Abstract
Considering numerous issues due to asphaltene precipitation during production and transportation of crude oil, this work aims to postpone the onset point of asphaltene and improve the stability of its colloids in heavy (S) and light oil (D) samples. For this purpose, water/oil interfacial tension (IFT) is measured to evaluate the effect of surfactants as asphaltene inhibitor on heavy and light oil samples from Iranian south fields. Onset of precipitation from oil samples is revealed by sudden change in IFT between deionized water and oil samples through addition of different fractions of n-heptane as precipitant. Surfactants are used to stabilize the asphaltene colloids and prevent asphaltene precipitation from crude oil. However, crude oils of different properties require investigations for appropriate surfactant at optimum concentration to delay asphaltene precipitation. In this work, three surfactants Linear dodecyl-benzene-sulfonic acid (L–DBSA), dodecyl resorcinol (DR) and nonylphenol (NP) are examined for their retarding capabilities of onset point of asphaltene precipitation in oil samples S and D. Also, the involved mechanisms for addition of surfactant to crude oil are analyzed and interpreted. Enhanced stability of crude oil and degree of postpone in onset point follows the order of L–DBSA > DR > NP for heavy oil S and NP > DR > L–DBSA for light oil D owing to nature and chemical structure of surfactants and also properties of crude oil samples. Furthermore, IFT fluctuations for light and heavy oil are similar but the faster onset point and different values of IFT for light oil arise from unstable nature and density difference.

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
INTRODUCTION
Asphaltene is the heaviest part of crude oil with structure comprising of carbon, hydrogen, oxygen, sulphur, nitrogen and metals such as nickel, vanadium and iron [1] that is dissolved in toluene and precipitated by alkanes.
At normal conditions, Asphaltene is in thermodynamic equilibrium with other species in crude oil. It may precipitate due to change in pressure, temperature or composition and cause flow assurance challenges during production from oil reservoirs [2, 3].
The onset point is recognized by start of asphaltene precipitation when paraffinic hydrocarbon is added to crude oil [4, 5].
The onset of asphaltene precipitation from crude oil can be inferred by sudden change in IFT of water/oil system [6]. IFT is defined as the force between two immiscible liquid phases [7, 8] which is measured by Pendant Drop experiments [9].
Surfactants have amphiphilic nature with hydrophilic and hydrophobic parts in their molecular structure. Therefore, they are employed as crude oil stabilizing agents to disperse asphaltene aggregates and inhibit their accumulation. Surfactants are wetting agents which adsorb on the water/oil interface and reduce the interfacial tension between two liquids [10–12]. In [13] the effect of selected surfactants on precipitation is examined. Furthermore, the results of the work on impacts of resins and DBSA on asphaltene precipitation achieved by [14] revealed the complex interactions between species in crude oil.
In [15] the effect of chemicals to inhibit asphaltene precipitation in Brazilian crude oil samples is examined. Results showed remarkable solubilization effect of DBSA that confirms the role of acid–base interactions in the process.
Razipour et al. [16] studied IFT behavior as result of surfactants addition to Iranian heavy oil sample and the outcome showed L-DBSA as most effective formula for improvement of oil stability against asphaltene precipitation. Nevertheless, the present work includes performance of NP at different concentrations and also enhances the reliability of results obtained previously for L-DBSA as the selected chemical.
It is attempted in this work to cover the lacking data and investigations for performance of surfactants in light oil due to intrinsic complexity. Therefore, a comparative study and interpretation are being executed to analyze surfactants including NP, DR, and L-DBSA for light and heavy crude oils.
This work measures IFT values for water/oil and analyzes the results in order to evaluate a variety of surfactants and attain the most effective formula at optimum concentration for inhibition of asphaltene precipitation in Iranian heavy and light oil samples.
MATERIALS AND METHODS
Oil Sample Preparation
The oil samples are collected from the surface separator which is used for separation of produced oil, gas and water from Iranian heavy (S) and light (D) oil fields.
SARA (Saturates, Aromatics, Resins and Asphaltene) analysis, composition and physical properties of heavy (S) and light (D) oil samples from Iranian oilfields are shown in Tables 1, 2 and 3. In addition, toluene and n-heptane are mixed at different volume ratios followed by addition of 9.03 wt % of extracted asphaltene extracted by ASTM D3279 approach from oil S to make synthetic fluid model for the first set of tests. Next, experiments are completed using the real oil samples.
