Abstract
The process of unsteady-state membrane gas separation (fast-permeant impurity removal) with a pulsed retentate flow operation was considered. A semiempirical mathematical algorithm was developed to describe this process taking into account its kinetic characteristics (total cycle time, stripping time and withdrawal time, withdrawal velocity) using the MathCad® software package. Based on the developed algorithm, the basic operational parameters that affect the separation efficiency of the unsteady-state process were analyzed. It was shown that the optimal ratio of the stripping time and the withdrawal one determined by the maximum efficiency criterion more corresponds to the minimum retentate concentration than to the maximum productivity. However, the developed algorithm allows to set the productivity minimum limit by introducing additional initial data into the calculation procedure. The mathematical modeling results correlate well with the experimental data.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
The development of gas separation technologies is associated with the solution of tasks to increase requirements for final products quality, energy efficiency, industrial and environmental safety. To overcome new technological challenges, it is possible to follow the integral trend, namely, to combine separate processes in hybrid or integrated circuits, including both traditional separation methods and ones based on membrane technologies [1–10]. Membrane gas separation is a rapidly developing energy-efficient technology [11–16], which provides advantages such as hardware design simplicity, high environmental safety, high level of energy saving while realizing the process at low material costs in contrast with the traditional methods mentioned above. The key directions in the development of membrane gas separation technologies are materials science, namely the synthesis of new highly selective polymer materials, chemical technology (including the development of new processes) and the operating conditions optimization of already existing separation schemes, for example, membrane cascades [13, 16–18]. One of the promising trends related to the optimization of membrane module operation is the development and optimization of cyclic membrane processes to improve the selectivity and productivity of membrane gas separation [19–27].
The first theoretical study considering the cyclic process of membrane gas separation was performed by Paul D. [19] in 1971. This process is based on steady cyclic pulsed inlet of feed with further sequential mass transport of binary gas mixture components through the polymeric membrane.
In 1991, Beckman I. [28] experimentally implemented the process theoretically proposed by Paul. He and co-workers separated the binary gas mixture of equally permeable components He/CO2. The difference in their diffusion coefficients provided the separation effect of the mixture which is not separable in a traditional membrane module. It should be noted that membrane thickness is crucial for such cyclic processes. This parameter is important for determining the necessary switching time between pulsed inlet of a feed mixture and components sequential withdrawal in the form of permeate. Hence, this gas separation method imposes a number of limitations on the choice and application of asymmetric polymeric membranes with a thin separation layer. Moreover, for this process, a forced membrane recovery stage is also important, meaning that the productivity of this cyclic gas separation mode inevitably decreases in comparison with a steady-state process.
Feng X. et al. [23] proposed an alternative approach to improve membrane separation process efficiency during cyclic operation. Their process is analogous to pressure swing adsorption. In a membrane module, alternating transmembrane pressure is created by cyclic inlet of feed followed by release of permeate product at elevated pressure. This cycle increases the driving force for the separation process. The pressure swing permeation achieves higher selectivity compared to that of steady-state operations, but the effective flow rate is quite low. Moreover, Feng’s process is not suitable for deep purification of gases due to the inevitable cross contamination of the product during flows switching.
In 2011, Favre E. [20] wrote a review devoted to the cyclic processes of membrane gas separation. It was shown that, in the operating modes of membrane apparatuses under non-stationary conditions, a significant increase in separation efficiency can be achieved in comparison with steady-state processes, but with loss of productivity. It was also noted that improving the separation efficiency is always associated with hardware design complication, increased operating costs and energy consumption.
