Abstract
The emerging fuel crisis necessitates a shift in focus towards alternative renewable forms, so that sustainable development can be achieved. Bio-oil is a promising alternative renewable source of energy which is a third generation bio-fuel. Algae are a popular candidate for bio-fuel production due to their high lipid contents, ease of cultivation and rapid growth rate. In this study, Hydrothermal liquefaction of Scenedesmus obliques biomass cultivated in photo-bio-reactor (PBR) from wastewater was studied. The influence of process parameters on the bio-oil yield and bio-oil upgrading was analysed. Different S. obliques biomass to water ratios (0.025, 0.05, 0.075 and 0.1 g/ml) were liquefied at diverse temperatures ranging from 200 to 340°C under 5 MPa N2 gas atmosphere. The influence of catalyst on bio-oil upgradation was studied at varying catalyst loading of the range 1–5 wt %. Bio-oil was analysed using Gas Chromatography Mass Spectroscopy (GC-MS) and Fourier Transform Infrared Spectroscopy (FTIR). Results showed a maximum bio-oil yield of 24.57 wt % at 300°C, 15 g/200 ml biomass load and 2.5 wt % NaOH at 60 min holding time. Also, it was found that the gas generated from liquefaction process contained 22 vol % Hydrogen gas, 18 vol % Carbon dioxide gas, 27 vol % Carbon monoxide gas, 22 vol % of methane gas and a small amount of other gaseous components (H2S). HTL bio-oil was upgraded and it resulted in 30.15 wt % yield with higher degree of C7−C21 range hydrocarbons in it.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
INTRODUCTION
During the last two decades, there have been a growing concern about the depletion of traditional fossil fuel, and hence, the use of renewable energy to overcome the world’s energy needs. Producing bio-fuel from oil crops and waste oil appeared to be insufficient, and the attention toward the use of microalgae has considerably increased [1]. Residual biomass from wastewater treatment processes such as high rate algal pond (HRAP) can be used potentially as fertilizers, animal feedstock and even bio-fuel [2]. Biodiesel is derived from organic oils, plants or animals by the process of Transesterification to obtain monoalkylesters [3].
Microalgae present a variety of advantages over terrestrial plants for the production of renewable fuels and chemicals. Chief among these is the fact that many species of algae exhibit high growth rates and can be grown on non-arable land using wastewater, thus avoiding undesirable competition with food crops for land and water resources [4]. Photo-bio-reactors (PBR) are used for efficient CO2 capture and maximum microalgae yield [5]. Microalgae is the better solution for the effective wastewater treatment through environmentally feasible and economic way [6].
Pyrolysis of blended cyclohexane and benzene fuel mixture in a flow tube reactor under supercritical pressure condition reveals that cyclohexane to a great extent easier to undergo thermal cracking than benzene [7]. The thermo-chemical conversion process employed in bio-fuel oil production is the hydrothermal liquefaction process. This process is much preferred over pyrolysis and produces oil products of desirable quantities [8].
The principal role of hydrothermal liquefaction is to decompose the bio-macromolecules in the aquatic biomass into smaller molecules that can then be further treated, if desired to produce specific liquid fuels [9]. Aquathermolysis could not only enhance the vis-breaking of heavy oil, simultaneously improve pyrolysis and remove some heteroatoms (S, O, and N), which ends up in better flow property and upgraded quality [10].
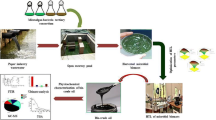
In this study, microalgae S. obliques was cultivated from municipal wastewater using an aerated photo-bioreactor. Microalgal biomass was processed through photo-bio-reactor liquefaction for bio-oil production. The HTL bio-oil was catalytically upgraded into liquid hydrocarbons. Gas chromatography-Mass Spectroscopy (GC-MS) and FTIR techniques were used for the characterization and elucidation of compounds in bio-oil.
MATERIALS AND METHODS
Wastewater and microalgae. Algae growth was isolated from water body near to SSN College of Engineering, Chennai according to standard protocols [9] and after examination it was confirmed to be S. obliques. PBR capacity of 1-L glass vessel made up of acryl material with 800 mL BG11 medium [6], 2000 lux light intensity using fluorescent lights and 0.2 vvm CO2 (2.5%) was used for microalgae cultivation. The algal growth in PBR was incubated for a time period of 15 days at room temperature of 27 ± 3°C. In this study, the wastewater was collected from the Chennai wastewater treatment plant situated in Perungudi, Chennai, Tamil Nadu, India. The nitrogen (N), phosphorus (P), and chemical oxygen demand (COD) of the wastewater were calculated using standard protocol [11].
