrometry). Nitrogen contents were studied by ASTM D 4629-08 method (Standard Test Method for Trace Nitrogen in Liquid Hydrocarbons by Syringe/Inlet Oxidative Combustion and Chemiluminescence Detection). Ramsbottom carbon tests were obtained by ASTMD524 (Standard Test Method for Ramsbottom Carbon Residue of Petroleum Products). The distillation curves and the fraction of the heavy oil were obtained by the ASTMD7169 method (Standard Test Method for Boiling Point Distribution of Samples with Residues Such as Crude Oils and Atmospheric and Vacuum Residues by High Temperature Gas Chromatography). The procedure is also known as simulated distillation. Each distillation fraction was assigned as follows: gasoline (IBP to 220°C), light gas oil (220 to 380°C), heavy gas oil (380 to 530°C), and residue (530°C to FBP).
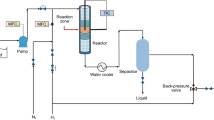
Activity tests. The experiments were carried out in a Parr batch reactor and submitted to an activation procedure (sulfidation) with a mixture of H2S/H2 (5/10 (vol/vol)) at a rate of 60 mL/min for 4 h at 3 MPa and 270°C. After sulfidation, each sample was purged with nitrogen and analyzed. In the Parr reactor, 200 g of heavy oil (Ku-Ma-Loob Zaap) mixed with 5 g of catalyst, then the reactor was purged with N2 and stabilized at the required reaction pressure, temperature, and stirring rate. The hydrogen pressure was increased to 10.8 MPa, and the reactor was heated to the reaction temperature of 380°C, at 1000 rpm stirring rate. The products were recovered at the end of each test (1 h). The physical and chemical properties of the products and feedstock were characterized according to the ASTM methods described in the previous section.
RESULTS AND DISCUSSION
Physicochemical characterization of the catalysts. The chemical composition of the as-synthesized catalysts was analyzed and verified by the atomic absorption technique [20]. As shown in Table 1, the total metal contents of the NiMoP, CoMoP, and NiCoMoP samples were 14.7, 15.8, and 16.8 wt % due to the variations in the nickel, cobalt, and molybdenum contents associated with the impregnation method. Phosphorus (P) content was maintained around 2 wt % in all three samples, therefore, an effect of P presence was not studied. However, P presence has proven to increase the hydrotreating activity due to the increased acidity of the catalyst. The hydrotreating promotion reached a maximum at 1.6 wt % of P, then higher P contents tended to decrease this activity [19]. It is already known that a trimetallic catalyst like NiMoWP/γ-Al2O3 showed superior hydrotreating activity than the bimetallic NiMoP/γ-Al2O3 and NiWP/γ-Al2O3 catalysts, at similar P contents (i.e. 1.6 wt %) [19].
Regarding the textural properties of the as-synthesized catalysts, it was observed (Table 2) that the surface area of the supported catalysts did not decrease significantly with the increase of the metallic content; however, to compare the surface area of the alumina source with the supported catalyst, the reduction averaged approximately 24% in the three samples. The same trend was observed by the pore volume, in this case averaging an 11% reduction [21].
TPR is a useful technique for studying the reducibility of species, mainly in hydrotreating catalysts. Generally, it is expected that close contact between two metals like those that Co and Mo atoms in the sulfide catalyst turn out essentially in an increase of activity level [22, 23].
In this study, the three catalysts were analyzed using TPR to give information on the effects of different metals and impregnation loading on the catalytic activity for upgrading heavy crude oil (Fig. 1). The profiles for the three catalysts in the oxide and sulfide forms were presented in the same figure for the purpose of comparison. Only the TCD signals are shown since no methane formation was observed for these samples. The total hydrogen consumption for these samples corresponded with the complete reduction of the metals.