In 2011, Vorotyntsev V. et al. [29] proposed and theoretically considered the process of membrane gas separation in a single membrane module operating in a pulsed retentate flow mode, which differs from the previously studied cyclic processes. This approach conceptually repeats the principles of batch rectification with periodical withdrawals [30–34]. The proposed process of membrane gas separation with the pulsed retentate flow mode is realized cyclically in two stages. In the first stage, the membrane module operates in a closed operating mode [35], when there is no a purified product withdrawal. At the same time, the module gradually establishes a state close to a steady one, and the limit stripping state for a fast-permeant impurity is achieved. In the second stage, the withdrawal of a purified slow-permeant target component is carried out by opening a pneumatic valve on the retentate line. Increase in efficiency is achieved due to the alternation of the closed operating mode with the short-term retentate withdrawal, since a periodic perturbation close to the steady state contributes to an increase in the driving force due to a more intensive process of mass transport on the feed side. This cyclic separation mode is more versatile than the pulse input mode of a feed mixture, since the latter is effective only if there is a different diffusion mobility of the separated components (time lag effect). Membrane thickness is not critical for the new process, and its complexity and operating costs are relatively less.
In 2017, Vorotyntsev V. et al. [36] experimentally verified the mathematical model of the unsteady-state membrane gas separation with pulsed retentate flow operation on the example of three binary gas mixtures with different selectivity values: CH4/N2 (αef = 2.7 ± 0.5); CO2/N2 (αef = 7.5 ± 1.5); N2O/N2 (αef = 10 ± 2). It was shown that, in the field of small-productivity, the separation efficiency for the gas mixtures (on the membrane Silar®) is higher in the case of the pulsed retentate mode than in the case of the steady one with the same productivity. The obtained results showed good agreement with the preliminary theoretical evaluation of the cyclic process behavior [17]. However, during experimental studies, effects were established that influence the separation factor. So, in the work [17] on the pulsed retentate mode, the mathematical model does not take into account the stages relaxation time. In the work [17], the correlation of stripping time and separation efficiency in the closed mode operation of the membrane module was obtained. Also, the dependence of final product purity on withdrawal time and, accordingly, on retentate withdrawal volume was established. Hence, based on the presence of kinetic factors that affect separation efficiency and corresponding performance, it is necessary to develop a multiparametric optimization model to quickly and efficiently select the best operating parameters that provide the necessary purification degree and target productivity.
The purpose of this work is a mathematical description of the cyclic membrane gas separation (on the example of a radial membrane module) taking into account the cycle time of pulsed retentate flow operation (total cycle time, stripping time and withdrawal time) and the analysis of the main parameters affecting separation efficiency: retentate withdrawal volume, withdrawal velocity, transmembrane pressure, flow ratio and membrane selectivity.
MATHEMATICAL MODELING
The operation principle of the radial membrane module is as follows (Fig. 1) [37, 38]. A flat-sheet membrane of the same size as the module internal diameter is placed on a porous stainless steel substrate inside the module, it separates a feed side (high pressure compartment, HPC) and permeate side (low pressure compartment, LPC), respectively. A feed flow is fed to the HPC of the membrane module and radially moves along the selective membrane surface from the periphery to the center as shown in Fig. 1. This principle excludes the “dead” zones in the module and allows to ensure laminar gas flow in the gap between the distribution disk and the membrane (the shaded area in Fig. 1). The membrane module operates in a mode close to ideal displacement. The gas linear velocity in the radial module changes to a lesser degree than in a plane-parallel module, so that the negative effect of longitudinal mixing decreases and this design shows higher separation efficiency [36]. The LPC is similar, but a permeate flow radially moves from the center to the periphery.
In the process with the pulsed retentate flow operation, the feed flow is fed to the HPC at a constant pressure, while the permeate flow is continuously pumped out of the LPC using a vacuum pump with a constant flow.
The operation cyclic mode of the membrane module is carried out in two stages.
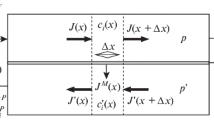
At the first stage, the membrane module operates in the closed mode with zero retentate flow (a pneumatic valve is closed), in this case, it means no product withdrawal. At this stage, in the HPC a state close to a steady one gradually establishes, while the feed flow is continuously fed at a constant pressure, the permeate is evacuated with the constant flow. Under these conditions, an impurity concentration profile is formed along the membrane in the HPC, as shown in Fig. 2. When the state close to the steady one (t0close) is reached, the maximum difference between the concentrations at the feed side entrance (C0 is an input concentration) and in the membrane center (C0out is an output concentration) is realized, where the purified retentate withdrawal occurs.