Microalgae biomass recovery. Mixotrophic mode of cultivation was carried out utilizing blend of wastewater (80%) and BG-11 medium (20%) for S. obliques biomass production. Microalgal biomass production in PBR was observed regularly in equal intervals by measuring optical density (OD680 nm) utilizing UV spectrophotometer [12]. Biomass recovery was carried out through flocculation process by varying the pH of the solutions to 10, 10.5, 11, 11.5 and 12 using concentrated NaOH solution. After the flocculation process the microalgal suspension was centrifuged at 50 rpm for 15 min. The difference between Total suspended solids (TSS) of wastewater and the supernatant obtained from the four flocculated samples after sedimentation at room temperature for 30 min and 24 h [13] was estimated to determine the flocculation efficiency. The recovered biomass was dried, powdered and kept at –20°C for further process.
Biomass characterization plays the important role in determining the effective process for desired product production. Moisture and fiery debris content of the microalgae biomass was determined using ASTM E 871 standard, and ASTM E 1755 standard. Pyrolytic behavior of recovered microalgal biomass was analyzed using a thermo-gravimetric analyzer (Shimadzu, TGA 50H). Around 10 mg of microalgal biomass was examined to study its weight degradation curve. The biomass was heated from temperature of 30 to 800°C at various heating rates of 10, 20, 30, 40 and 50°C/min and kept hold at final temperature for 10 min [14].
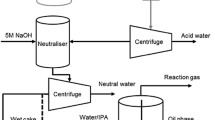
Hydrothermal liquefaction process. The equipment used for the experiment was a 250 mL capacity stainless steel closed reactor equipped with electric heater and a cooling unit. Different S. obliques biomass to water proportions (0.025, 0.05, 0.075 and 0.1 g/mL) were liquefied at varying reaction temperatures ranged from 200 to 340°C under 5 MPa nitrogen environment for 60 min. Different catalyst loadings of 2.5–8 wt % NaOH were added to the reactor. Homogeneous suspension was obtained inside the reactor by mixing at 720 rpm using a variable speed controller. After the experiments, the reactor was cooled down to room temperature using cold running tap water. Later, the solid residue left-out, crude oil and the gas generated were analyzed and quantified [15].
Bio-oil extraction. Solvent extraction technique was carried out to recover the maximum bio-oil from the crude by just leaving 0.5−0.7 % in the biomass [16]. Chloroform, hexane, acetone and benzene either separately or in blended proportions used for bio-oil extraction process [17]. Due to low risk and cost hexane was used in this study [18]. Hexane was added in equal proportions to the crude oil obtained from liquefaction process for bio-oil extraction. The organic phase obtained after separation was collected, pre-weighed and subjected to vacuum refining to confirm zero amount of hexane in it. The oil yield (wt %) was then decided utilizing (Eq. 1) and further the physicochemical properties of the bio-oil is examined.
Upgrading of bio-oil. The bio-oil obtained from liquefaction was upgraded into hydrocarbons rich liquid fuels using catalyst in a high-pressurized reactor. Bentonite was used as catalyst and its preparation was reported in our previous study [19]. Diverse catalyst loadings of 0.2–1 wt % were added in accordance with 100 mL of bio-oil in the reactor. Catalytic upgrading was carried out at varying temperature from 240 to 320°C for a holding time of 120 min at 750 rpm under nitrogen environment. After upgrading process the reactor was cooled down to room temperature using ice-cold water. The upgraded bio-oil was filtered and vacuum refined to recover high grade liquid hydrocarbons. The upgraded bio-oil yield was calculated using Eq. (2):
Characterization of sample. Agilent 7890 GC outfitted with an Agilent 7683B auto-injector, a HP-5 section of (100 m × 320 µm × 0.25 µm) and a fire ionization indicator (FID) was used to analyze the produced bio-oil. The organic constituents present in the bio-oil were identified using FTIR analysis. Elemental analyses like carbon, hydrogen, nitrogen, sulphur and oxygen were performed using Thermo Scientific flash 2000 auto-analyser (Thermo Fisher Scientific, USA).