According to Fig. 1, the reduction of CoMoP oxide took place, essentially, in one asymmetric peak that started at 400°C and continued the reduction up to 620°C; the reduction of the CoMoP sulfide was easier, beginning at 240°C and finishing at 510°C, with a visible shoulder at 295°C. The bimetallic NiMoP oxide, exhibited a shoulder with a very broad band appearing in the 400–870°C temperature region, whereas the sulfide form presented a similar TPR profile starting at a lower temperature (325°C), both bands, the oxide and sulfide NiMoP seems not to have finished reacting until the end of the experiment. According to Al-Dalama and Stanislaus [24], the reduction of the Ni starts at environs 330°C while the reduction of different polymolybdates is spread over the range of 400–520°C and a high temperature peak is attributed to the reduction of tetrahedral Mo4+ species that are strongly bonded to the support. Arnoldy et al. [25] reported that the reduction of CoMoP started around 320°C and depending on the temperature of calcination and the interaction of metals, the reduction was affected. In the NiMoP catalyst, the broad band can be interpreted as an overlapping of different species that interact with themselves and with the support.
On the other hand, the reduction of the oxide form of the trimetallic catalyst commenced above 370°C and proceeded in a series of peaks to about 870°C where the run ended. Two well defined peaks (350–563 and 563–730°C) were detected, and it was presumed that a third unfinished peak can be observed starting at 730°C. During the reduction of the trimetallic sulfide catalysts, one shoulder and a big peak were visible, starting at 145°C and finishing at 475°C. Obviously, the reduction of the trimetallic catalyst was easier than the reduction of the bimetallic catalyst, and this effect increased when the catalytic samples were sulfide.
Then, the results obtained during the hydroprocessing reactions could be mainly explained by the reducibility of the catalyst, i.e., the easier the reducibility, the more active the catalyst is. It has already been reported [26, 27] that the reducibility of metal oxide decreases in general with an increase in the strength of the metal-oxygen bond. Therefore, if the reducibility of the three metallic sample increases with respect to that of the bimetallic material, the synergic effect among metals decreases and the sulfidation of the metals could be stronger because of the reduced interactions, therefore, its catalytic activity is expected to increase.
Nevertheless, it is important to keep in mind that there is a correlation between the number of active sites based on the detector signal and how much hydrogen reacts, and this can be dependent on the total metal content [28].
Effect of the catalyst in the hydroprocessing of heavy oils. The feedstock used in the present study showed physicochemical properties that are characteristic of heavy oil with an API low gravity and high sulfur, nitrogen, resin, and asphaltene contents (Table 3) [29]. The three catalysts were sulfided before reaction, because the sulfided phase is the active form for hydrotreating oil streams [30]. After the reaction, the products always presented upgradeability in their properties by showing reductions in viscosity and carbon Ramsbottom values and increasing the API gravity (Table 3). Specifically, regarding the API gravity, after the reaction, an increment of 68–71% was observed when bimetallic catalysts were used, while an increment of 94% was achieved with the trimetallic one. The increment in API gravity is directly related to a significant decrease in viscosity [31]. The viscosity at 16 °C dropped from 15416 to 197 cSt with NiMoP, 117 cSt with CoMoP, and with the trimetallic catalyst down to 78 cSt. These results clearly showed that although the bimetallic catalysts performed well, the catalytic activity significantly improved when the trimetallic catalyst was used. Regarding the Ramsbotton test, measurements of both the heavier crude oil and fuel products are important because these values will indicate the tendency to form carbon deposits under high temperature conditions [32]. Consequently, in addition to the reduction of contaminants like sulfur and nitrogen, heavy oil hydroprocessing offers the reduction of other harmful products like coke. It was possible to reduce 51 wt % of the Ramsbotton carbon with the trimetallic catalyst, while with the CoMoP and NiMoP catalyst, the decrement was 47 and 33 wt %, respectively. The trimetallic catalyst showed the lowest sulfur and nitrogen content, going from 5.13 and 0.78 wt % in the feedstock to 1.5 and 0.41 wt %, respectively. All the observed changes in properties were due to hydrogenation, and hydrocracking reactions [29, 30].