Two-step cycle of the pulsed retentate flow mode for a single plane-parallel membrane module compared to the steady-state operation: functioning flows are shown by thick likes; grey line represents an exemplary feed-side concentration profile of a fast-permeant impurity; shaded area represents a retentate sample to be withdrawn.
At the second stage, the pneumatic valve opens, and a small amount of purified gas is taken from the module feed side (Fig. 2) [35]. It is assumed that, during the valve opening (topen), the impurity concentrations profile shifts along the membrane (Fig. 2). When carrying out the process with the pulsed retentate flow operation, it is important to withdraw only a part of the feed side volume in order to achieve the lowest impurity concentration in the retentate withdrawal (Cavg.out).
The process proceeds cyclically with the alternation of the closed mode and the pulsed retentate flow operation at a constant transmembrane pressure with minor fluctuations arising from the cyclic withdrawal. The total time of one cycle (the both stages) consists of the closing and opening time of the pneumatic valve: tcycle = tclose + topen. Since the retentate withdrawal is realized only during the valve opening, the average retentate flow is defined as the value numerically equal to the ratio of the withdrawal volume (at the time of the valve opening) to the total cycle time.
Next, the cyclic membrane gas separation process in the HPC of the radial module, in which stripping a fast-permeant impurity occurs, is considered. The choice of this side is based on the fact that, it is most important for characterizing the process with the pulsed retentate flow operation, since the retentate flow Lout and the feed one Lin depending on it (depending on the cycle time) change there [36]. On the permeate side (LCP), the flow Lm does not change in contrast to the impurity concentration in it, which, in turn, will be taken into account in optimizing the process.
The algorithm for calculating the process was carried out based on the following assumptions:
(1) the gas dynamics in the membrane module is completely determined by the target component, and the impurity influence can be neglected, since its concentration is much lower than the target component concentration;
(2) the membrane module operates in the ideal displacement mode (longitudinal mixing is negligible in the HPC);
(3) membrane permeability remains constant in time and is the same for both pulsed and steady-state modes;
(4) gas mixture concentration in a direction perpendicular to the membrane surface is constant;
(5) pressures in the feed and permeate side are assumed to be constant;
(6) the process is carried out under isothermal conditions;
(7) ideal gas behavior is assumed.
The radius orientation of the radial module (and, correspondingly, of the membrane) l coincides with the x axis, along which the fast-permeant impurity component of a gas mixture strippes (Fig. 2). On this axis, the segment l' reflects a cylinder base radius, the volume of which is equivalent to the retentate withdrawal volume. In turn, the value (l – l')/l reflects the withdrawal ratio.
The impurity concentration in the gas mixture decreases (in the mode close to ideal displacement) along the working membrane surface in the HPC from the periphery (input) to the center (output) because the fast-permeant impurity component goes under the membrane. In the closed mode (there is no retentate withdrawal), the gas mixture in the HPC also moves along the membrane surface from the periphery to the center due to the distribution disk presence, so that the impurity concentration profile is established along the membrane surface from the initial (at the periphery) to the minimum (in the center). Based on this and the experimental data obtained [36], it was assumed that, during establishing the state close to the steady one in the closed mode, the impurity concentration gradient is distributed according to the exponential law (in the HPC of the radial membrane module):
where k > 0, a < 0—equation coefficients of an exponential curve in general form. To find these coefficients, the following boundary conditions were considered:
The coefficient a was found using the following transformations:
The resulting expressions for the coefficients k and a were substituted into the equation (1):
Analogous transformations were performed to obtain the equation for the dependence of the impurity concentration at the outlet of the membrane module on the stripping time Cout(tclose):
Based on the equation (2), the expression for \({C}_{{{\text{out}}}}^{'}\) was obtained:
The retentate withdrawal volume is equivalent to the cylinder volume with a base radius l' and a height hm (a module HPC height). This volume is withdrawn during the module opening time topen. Proceeding from this, l' was defined by the following expression:
where Vout—the retentate withdrawal volume, which was defined as: Vout= vouttopen,, vout—the withdrawal velocity.