Surfactants
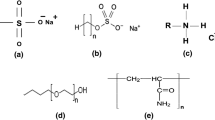
Anionic surfactants are appropriate inhibitor for asphaltene precipitation [15, 17, 18]. Three surfactants as follow are selected, designed and manufactured based on the oil properties in this work;
– DR (Dodecyl Resorcinol ); selected
– L-DBSA (Linear Dodecyl-Benzene-Sulfonic acid ); designed and manufactured
– NP (Nonylphenol); selected
Table 4 illustrates the chemical structure and properties of above surfactants.
Experimental Method
Pendant Drop method is applied to measure the IFT of deionized water-synthetic oil and deionized water/oil samples considering the effect of surfactant.
Two drop-related parameters, equatorial diameter (D) and diameter (d) at the distance D from the top of the drop, are involved for determination of IFT from Pendant Drop results. Oil drops submerged in water for IFT measurements by Pendant Drop method and related parameters are shown in Fig. 1.
Pendant Drop [9].
Data obtained from Pendant Drop tests are used in the following equation to calculate the IFT [9].
γ = ΔρgD2/H, (1)
where γ denotes IFT, Δρ is density difference, g represents gravity acceleration and H as the shape dependent parameter depends on “shape factor,” S = d/D.
Also, the values of 1/H is determined by the following correlation,
1/H = B4/SA + B3S3 – B2S2 + B1S – B0, (2)
where B0, B1, B2, B3, B4, and A are empirical constants for a certain range of S, which are shown in Table 5.
RESULTS AND DISCUSSION
Experimental Results—Synthetic Oil
First series of experiments is implemented by Synthetic oil where fractions of precipitant, n-C7, are added to toluene with asphaltene at 9.03 wt % and water. Measurements are begun with 10 mL of toluene plus 9.03 mass fraction of asphaltene/deionized water and continued with samples at higher n-C7 volume ratio (Fig. 2, run 1).
According to results in Fig. 2, the abrupt change in IFT values is observed at 20 vol % of n-C7. Also, asphaltene precipitation onset is thought to be in the range of 20– 40 vol % for n-C7. It should be noted that IFT between toluene and water increases monotonically when more n-C7 is added [6]. Meanwhile, IFT measurement for water and synthetic oil plus n-heptane displays non-uniform variation which is related to precipitation of asphalene. n-C7 causes the asphaltene to precipitate where the onset point of asphaltene precipitation accounts for IFT fluctuations.
IFT measurement for synthetic oil/toluene [16] (1) run 1 and (2) run 2.
The repetition of measurements in the range of 20– 40 vol % at 25, 27 and 35 percent of n-C7 are shown in Fig. 2 (run-2). Results indicate the point of 25 vol % of n-C7 with IFT of 27.352 mN/m as the onset of asphaltene precipitation.
Mechanistic Analysis of IFT
IFT measurement is also performed for oil samples S- and D-deionized water and addition of n-C7 fractions as the precipitating agent (Fig. 3).
Properties and surface behavior of asphaltene explained below justify the observed fluctuations of IFT data in curve 2 of Fig. 3:
1. Addition of n-C7 leads to asphaltene precipitation to start at point І as the onset point which is followed by increase in asphaltene bulk concentration and IFT when more n-C7 is added. The steady trend and minor variations of IFT in the interval between points ІІ and ІІІ is due to the equilibrium between asphaltene-adsorption and -desorption at the water/oil interface.
2. However, asphaltene precipitation is intensified when more n-C7 is added from point ІІІ to ІV. But the unsaturated water/oil interface causes the asphaltene particles to migrate from bulk to the interface. Therefore, IFT is reduced when asphaltene as surface active agents (surfactant) is accumulated at the interface. This process ends up with creation of a solid-like film on the interface due increasing in surface concentration of asphaltene.
3. Eventually, large vol % of n-C7 between points ІV and V results in considerable asphaltene precipitation. Also, weak transfer of asphaltene from bulk environment to saturated interface and resulted increase in bulk concentration along with resistance of solid-like interface against phase mixing cause the IFT of water/oil to increase.