The composition of the starting heavy crude oil (HCO) was determined using a SARA analysis (Table 4). The results showed a high resin content, followed by asphaltenes, aromatics, and to a lesser extent, saturated hydrocarbon type compounds. Once the crude oil was treated with the different catalysts, it was observed that the aromatic and saturated hydrocarbons presence increased to the detriment of the asphaltenes and resins presence. Resin plus asphaltene conversion was 49, 66, and 72% for NiMoP, CoMoP, and NiCoMoP, respectively, increasing the presence of the saturated and aromatic compounds to 30, 40, and 46%.
It was determined that the highest amount of aromatic content (51.1 wt %) was obtained with the trimetallic catalyst while the lowest quantity was displayed by the NiMoP catalyst (46.5 wt %). It is important to note that the amount of metal is not directly proportional to the results observed in the processed products, however, a relation between the metal content and the catalytic activity cannot be ignored [28]. Furthermore, if the price of metal is considered, the cost of the catalyst could hardly be compensated by the content of aromatic compounds in the product.
By correlating the catalytic activity with the reducibility of metals in their oxide and sulfide form shown by TPR, it was found that when the metals were reduced at lower temperatures, the hydrodesulfurization activity increased [33] and if the reducibility decreases the interaction between metal and support is lower which could favor a deeper hydrodesulfurization process [26, 27].
By means of the simulated distillation, using the method ASTM-D7169, the distillation curves of the hydroprocessed products were obtained, which made it possible to evaluate the quality of the products obtained after reaction with the different catalysts (Table 5). The best yield towards gasoline and light gas oil was achieved with the trimetallic catalyst, increasing from 7 and 22 vol % in the feedstock to 16 and 39 vol % in the product. While the NiMoP catalyst presented the lowest activity, reaching 10 and 30 vol % of gasoline and light gas oil.
CONCLUSIONS
The use of hydroprocessing catalysts for upgrading heavy crude oil was shown. Three different catalysts (NiMoP/γ-Al2O3, CoMoP/γ-Al2O3, and NiCoMoP/γ-Al2O3) were evaluated, having different metals and contents. It was demonstrated that the combined effect shown by the TPR (reducibility) of the three metals coupled with their high metallic contents favored the highest activity in removing sulfur and nitrogen compounds, in converting asphaltene and resin in aromatic and saturated compounds and reducing the API gravity and viscosity. Concerning the bimetallic catalyst, the NiMoP catalyst showed slightly better performance than the CoMoP sample. Phosphorus presence was not discussed because the three catalysts presented almost the same amount of the compound (i.e., 2 wt %). Although, it is known that phosphorus increases hydrotreating activity due to the increased acidity of the catalyst, the phosphorous trimetallic catalyst was more active than the phosphorous bimetallic ones. Therefore, the increased activity did not depend only on the phosphorous presence. The better performance of the trimetallic catalyst over bimetallic ones can be explained by the fact that the reducibility of the metal plays a key role in the catalytic activity of the samples; the lower reducibility, the lower interaction metal-support, and the higher catalytic activity also contributed to the increase in activity as expected. Surface area and pore volume had no effect because of their insignificant differences among samples.
Nevertheless, considering the difference in the upgradeability of heavy crude oil with the metal content and metal price, it could be economically more convenient to use a bimetallic catalyst. The use of hydroprocessing catalysts is an interesting alternative for upgrading heavy crude oil.