RESULTS AND DISCUSSION
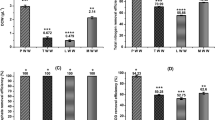
Wastewater treatment using algae. The significant reduction in the ammoniacal nitrogen and nitrate nitrogen was observed in the diluted wastewater compared to the original wastewater, which has also been reported by Han et al. [20]. In a similar fashion, Kshirsagar [21] found that for a retention period of 15 days, the removal of nitrate with Chlorella vulgaris and Scenedesmus quadricauda was 78.1 and 70.3% respectively. In this study, about 86.9 and 91.8% removal of ammoniacal nitrogen was observed on the 7th and 15th days, whereas in the case of nitrate, it was monitored to be 54.6 and 89.3% on 7th and 15th day respectively. Thus the growth of microalgae is vital by nitrogen stripping via the processes of decay and uptake [22]. Microalgae based bio treatment of wastewater obtained from this study was compared with those which utilised other S. obliques species.
Microalgae growth and biomass recovery. The biomass recovered from PBR was weighed and the yield was around 3.8 g/L. Like bacteria, microalgae also displayed four development stages – lag, exponential, stationary and death phase [23]. Figure 1 depicts the amount of biomass recovered after flocculation process. The biomass recovery after 30 min was around 84.4% in pH 12 and 18% in pH 10 respectively. When it was kept for sedimentation for 24 h, the biomass recovery increased. After 24 h the biomass recovery was 29% at pH 10 and at pH 12 it was around 91.2%. Bio-molecules (protein, fats, and so on) at pH 12 tends to precipitate. This process helps in higher degree of flocculation and faster sedimentation [24].
Biomass characterization. The moisture content of S. obliques biomass was 10.7 ± 0.2 wt % (wet premise) and 11.5 ± 0.3 wt % of fiery debris (ash) content. The higher heating value (HHV) was around 18.2 ± 0.4 MJ/kg. Microalgal biomass possessed higher nitrogen content and less oxygen content than terrestrial plants [25]. The protein content was 54.62% and fat content was around 12.32% individually in the microalgal biomass. Figure 2 portrays the weight reduction curve of S. obliques at various heating rates under nitrogen atmosphere. The TGA curve can be recognized by three phases’ likely primary stage (40–200°C), secondary stage (200–600°C) and third stage (600–800°C). Primary stage corresponds to loss of moisture due to slight warm conditions. The weight reduction at this stage was 10.7 ± 0.2, which was equivalent to the moisture substance of biomass. The second stage occurs due to pyrolysis of sample, and the third stage occurred due to slow degradation of carbonaceous material. A large portion of the organic materials was disintegrated during the second stage. The major mass disintegration happens between 200−350°C, in other macro algae’s [26]. The mass loss beyond 600°C may be due to lipid degradation.
Liquefaction process. Apart from bio-oil, liquefaction process yielded gas and solid derivatives. Solids mostly contain charred bio-matter. In the present study, the yield of biochar was approximately 2 g/15 g algae. The yield of the final liquefied products and the gas mixture composition depends upon many parameters like, temperature, biomass source, biomass size, heating rate, operating pressure and reactor configuration [27]. According to the above parameters, it was found that the gas generated from liquefaction process contained 22 vol % hydrogen gas, 18 vol % carbon dioxide gas, 27 vol % carbon monoxide gas, 22 vol % of methane gas and a small amount of other gaseous components (H2S). All these values were calculated without N2 component since it was purged during liquefaction process.
Effect of reaction temperature. Hydrothermal liquefaction was carried out using varying catalyst loads likely 2.5, 5 and 8 wt % under different temperature range (200–340°C). Increase in catalyst loading (5 and 8 wt %) resulted in a lesser bio-oil yield than 2.5 wt % of catalyst load. This may be due to insufficient biomass to water ratio loading rates. The influence of reaction temperature on the bio-oil yield from S. obliques biomass was studied at biomass loading of 15 g/200 ml and a catalyst loading of 2.5 wt % for holding time of an hour is given in Fig. 3. The bio-oil yield beyond 320°C tends to be in decreasing pattern. This may be due to higher heating temperature and insufficient solvent in the reactor.
Effect of Biomass loading.Figure 4 demonstrates the effects of biomass loading (0.025, 0.05, 0.075, and 0.1 g/mL) on bio-oil yield at a temperature of 300°C, a catalyst loading of 2.5 wt % and a holding time of about 60 min. From the results it was depicted that biomass loading had significant increase in bio-oil yield when the biomass loading was increased to 0.075 from 0.025 g/mL respectively. The bio-oil yield decreased at biomass loading of 0.1 g/mL due to degradation of biomass by condensation, cyclization and polymerization reactions. In similar fashion, Biswas et al. [28] reported that maximum bio-oil yield was 23.8 wt % when hydrothermal liquefaction of algae (Sargassum tenerrimum) was performed with ethanol as solvent at a temperature of 280°C.