Trend of IFT for light oil D confirms analogous trend with the differences in faster onset point because of unstable nature and also higher values for IFT due to more density difference with water versus heavy oil S.
Effect of Surfactants on Onset of Asphaltene Precipitation
Heavy oil sample S. Surfactants DR, L-DBSA, and NP are examined for their effect on onset point of asphaltene in oil sample S.
Initially, 0.02 wt % of surfactant DR is used in 10 cc of heavy oil sample and n-C7 vol % of 34 and IFT of 10.826 mN/m is found as the onset point for asphaltene precipitation.
Also, onset point of asphaltene occurs at 37 vol % of n-C7 and IFT of 5.627 mN/m when experiment is repeated with 0.05 wt % of DR. As a result, asphaltene stability experienced minor improve due to increase of DR from 0.02 to 0.05 wt %.
Any additional increase in concentration of DR above 0.05 wt % yielded severe instability of oil drop and interface between oil and water. Hence, it was not practical to obtain the similar trend for IFT versus Heptane volume fraction.
Although the optimum concentration of 0.25 wt % L-DBSA is determined based on initial investigation among 0.1, 0.25, and 0.5 wt % [16], it is still important to narrow down the accuracy of tests through repeating the stability tests and IFT measurements at close values of 0.20 and 0.30 wt % L-DBSA. This helps to consider the future needs and precision which is required for field application if a more accurate value other than 0.25 wt % L-DBSA for optimum concentration exists.
As shown in Fig. 4, when concentration of L-DBSA is reduced from 0.25 to 0.20 wt %, onset point occurs at 38 vol % of n-C7 indicating weaker performance against 0.25 wt % of L-DBSA with onset point of 41 vol % of n-C7. Also, significant drop in IFT to 6.443 mN/m at onset point is observed when the experiment is conducted by increased concentration of L-DBSA from 0.25 to 0.30 wt %. However, the change in onset point is not impressive and therefore the optimum concentration for asphaltene stability remains as 0.25 wt %.
Finally, the experiments of nonylphenol (NP) surfactant are repeated at mass concentrations of 0.1 and 0.25 to validate the results. According to Fig. 5, NP reduces the IFT however it is not appropriate in terms of asphaltene stability as the onset point of asphaltene is postponed from 31 vol % of n-C7 for heavy oil sample to 34 vol % of n-C7 for sample with 0.25 wt % of NP.
According to Fig. 6, L-DBSA as the best surfactant is expected to postpone the onset point at higher fraction of n-C7. It plays a main role in control of asphaltene stability through appropriate IFT reduction at optimum concentration of 0.25 wt %. However increase of its fraction from 0.25 to 0.5 wt % does not change the onset point significantly, IFT undergoes a severe drop which is not optimal and desirable.
According to results, the examined surfactants for postpone of onset point and enhance of oil stability are effective in the order of L-DBSA > DR > NP and the optimum concentration for L-DBSA is found to be 0.25 wt %.
Light oil sample D. Due to characteristic discrepancies between light and heavy oil in terms of density, viscosity, chemical constitutes and structure, etc., asphaltene content in light oil samples show different stability and behavior. Existence of higher fraction of light components in light oil compared to heavier oil samples results in less solvency property of oil and higher instability of asphaltene fraction which yield precipitation [19, 20]. Therefore, it is essential to compare the performance of surfactants required for enhanced asphaltene stability in heavy and light oil and find the suitable and compatible surfactant for light oil.
Accordingly, stability test and IFT measurements are performed for light oil sample D from an Iranian oil field. Figure 7 compares the effectiveness of the selected surfactant, L-DBSA at 0.25 wt % for oil sample S with oil sample D. As shown in the figure, L-DBSA could not enhance the stability of light oil to the same extent as heavy oil sample. The onset point in sample D undergoes a minor increase from 15 to 17 heptane vol % while it experiences a significant postpone from 31 to 41 heptane vol % in sample S.
In addition, surfactant DR at 0.25 wt % is tested for light oil simple D where the results in Fig. 8 show unimpressive postpones of onset point from 15 heptane vol % (curve 1) to 18 heptane vol % (curve 2).