REFERENCES
Owen, N.A., Inderwildi, O.R., and King, D.A., Energy Policy, 2010, vol. 38, pp. 4743–4749. https://doi.org/10.1016/j.enpol.2010.02.026
Hart, A., J. Petrol. Explor. Prod. Technol., 2014, vol. 4, pp. 327–336. https://doi.org/10.1007/s13202-013-0086-6
Duyck, C., Miekeley, N., Porto da Silveira, C.L., Aucélio, R.Q., Campos, R.C., Grinberg, P., and Brandão, G.P., Spectroschim. Acta B, 2007, vol. 62, pp. 939–951. https://doi.org/10.1016/j.sab.2007.04.013
Speight, J.G., Heavy and Extra-Heavy Oil Upgrading Technologies. 1st ed. Gulf Professional Publishing, UK, 2013. ISBN:978-0-12-404570-5
Gawel, I., Bociarska, D., and Biskupski, P., Appl. Catal. A: Gen., 2005, vol. 295, pp. 89–94. https://doi.org/10.1016/j.apcata.2005.08.001
Mukhamatdinov, I.I., Khaidarova, A.R., Zaripova, R.D., Mukhamatdinova, R.E., Sitnov, S.A., and Vakhin, A.V., Catalysts, 2020, vol. 10, pp. 114–120. https://doi.org/10.3390/catal10010114
Leyva, C., Rana, M.S., Trejo, F., and Ancheyta, J., Ind. Eng. Chem. Res., 2007, vol. 46, pp. 7448–7466. https://doi.org/10.1021/ie070128q
Leyva, C., Rana, M.S., Trejo, F., and Ancheyta, J., Catal. Today, 2009, vol. 141, pp. 168–175. https://doi.org/10.1016/j.cattod.2008.03.030
Ancheyta-Juarez, J., Bentacourt-Rivera, G., MarroquinSánchez, G., Pérez-Arellano, A.M., Maity, S.K., Córtez, Ma.T., and del Río-Soto, R., Energy Fuels, 2001, vol. 15, pp. 120–127. https://doi.org/10.1021/ef000141m
Cáceres, C., Fierro, J.L.G., López Agudo, A., Severino, F., and Laine, J., J. Catal., 1986, vol. 97, pp. 219–227. https://doi.org/10.1016/0021-9517(86)90052-7
López-Agudo, A., Fierro, J.L.G., Cáceres, C., Laine, J., and Severino, F., Appl. Catal., 1987, vol. 30, pp. 185–188. https://doi.org/10.1016/S0166-9834(00)81024-6
Homma, T., Echard, M., and Leglise, J., Catal. Today, 2005, vol. 106, pp. 238–242. https://doi.org/10.1016/j.cattod.2005.07.187
Ninh, T.K.T., Laurenti, D., Leclerc, E., and Vrinat, M., Appl. Catal. A: Gen., 2014, vol. 487, pp. 210–218. https://doi.org/10.1016/j.apcata.2014.07.042
Lee, D.K., Lee, I.C., and Woo, S.I., Appl. Catal. A: Gen., 1994, vol. 109, pp. 195–210. https://doi.org/10.1016/0926-860X(94)80118-5
Mikhail, S. and Riad, M., Fuel Process. Technol., 2014, vol. 128, pp. 482–489. https://doi.org/10.1016/j.fuproc.2014.07.044
Klimov, O.V., Nadeina, K.A., Dik, P.P., Koryakina, G.I., Pereyma, V.Yu, Kazakov, M.O., Budukva, S.V., Gerasimov, E.Yu., Prosvirin, I.P., Kochubey, D.I., and Noskov, A.S., Catal. Today, 2016, vol. 271, pp. 56–63. https://doi.org/10.1016/j.cattod.2015.11.004
Badoga, S., Ganesan, A., Dalai, A. K., and Chand, S., Catal. Today, 2017, vol. 291, pp. 160–171. https://doi.org/10.1016/j.cattod.2017.01.005
Mashayekhi, M., Soltanali, S., Mohadecy, S.R.S., and Rashidzadeh, M., Pet. Chem., 2020, vol. 60, pp. 785–793. https://doi.org/10.1134/S0965544120070099