The average concentration Сavg.out in the retentate withdrawal volume was calculated by the mean value theorem for definite integrals:
RESULTS AND DISCUSSION
Since the algorithm provides for and takes into account the possibility of scaling a separation cell and a membrane area, and also the possibility of changing the velocities of input and output flows, the membrane module productivity was considered as a relative value indicating the percentage of the retentate flow to the feed one:
where Lin—the feed flow, Lout—the retentate flow:
The equation of material balance:
where Lm—the permeate flow.
The equations (3) and (4) are the algorithm basis for optimizing the pulsed retentate flow operation mode. However, optimization implies accounting for all parameters of the simulated process, and its implementation only on the basis of analytical calculations is difficult. In this connection, all calculations were carried out using matrix operations in the software package MathCad®.
Matrix dimension was set based on the maximum allowable values of topen (number of rows) and tclose (number of columns). The maximum opening time was limited by the time required to withdraw the total volume of the module feed side:
where Vm – the module feed side volume. The maximum closing time was limited by the module operation transiting time to the steady-state mode:
To be able to compare calculated data with empirical ones, the matrix pitch relative to the opening topen and closing tclose time of the membrane module was one second (based on instrumental experimental limitations related to the minimum switching time of the pneumatic valve).
The optimization calculations was considered on the example of dinitrogen oxide removal from the system N2/N2O, for which, according to the data of [36], the greatest increase in the separation efficiency was obtained in the cyclic mode in comparison with the steady-state one.
The case under consideration was determined by the following initial conditions: рHPC = 120 kPa, рLPC = 8 kPa, vout = 0.0006 L/s – the withdrawal velocity, Cin = 0.01 (1 vol %)—the impurity concentration in the feed flow, С0out= 0.000001 (0.0001 vol %)—the minimal impurity concentration in the retentate flow for the closed mode (was determined experimentally), hm = 0.001 m—the module HPC height, l = 0.126 m—the membrane module radius, \({{V}_{{\text{m}}}} = \pi {{l}^{2}}{{h}_{{\text{m}}}}\)—module HPC volume (is calculated on the basis of geometry features; in this case, it is a cylinder with a volume of Vm = 0.050 l), topenMAX = 80 s—the maximum withdrawal time, tcloseMAX = 15 s— the maximum stripping time (was determined experimentally).
For all possible combinations of the opening topen and closing tclose time of the membrane module operating in the pulsed retentate flow mode, using the matrix operations, the average values of impurity concentration in the retentate flow Сavg.out and the relative productivity P, the optimal combination of which was determined by the efficiency criterion E:
where EC—a value describing the ratio of the minimum average impurity concentration in the retentate flow Cavg.outMIN to all possible its values Cavg.out:
ЕР—a value describing the ratio of all possible values of the system’s productivity Р to its maximum value РMAX:
The physical meaning of the E efficiency criterion components is as follows:
(1) the closer the value of EC to 1, the greater the approximation of the average impurity concentration in the retentate flow to the minimum;
(2) the closer the value of EP to 1, the greater the approximation of the relative productivity to the maximum.
Thus, the maximum value of the efficiency criterion E corresponds to the optimal pulsed retentate operation mode of the system.
In the calculations for the gas system under study, the maximum value of the efficiency criterion EMAX was 0.1374. This maximum corresponds to the impurity concentration of N2O in the retentate flow Сavg.out = 1.68 ppm (1.68 × 10–4vol %) and the relative productivity P = 0.1 (10%), which, in turn, correspond to the pulsed retentate operation mode at topen = 4 s, tclose = 15 s. Figure 3 shows the plane graphs of the EC and EP values, as well as the efficiency criterion E, the maximum of which determines the value EMAX depending on topen and tclose.