Upgraded bio-oil yield. Catalytic upgrading of HTL bio-oil obtained from NaOH processed S. obliques biomass resulted in higher upgraded liquid hydrocarbons yield around 30.15 wt % of upgraded bio-oil at a reaction temperature of 280°C and catalyst load 0.8 wt % of HCl activated catalyst respectively (Fig. 5). The HHV value was 42.07 MJ/Kg respectively under above similar conditions. At higher temperatures insufficient solvent would have resulted in decreased the upgraded bio-oil yield. Microalgae bio-oil upgrading studies showed 39.6–41.9 MJ/Kg of HHV for Scenedesmus sp. in presence of Pt/Al2O3 and HZSM-5 as catalysts [29].
Characterization of bio-oil.Physiochemical properties of bio-oil. The physical properties of bio-oil and fractionated bio-oils are discussed in Table 1. Filatov et al. [30] reported the presence of hetero compounds in the high viscous oil from Ashal’chinskoe field with similar findings related to present study. The presence of hydrocarbons benefits the bio-oil for the usage as transportation fuel.
The main absorbance bands of bio-oil that reveals the specific functional groups and the presence of related class of compounds is discussed. The operating conditions did not affect the main organic components present in the bio-oil. The FTIR spectra were the same in all operating conditions tested. The band at 1404.36 cm–1 are related to CH2 bending and CH3 bending vibrations which shows the presence of alkanes. The O–H stretching vibration appearing in the frequency 3349.01 cm–1 can be caused by the presence of water impurities or alcohols. The C–H bending vibrations in 595.48, 549.99, 487.18, 473.03, 465.49, and 459.21 cm–1 may be due to the presence of esters and aromatic compounds. The C=O vibration in the range 3349–1634.67 cm−1 indicates the presence of carboxylic acids, ketones or aldehydes. The band at 1554.15 cm–1 can be attributed to a C−H stretching vibration that indicates the presence of alkanes in the bio-oils. Unsaturated hydrocarbons (alkenes, alkynes and aromatic hydrocarbons) were present in bio-oil. However, upgradation is essential to convert this bio-oil to transportation fuel.
GC-MS analysis of bio-oil. Gas Chromatography-Mass Spectrometry (GC-MS) depicts the composition and nature of compounds present in the bio-oil. Hydrothermal liquefaction works based on decomposition of biomass under higher temperature and high pressure. This provides supercritical water conditions in which the reaction rates are enhanced. Beyond this temperature conditions (400–700°C) it leads to hydrothermal gasification process. Around sixteen major compounds were distinguished in the bio-oil obtained through liquefaction process of S. obliques biomass. Table 2 elaborates the various compounds identified in bio-oil through GC-MS analysis.
A sterol compound (stigmastanol) found in major plant sources was also distinguished in the microalgal bio-oil. Presence of aromatic compounds in bio-oil enhances its octane number. Higher H/C ratio and lesser O/C ratio are the major factors determine the usage of this bio-oil as transportation fuels. The low (C12–C14) and high (C22–C30) molecular weight compounds identified in the bio-oil are majorly the derivatives of n-alkanes. Among aromatic compounds, polycyclic aromatic hydrocarbons (PAHs) were below the detection limit. Saturated hydrocarbons were bonded via ester and sulfide bridges and had n-alkanes (around 94.9 and 85.9%, respectively) in the bio-oil obtained from oilfield [31]. The amine compounds detected in the bio-oil would have attributed due to decarboxylation reactions. The aromatics, pyridines and amides were below the detection limit. The hexadecane and octadecane detected in upgraded bio-oil are alkane hydrocarbons, which was the important measure of diesel fuel.
CONCLUSIONS
The following conclusions were made out of this study:
—S. obliques has the capability to remove 91.8% of N–NH3 and 88% of COD from municipal wastewater. The biomass growth observed was 3.8 g/L.
—Hydrothermal liquefaction yielded a maximum of 24.57 wt % bio-oil at reaction temperature 300°C, reaction time of 60 min and 2.5 wt % of catalyst load.
—Catalytic upgrading of HTL bio-oil resulted in 30.15 wt % of upgraded bio-oil yield.
—GC-MS revealed that upgraded bio-oil mainly contained C7−C21 range hydrocarbons.
—This preliminary study has shown that hydrothermal liquefaction of microalgae S. obliques can potentially produce bio-oil and also value-added platform chemicals.