Sigurdson, S., Sundaramuthy, V., Dalai, A.K., and Adjaye, J., J. Mol. Catal. A: Chem., 2008, vol. 291, pp. 30–37. https://doi.org/10.1016/j.molcata.2008.05.011
Ferreira, S.L.C., Bezerra, M.A., Santos, A.S., dos Santos, W.N.L., Novaes, C.G., de Oliveira, O.M.C., Oliveira, M.L., García, R.L., Trends Anal. Chem., 2018, vol. 100, pp. 1–6. https://doi.org/10.1016/j.trac.2017.12.012
Scholten, J.J.F., Stud. Surf. Sci. Catal., 1979, vol. 3, pp. 685–714. https://doi.org/10.1016/S0167-2991(09)60244-5
Topsøe, H., Clausen, B.S., Candia, R., Wivel, C., and Mørup, S., J. Catal., 1981, vol. 68, pp. 433–452. https://doi.org/10.1016/0021-9517(81)90114-7
Nikulshin, P.A., Tomina, N.N., Pimerzin, A.A., Stakheev, A.Yu., Mashkovsky, I.S., and Kogan, V.M., Appl. Catal. A: Gen., 2011, vol. 393, pp. 146–152. https://doi.org/10.1016/j.apcata.2010.11.033
Al-Dalama, K. and Stanislaus, A., Thermochim. Acta, 2011, vol. 520, pp. 67–74. https://doi.org/10.1016/j.tca.2011.03.017
Arnoldy, P., Franken, M.C., Scheffer, B., and Moulijn, J.A., J. Catal., 1985, vol. 96, pp. 381–395. https://doi.org/10.1016/0021-9517(85)90308-2
Quincoces, C.E., Perez de Vargas, S.L., Gonzalez, M.G., Diaz, A., and Montes, M., Stud. Surf. Sci. Catal., 1998, vol. 119, pp. 837–842. https://doi.org/10.1016/S0167-2991(98)80536-3
Misono, M., Stud. Surf. Sci. Catal., 2013, vol. 176, pp. 25–65. https://doi.org/10.1016/B978-0-444-53833-8.00002-8
Pirola, C., Galli, F., and Patience, G.S., Can. J. Chem. Eng., 2018, vol. 93, pp. 2317–2320. https://doi.org/10.1002/cjce.23317
Speight, J.G., in Enhanced Recovery Methods for Heavy Oil and Tar Sands, 2009, pp. 95–132. https://doi.org/10.1016/B978-1-933762-25-8.50009-2
Robinson, P., Tutorial on Hydroprocessing: Hydroprocessing Catalysts and Processes. Conference: 2012 AIChE Spring National Meeting. https://www.researchgate.net/publication/267345317
Sánchez-Minero, F., Sánchez-Reyna, G., Ancheyta, J., and Marroquin, G., Fuel, 2014, vol. 138, pp. 193–199. https://doi.org/10.1016/j.fuel.2014.08.022
Speight, J.G., in Enhanced Recovery Methods for Heavy Oil and Tar Sands, 2009, pp. 261–294. https://doi.org/10.1016/B978-1-933762-25-8.50013-4
Scheffer, B., Arnoldy, P., and Moulijn, J.A., J. Catal., 1988, vol. 112, pp. 516–527. https://doi.org/10.1016/0021-9517(88)90167-4
ACKNOWLEDGMENTS
The authors acknowledge the Mexican Petroleum Institute for the permission granted to publish this work.
Funding
The authors are grateful for the financial support of the SENER Hydrocarbon Fund provided by CONACYT Mexico.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare no conflict of interest requiring disclosure in this article.
Rights and permissions
About this article
Cite this article
Schacht-Hernández, P., Romo, P.P. & Laredo, G.C. Comparative Catalytic Study for Upgrading Mexican Heavy Crude Oil. Pet. Chem. 63, 510–517 (2023). https://doi.org/10.1134/S0965544123040059
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0965544123040059