Abstract
Comprehensive petrological and isotope-geochemical study of marble was carried out in the upper part of the Sortavala Group of the northeastern lense at the Ruskeala deposit in the Northern Ladoga area. The petrological study showed that the carbonate rocks of the Sortavala Group underwent medium-temperature low-pressure amphibolite-facies metamorphism. The mineral assemblages of Ruskeala marbles were formed at temperature of 550–600°C and pressure of ~3–5 kbar in equilibrium with a mixed water-carbon dioxide fluid with \({{X}_{{{\text{C}}{{{\text{O}}}_{2}}}}}\) ~ 0.5–0.8. Dolomite marbles contain admixture (up to 2%) of finely disseminated carbonaceous matter and about 8–15% of calcite. Dolomites contain small amounts of Mn (70–110 ppm) and Fe (1600–3600 ppm) and high content of Sr (122–256 ppm). The initial 87Sr/86Sr ratio in the dolomites is within 0.70465–0.70522, δ13C values vary between +0.6…+1.9 ‰, and δ18О within –13.2 … –10.2‰ (V-PDB). Calcite marbles are free of carbonaceous matter, have very low contents of Mg (0.2–0.8%), Mn (10–90 ppm) and Fe (160–640 ppm) and very high Sr concentration (850–2750 ppm). The initial 87Sr/86Sr ratios in the calcite marbles range from 0.70482 to 0.70489, δ13С from 1.5 to 2.1‰, and δ18О from –10.9 to ‒8.1 ‰ (V-PDB). Tremolite-bearing marbles have higher 87Sr/86Sr ratio (up to 0.70522), while their δ13С and δ18О values decrease to 0.1‰ and –12.2‰, respectively. The metamorphism of Ruskeala carbonate even under medium-temperature amphibolite-facies conditions was essentially isochemical process, which retained the unique Sr and C chemostratigraphic potential of calcite and dolomite marbles for the reconstruction of 87Sr/86Sr and δ13C in the Paleoproterozoic seawater. The 87Sr/86Sr ratio in the 1.9–2.0 Ga Svecofennian ocean was 0.70463–0.70492, and δ13С value +1.5 ± 1‰. New Sr-isotope data record an increase in radiogenic Sr input in the ocean about 2 billion years ago, which was probably related to the growth of continental crust and more intense weathering. The δ13С in Ludicovian carbonates mark the beginning of C-isotope stasis in ocean after the Lomagundi-Jatulian anomaly of 13Ccarb.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Sedimentary and metasedimentary carbonate rocks provide information on the chemical and isotope composition of ocean in the geological past. This information is of great fundamental significance because the long-term isotope-geochemical variations of seawater are controlled by changes in global integrated fluxes into ocean. These changes were related to the evolution of crust, hydrosphere, atmosphere, and biosphere. A disturbance of balance within and between the geospheres affected the composition of ocean, in which chemogenic sediments have been accumulated and recorded important isotope-geochemical changes. In addition, obtained Sr and C isotope variation curves in the ocean can be used as a new tool for dating and correlation of marine chemogenic sediments, i.e., for isotope chemostratigraphy.
An idea to use 87Sr/86Sr ratio in marine chemogenic sediments for geochronometry and chemostratigraphy was proposed in the mid-20th century (Wickman, 1948). It was based on the fact that the 87Sr/86Sr ratio in the ocean is formed during crustal weathering and uniformly increases with time. First attempts to bring this hypothesis into practice were based on studying three samples of the Precambrian metacarbonate rocks from the Bulawayan, Grenville, and Belt provinces (Gast, 1955) and marbles of the Baltic Shield (Ruskeala deposit in the Ladoga region and Mramornyi Island in the White Sea) (Gerling and Shukolyukov, 1957). The pioneering studies showed that the growth of 87Sr/86Sr in the chemogenic rocks of the Earth is very insignificant and could not be measured accurately on mass spectrometers used at that time. In addition, the authors assumed that Precambrian rocks were “contaminated by Sr of unknown origin”. This fact in combination with a poor knowledge of strontium geochemical cycle in oceans and linked marine basins has delayed for a long time the implementation of idea of isotope chemostratigraphy.