As a result, it is vital to look for an effective surfactant to improve the stability of asphaltene and delay the precipitation in light oil samples.
Figure 9 demonstrates the retarding effect of surfactant NP at 0.25 wt % for oil samples S and D. In contrast to L-DBSA, the onset point of precipitation for oil sample D is shifted away significantly from 15 to 21 heptane vol %. This indicates higher performance of NP in light oil D compared to heavy oil S where onset point experienced only 3 units increase in heptane vol % from 31 to 34 by adding NP to S oil.
Figure 10 compares the retarding effect on onset point of asphaltene precipitation for light oil sample D when 0.25 wt % of L-DBSA, DR and NP are used as inhibitors. Effectiveness of surfactants in delaying the onset point follows the ascending order of 15, 17, 18, and 21 heptane vol % for oil sample (no surfactant), L-DBSA, DR and NP, respectively where the corresponding measured IFT is 29.968, 24.464, 21.763, and 17.162. Therefore, NP is the candidate surfactant for light oil. This could be explained by the nature and chemical structure of NP that assist stable conditions for asphaltene in oil.
In order to apply NP for light oil, it is essential to find out the optimum concentration of surfactant considering the required postpone of onset point of asphaltene and also provision cost of surfactant. Accordingly, surfactant NP at concentrations of 0.20, 0.25, 0.30, 0.35, and 0.45 wt % is examined on light oil D. Based on the results in Fig. 11, IFT is dropped through addition of NP where reduction level is proportional to concentration of surfactant NP. However the onset point is increased considerably from 21 to 28 heptane vol % by increase of concentration from 0.25 to 0.30 wt %, the subsequent increase on NP concentration does not result in more significant delay of onset point.
Thus, the optimum concentration for NP is 0.30 wt % with onset coordination of 28 and 13.635 for heptane vol % and IFT, respectively. Any higher concentration above 0.30 wt % of NP does not yield justifying increase of onset point and is not economically favorable.
CONCLUSIONS
Addition of n-C7 to oil samples causes asphaltene precipitation and affects IFT as surface property. Experimental results confirm fluctuations in IFT when n-C7 is added to oil samples that can be used to determine the onset point.
Chemical structure and polar group of surfactants define their inhibition capacity. The hydrophobic end in structure of surfactants makes more stable layer around asphaltene which results in prevention of asphaltene precipitation. Thus, when surfactants are added to crude oil, asphaltene colloids are formed that enhances the stability.
Furthermore, asphaltene is the most polar part in crude oil and polar ends in surfactant as inhibitor causes it to adsorb on asphaltene surface and improve its stability. In fact, preventing asphaltene to accumulate occurs through interaction between polar surfactant molecules and polar asphaltene. As a result, the inhibition capacity of surfactant is measured by the delay time on asphaltene precipitation onset.
Experimental results confirm the order L-DBSA > DR > NP for stability enhancement of asphaltene from heavy oil sample Sand NP > DR > L-DBSA for light oil sample D. Also, optimum concentration representing improved stability of asphaltene in oil sample S with 9.03 wt % asphaltene is 0.25 wt % of L-DBSA however the sensitivity analysis at concentrations of 0.20 and 0.30 wt % do not show better performance in terms of retarding the onset point of precipitation. This value for sample D with 0.97 wt % asphaltene is 0.30 wt % of NP at 28 vol % of n-C7.
It is worth noting that the general trend of IFT remains similar for light and heavy oil however differences are observed for onset point which occurs faster in light oil due to less stability of asphaltene and also less values of IFT owing to less density difference with water compared to light oil.