It was revealed that, in determining optimal opening and closing times, the criterion maximum is always shifted to the minimum concentration, and not to the maximum productivity of the pulsed retentate operation mode. This is due to analytical expressions of the dependence of concentration and productivity on the cycle duration (the concentration on the cycle duration is described by an exponential function, and the productivity dependence is described by a hyperbolic one). The cycle time effect on concentration predominates over the cycle time effect on productivity.
However, the developed algorithm for calculating the efficiency criterion E allows to shift the relative productivity boundary towards higher values by entering additional constraints that set the permissible minimum PMIN in the dominance field (concerning the separation efficiency) of the pulsed retentate operation mode over the steady-state one [36].
For the N2/N2O system (at the withdrawal velocity vout = 0.0006 L/s), when changing the value PMIN from 0.05 (5%) to 0.2 (20%) at a pitch of 0.05 (5%), the following shift of the optimum of the pulsed retentate operation mode was observed:
(a) topen = 4 s, tclose = 15 s, P = 0.064, C = 1.68 ppm;
(b) topen = 8 s, tclose = 15 s, P = 0.101, C = 3.05 ppm;
(c) topen = 19 s, tclose = 15 s, P = 0.153, C = 10.15 ppm;
(d) topen = 48 s, tclose = 14 s, P = 0.200, C = 119.70 ppm.
Thus, the developed algorithm describing the pulsed retentate process allows to calculate at what relative productivity, which concentration (as close as possible to the minimum) can be obtained for each system separately, which describes the chosen criterion in its maximum significance.
It is also shown that, when the minimum concentration is reached, both parameters, namely, the stripping time (tclose) and the withdrawal time (topen), equally affect the system optimum.
In addition, the developed algorithm allows to vary the values of the relative productivity P by changing the withdrawal velocity vout within a constant transmembrane pressure.
So, for the N2/N2O system, with an increase in the withdrawal velocity vout from 0.0006 L/s to 0.0017 L/s, the optimal values of topen and tclose for the new separation conditions were 2 and 15 s, respectively.
In order to verify the developed algorithm, based on the results of mathematical calculations, curves were constructed describing the dependence of the separation efficiency \(F = {{{{C}_{{{\text{in}}}}}} \mathord{\left/ {\vphantom {{{{C}_{{{\text{in}}}}}} {{{C}_{{{\text{avg}}{\text{.out}}}}}}}} \right. \kern-0em} {{{C}_{{{\text{avg}}{\text{.out}}}}}}}\) from the module relative productivity (Fig. 4) for three different withdrawal velocities and, respectively, three productivity areas (curves 1, 2, and 3). The results of the calculation correlate well with the experimental data obtained in work [36]. Noticeable discrepancies can be noted in the relative productivity range from 0.1 to 0.2. This is due to the fact that the operating conditions of the cyclic separation process, in which experimental data were obtained in [35], did not correspond to the optimal ones determineв by the algorithm, and therefore the calculation results predict a higher separation efficiency in this area. Thus, the proposed mathematical apparatus describing the cyclic gas separation process in the membrane module with pulsed retentate allows to optimize operating conditions for a single membrane module.
Graphs comparison of the separation factor dependence on the relative productivity obtained on the basis of the experimental realization of the steady-state (a) and unsteady-state (b) modes [32] and mathematical calculations at different withdrawal velocity: (1) 0.0006 L/s, (2) 0.001 L/s, (3) 0.0017 l/s.
In addition to introducing the additional constraints and varying the relative productivity values P by changing the withdrawal velocity, there is also the possibility of implementing a single-stage installation with a sequential arrangement of membrane modules [17] (Fig. 5). This approach allows to increase the relative productivity of the membrane apparatus without the purity loss of withdrawn retentate.