REFERENCES
J. K. Pittman, A. P. Dean, and O. Osundeko, Bioresour. Technol. 102, 17 (2011).
L. Christenson and R. Sims, Biotechnol. Adv. 29, 686 (2011).
A. Demirbas, Energy Policy 35, 4661 (2007).
C. Zhao, T. Bruck, and J. A. Lercher, Green Chem. 15, 1720 (2013).
J. S. Deschenes, A. Boudreau, and R. Tremblay, Algal Res. 10, 80 (2015).
C. Alcántara, E. Posadas, B. Guieyss, and R. Muñoz, Handbook of Marine Microalgae: Biotechnology Advances, Ed. by S.-K. Kim (Elsevier, Amsterdam, 2015), p. 439. https://doi.org/10.1016/B978-0-12-800776-1.00029-7
H. Zhou, X. Gao, P. Liu, et al., Pet. Chem. 57, 71 (2017).
P. Chen, M. Min, Y. Chen, et al., Int. J. Agric. Biol. Eng. 2 (4), 1 (2009).
T. M. Mata, A. A. Martins, and N. S. Caetano, Renew. Sustain. Energy Rev. 14, 217 (2010).
G. Chen, W. Yuan, Y. Bai, et al., Pet. Chem. 57, 389 (2017).
DR/890 Colorimeter: Procedures Manual (Hach, Loveland, CO, 2013).
N. M. Nasir, N. S. A. Bakar, F. Lananan, S. H. A. Hamid, S. S. Lam, A. Jusoh, Bioresour. Technol. 190, 492 (2015).
N. Drira, A. Piras, A. Rosa, et al., Bioresour. Technol. 206, 239 (2016).
S. Thangalazhy-Gopakumar, S. Adhikari, S. A. Chattanathan, and R. B. Gupta, Bioresour. Technol. 118, 150 (2012).
V. V. Teplyakov, M. G. Shalygin, A. A. Kozlova, A. V. Chistyakov, M. V. Tsodikov, and A. I. Netrusov, Pet. Chem. 57, 747 (2017).
Z. T. Harith, F. M. Yusoff, M. S. Mohamed, et al., Afric. J. Biotechnol. 8, 5971 (2009).
N. S. Topare, S. J. Rout, V. C. Renge, et al., Int. J. Chem. Sci. 9, 1746 (2011).
S. Rajvanshi and M. P. Sharma, J. Sustain. Bioenergy Syst. 2 (3), 49 (2012).
J. Arun, K. P. Gopinath, P. SundarRajan, M. Jose-lyn-Monica, and V. Felix, Bioresour. Technol. 274, 296 (2019).
X. Han, Y. S. Wong, M. H. Wong, and N. F. Y. Tam, J. Hazard. Mater. 146, 65 (2007).
A. D. Kshirsagar, Int. J. Life Sci. Biotechnol. Pharm. Res. 2, 339 (2013).
E. J. Olguin, S. Galicia, G. Mercado, and T. Perez, J. Appl. Phycol. 15, 249 (2003).
E. Ryckebosch, K. Muylaert, and I. Foubert, J. Am. Oil Chem. 89, 189 (2012).
J. S. Garcia-Pérez, A. Beuckels, D. Vandamme, et al., Algal Res. 3, 24 (2014).
P. McKendry, Bioresour. Technol. 83, 37 (2002).
S.-S. Kim, H. V. Ly, J. Kim, et al., Bioresour. Technol. 139, 242 (2013).
A. L. Stephenson, E. Kazamia, J. S. Dennis, et al., Energy Fuels 24, 4062 (2010).
B. Biswas, A. A. Kumar, Y. Bisht, et al., Bioresour. Technol. 242, 344 (2017).
D. L. Barreiro, B. R. Gómez, F. Ronsse, U. Hornung, A. Kruse, and W. Prins, Fuel Process. Technol., 148, 117 (2016).
D. A. Filatov, E. B. Krivtsov, N. N. Sviridenko, et al., Pet. Chem. 57, 649 (2017).
T. V. Cheshkova, E. Yu. Kovalenko, N. N. Gerasimova, et al., Pet. Chem. 57, 31 (2017).
ACKNOWLEDGMENTS
The authors wish to acknowledge the SSN trust for financially supporting this study.
Author information
Authors and Affiliations
Corresponding authors
Additional information
The article is published in the original.
Rights and permissions
About this article
Cite this article
Arun, J., Gopinath, K.P., Shreekanth, S.J. et al. Effects of Process Parameters on Hydrothermal Liquefaction of Microalgae Biomass Grown in Municipal Wastewater. Pet. Chem. 59, 194–200 (2019). https://doi.org/10.1134/S0965544119020026
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0965544119020026