REFERENCES
Keshmiri, K., Huang, H., and Nazemifard, N., Fuel, 2019, vol. 239, pp. 841–851. https://doi.org/10.1016/j.fuel.2018.11.044
Ghala, G.T., Sulaiman, S.A., and Japper-Jaafar, A., Fluid Mech., 2017, vol. 251, pp. 69–87. https://doi.org/10.1016/j.jnnfm.2017.11.008
Memon, A., Borman, C., Mohammadzadeh, O., Garcia, M., Judith, D., Tristancho, R., and Ratulowski, J., Fuel, 2017, vol. 206, pp. 258–275. https://doi.org/10.1016/j.fuel.2017.05.024
Mahdavi, E., Zebarjad, F.S., Taghikhani, V., and Ayatollahi, Sh., J. Chem. Eng. Data, 2014, vol. 59, no. 8, pp. 2563–2569. https://doi.org/10.1021/je500369e
Oh, K. and Deo, M.D., Energy Fuels, 2002, vol. 16, pp. 694–699. https://doi.org/10.1021/ef010223q
Mousavi-Dehghani, S.A., Riazi, M.R., Vafaie-Sefti, M., and Mansoori, G.A., J. Pet. Sci. Eng., 2004, vol. 42, pp. 145–156. https://doi.org/10.1016/j.petrol.2003.12.007
Kumar, B., A Thesis submitted to the Department of Chemical and Petroleum Engineering, Calgary, Canada, 2012, vols. 6–8, pp. 23–24.
Nikseresht, S., Riazi, M., Amani, M.J., and Tabrizi, F.F., Colloid Interface Sci. Commun., 2020, vol. 34, article 100217. https://doi.org/10.1016/j.colcom.2019.100217
Drelich, J., Fang, C., and White, C.L., Encyclopedia of Surface and Colloid Science, 2002, pp. 3152–3166.
Mishra, M., Muthuprasanna, P., Prabha, K.S., Rani, P.S., Satish, I.A., Chandiran, I.S., Arunachalam, G., and Shalini, S., Int. J. PharmTech Res., 2009, vol. 1, pp. 1354–1365.
Hiemenz, P.C. and Rajagopalan, R., Principles of Colloid and Surface Chemistry, New York: Marcel Dekker, 1997, 3rd ed.
Saeedi Dehaghani, A.H., Soodbakhsh Taleghani, M., Badizad, M.H., and Daneshfar, R., Colloid Interface Sci. Commun., 2019, vol. 33, article 100202. https://doi.org/10.1016/j.colcom.2019.100202
Al-Sahhaf, T.A., Fahim, M.A., and Elkilani, A.S., Fluid Phase Equilibria, 2002, vol. 194–197, pp. 1045–1057. https://doi.org/10.1016/S0378-3812(01)00702-6
Goual, L. and Firoozabadi, A., AIChE J., 2004, vol. 50, no. 2, pp. 470–479. https://doi.org/10.1002/aic.10041
Junior, L.C.R., Ferreira, M.S., and Ramos, A.C.D., J. Pet. Sci. Eng., 2006, vol. 51, nos. 1–2, pp. 26–36. https://doi.org/10.1016/j.petrol.2005.11.006
Razipour, M., Samipour Giri, M., and Majidian, N., Energy Sources, Part A: Recovery, Utilization, and Environmental Effects, 2020. https://doi.org/10.1080/15567036.2020.1752332
Gonzalez, G. and Middea, A., Colloids Surf., 1991, vol. 52, pp. 207–217. https://doi.org/10.1016/0166-6622(91)80015-G
Chang, Ch. and Fogler, H.S., SPE Int. Symp. on Oilfield Chemistry, New Orleans, LA, U.S.A., March 2–5, 1993. https://doi.org/10.2118/25185-MS
Hajizadeh, A., Rostami, R.R., Amani, M., and Shedid, A., 32nd Annual SPE Int. Technical Conf. and Exhibition, Abuja, Nigeria, August, 4–6, 2008. https://doi.org/10.2118/119725-MS
Wei, J., Li, J., Zhou, X., and Zhang, X., Petrol. Sci. Technol., 2020, vol. 38, pp. 116–123. https://doi.org/10.1080/10916466.2019.1684947
ACKNOWLEDGMENTS
The authors deeply acknowledge Chemical Engineering Department, Faculty of Engineering at North Tehran Branch of Islamic Azad University.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare no conflict of interest requiring disclosure in this article.
Additional information
Translated from Neftekhimiya, 2021, Vol. 61, No. 5, pp. 642–651 https://doi.org/10.31857/S0028242121050075.
Rights and permissions
About this article
Cite this article
Razipour, M., Giri, M.S. & Majidian, N. IFT Assisted Enhancement of Asphaltene Stability in Light/Heavy Oil Using Surfactants. Pet. Chem. 61, 1019–1026 (2021). https://doi.org/10.1134/S0965544121090097
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0965544121090097