From the point of view of the mathematical description, the process taking place in a multimodule installation is analogous to the one-module one and is described by the equations (3) and (4). However, the feed flow formation Lin largely depends on the number of modules in the installation, since, in terms of material balance, it is directed to compensate the retentate flow Lout and all permeate flows Lm:
where N—a number of modules in the membrane installation.
Based on the algorithm obtained for the N2/N2O system, it was shown that, for the same relative productivity, according to the separation efficiency \(F = {{{{C}_{{{\text{in}}}}}} \mathord{\left/ {\vphantom {{{{C}_{{{\text{in}}}}}} {{{C}_{{{\text{avg}}{\text{.out}}}}}}}} \right. \kern-0em} {{{C}_{{{\text{avg}}{\text{.out}}}}}}},\) the three-module installation always exceeds the two-module one, and the two-module installation is always exceeding the single-module one (Fig. 6). In addition, it is possible to vary the retentate flow and thereby increase the productivity analogically to the one-module installation.
CONCLUSION
The semiempirical mathematical algorithm for describing the process of membrane gas separation with pulsed retentate was developed taking into account its kinetic characteristics (total cycle time, stripping time and withdrawal time). The algorithm was implemented using the software package MathCad®. The results of mathematical calculations are in agreement with the experimental data.
Based on the developed algorithm, the basic operational parameters that affect the separation efficiency of the unsteady-state membrane separation of the gas mixture (with the fast-permeant impurity) were analyzed. To assess the cyclic membrane gas separation process, the efficiency criterion was introduced, the physical meaning of which is reduced to the choice of the optimal ratio of the stripping and withdrawal times for pulsed retentate providing a minimum impurity concentration in the withdrawn product sample and maximum productivity. It was revealed that the optimum of the pulsed retentate operation mode is always shifted to the minimum concentration, and not to the maximum productivity. This is due to analytical expressions of the dependence of concentration and productivity on the cycle duration of the module operation. However, it should be noted that the developed algorithm allows to vary the minimum allowable productivity value by entering additional conditions into the calculation procedure.
Thus, the developed algorithm for describing the membrane gas separation process with pulsed retentate allows to quickly estimate the final product purity and the possible productivity, determine the optimum ratio of the kinetic parameters for the module cyclic operating mode, and introduce additional initial conditions to optimize the process.
REFERENCES
B. van der Bruggen, Ind. Eng. Chem. Res. 52, 10335 (2013).
B. Belaissaoui, Y. L. Moullec, D. Willson, and E. Favre, J. Memb. Sci. 415–416, 424 (2012).
V. M. Vorotyntsev, V. M. Malyshev, I. V. Vorotyntsev, and S. V. Battalov, Theor. Found. Chem. Eng. 50, 459 (2016).
J. Pohlmann, M. Bram, K. Wilkner, and T. Brinkmann, Int. J. Greenh. Gas Control. 53, 56 (2016).
Q. Kang, B. van der Bruggen, R. Dewil, et al., Sep. Purif. Technol. 149, 322 (2015).
P. Luis, A. Amelioa, S. Vreysen, et al., Appl. Energy. 113, 565 (2014).
C. Makhloufi, E. Lasseuguette, J. C. Remigy, et al., J. Memb. Sci. 455, 236 (2014).
C. Servel, D. Roizard, E. Favre, and D. Horbez, Ind. Eng. Chem. Res. 53, 7768 (2014).
T. S. Anokhina, A. A. Yushkin, P. M. Budd, and A. V. Volkov, Sep. Purif. Technol. 156, 683 (2015).
A. Trusov, S. Legkov, L. J. P. van den Broeke, et al., J. Memb. Sci. 383, 241 (2011).
R. W. Baker, Ind. Eng. Chem. Res. 41, 1393 (2002).
W. J. Coros, J. Memb. Sci. 83, 1 (1993).
R. Pathare and R. Agrawal, J. Memb. Sci. 364, 263 (2010).
V. M. Vorotyntsev, P. N. Drozdov, I. V. Vorotyntsev, et al., Pet. Chem. 54, 491 (2014).
I. V. Vorotyntsev, A. A. Atlaskin, M. M. Trubyanov, et al., Desalin. Water Treat. 75, 305 (2016).
I. V. Vorotyntsev, D. N. Shablykin, P. N. Drozdov, et al., Pet. Chem. 57 (2), 172 (2017).
V. M. Vorotyntsev, P. N. Drozdov, I. V. Vorotyntsev, and S. V. Battalov, Pet. Chem. 54, 698 (2014).
V. M. Vorotyntsev, P. N. Drozdov, I. V. Vorotyntsev, and D. E. Tsygorov, Theor. Found. Chem. Eng. 43, 404 (2009).
D. R. Paul, Ind. Eng. Chem. Process Des. Dev. 10, 375 (1971).
L. Wang, J.-P. Corriou, C. Castel, and E. Favre, J. Memb. Sci. 383, 170 (2011).
A. Higuchi and T. Nakagawa, J. Appl. Polym. Sci. 37, 2181 (1989).
J.-P. Corriou, C. Fonteix, and E. Favre, AIChE J. 54, 1224 (2008).
X. Feng, C. Y. Pan, and J. Ivory, AIChE J. 46, 724 (2000).
Y. Chen, D. Lawless, and X. Feng, Sep. Purif. Technol. 125, 301 (2014).
D. D. Nikolić and E. S. Kikkinides, Adsorption 21, 283 (2015).
A. Shishov, A. Penkova, A. Zabrodin, et al., Talanta 148, 666 (2016).
V. M. Vorotyntsev, P. N. Drozdov, I. V. Vorotyntsev, et al., Pet. Chem. 51, 595–600 (2011).
I. N. Beckman, A. B. Shelekhin, and V. V. Teplyakov, J. Memb. Sci. 55, 283 (1991).
V. M. Vorotyntsev, P. N. Drozdov, I. V. Vorotyntsev, et al., Pet. Chem. 51, 492 (2011).
G. M. Howard, AIChE J. 16, 1030 (1970).
V. M. Vorotyntsev, G. M. Mochalov, M. A. Kolotilova, et al., Theor. Found. Chem. Eng. 42, 197 (2008).
V. M. Vorotyntsev, G. M. Mochalov, M. M. Trubyanov, and D. N. Shablykin, Theor. Found. Chem. Eng. 48, 55 (2014).
M. M. Trubyanov, G. M. Mochalov, V. M. Vorotyntsev, and S. S. Suvorov, Russ. J. Appl. Chem. 86, 1854 (2013).
M. M. Trubyanov, G. M. Mochalov, V. M. Vorotyntsev, and S. S. Suvorov, Sep. Purif. Technol. 135, 117 (2014).
P. N. Drozdov and I. V. Vorotyntsev, J. Theor. Found. Chem. Eng. 37, 491 (2003).
M. M. Trubyanov, P. N. Drozdov, A. A. Atlaskin, et al., J. Memb. Sci. 530, 53 (2017).
P. N. Drozdov, Y. P. Kirillov, E. Y. Kolotilov, and I. V. Vorotyntsev, Desalination 146, 249 (2002).
V. M. Vorotyntsev, P. N. Drozdov, I. V. Vorotyntsev, et al., Desalination 200, 232 (2006).
ACKNOWLEDGMENTS
The work was supported by the Russian Science Foundation, project no. 17-79-10464.
Author information
Authors and Affiliations
Corresponding author
Additional information
1The article was translated by the authors.
Rights and permissions
About this article
Cite this article
Battalov, S.V., Sazanova, T.S., Trubyanov, M.M. et al. Modeling of Fast-Permeant Component Removal from Gas Mixture in a Membrane Module with Pulsed Retentate. Pet. Chem. 58, 806–814 (2018). https://doi.org/10.1134/S0965544118090049
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0965544118090049