Several decades were required to reach sufficient accuracy of mass-spectrometric measurements. It has been established by that time that the 87Sr/86Sr ratio in ocean shows no a monotonous increase, but is controlled by two global fluxes from isotopically different reservoirs: low 87Sr/86Sr mantle and high 87Sr/86Sr continental reservoirs (Spooner, 1976). First studies of carbon isotope composition in the chemogenic sediments revealed the correlation of 13С/12С variations with paleoclimatic, biotic, and volcanic events and showed their chemostratigraphic potential (Galimov et al., 1975; Schidlowski et al., 1976; Veizer and Hoefs, 1976; Scholle and Arthur, 1980; Veizer et al., 1980).
The reconstruction of the Precambrian (2–3 Ga) history of oceans requires the study of mainly metasedimentary carbonate rocks exposed on the Archean–Proterozoic shields and orogenic belts. The chemical composition of metamorphosed carbonates represents not only characteristics inherited from primary carbonate sediments, but also features caused by diagenetic and metamorphic recrystallization. The transformation of sedimentary limestones and dolomites into marbles occurs at high temperatures and pressures, and fluid assistance and causes a change of their texture and formation of new minerals. In order to decipher the chemical and isotope composition of seawater that precipitated the carbonates, it is neccassary to undo this postsedimentation reworking. Numerous works have demonstrated that metamorphosed carbonates and even marbles of the amphibolite facies under certain conditions can retain Sr- and С-isotope signatures of primary sediment up to the present time (Ghent and O’Neil, 1985; Baker and Fallick, 1988; Veizer et al., 1990; Boulvais et al., 1998; Gorokhov et al., 1998, 2016; Melezhik et al., 2002, 2003, 2005; Bolhar et al., 2002; Ovchinnikova et al., 2007; Dufour et al., 2007; Satish-Kumar et al., 2008; Maheshwari et al., 2010; Frimmel, 2010; Kuznetsov et al., 2010, 2018; Sial et al., 2019, and others).
In this work, we attempted to reconstruct the C- and Sr-isotope characteristics of carbonate sediments of the Svecofennian (Ludicovian) ocean using a complex approach based on the (1) assessment and specification of metamorphic grade of the studied carbonate sequence using mineral assemblages of silicate-carbonate and silicate rocks associated with marbles; and (2) identification of samples with least disturbed isotope systems using geochemical criteria. The study was carried out for metamorphosed carbonate rocks of the Sortavala Group, Northern Ladoga area–marbles of the Ruskeala deposit. The Ruskeala marble was one of the first sample used for the recovery of isotope characteristics of an ancient ocean. Thus, this paper is a continuation of study of our outstanding Russian geologists E.K. Gerling and Yu.A. Shukolyukov (1957).
GEOLOGICAL OVERVIEW
The Ruskeala marble deposit is located near the town of Sortavala. This deposit is the oldest and largest deposit of face marbles in the Northern Ladoga area. Owing to the high decorative quality, Ruskeala marbles have been widely used in architecture and home décor since mid-17th century.
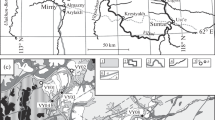
The Northern Ladoga area is located on the southeastern flank of the Raahe-Ladoga Suture Zone (Fig. 1), which is a junction zone of two large structures of the Fennoscandian Shield: Archean Karelian Craton and Paleoproterozoic Svecofennian accretionary orogen (Geologiya i petrologiya …, 2000; Bushmin and Glebovitsky, 2016). The lower structural-stratigraphic level is made up of Archean granite gneisses of the Karelian Craton margin, which are overlain by the Sortavala and Ladoga groups. The Sortavala Group (Kratts, 1963; Svetov and Svridenko, 1992; Shuldiner et al., 1996) consists predominantly of metamorphosed mafic and occasionally intermediate lavas and tuffs, which are alternated with two carbonate horizons (Kratts, 1963; Kitsul, 1963) and thin terrigenous interlayers. The Ladoga Group consists mainly of metaterrigenous rocks formed after greywacke–clayey turbidite sediments (Demidov and Kratts, 1958).
(a) Raahe–Ladoga Suture Zone (R–L) in the simplified tectonic scheme of the Fennoscandian shield (Bushmin and Glebovitsky, 2016) and geological structure of the Northern Ladoga area (b). Inset (b). Archean: (1) Karelian Craton; (2) granite gneiss domes. Paleoproterozoic: (3) metavolcanic rocks and metasediments, Sortavala Group; (4) metaterrigenous rocks, Ladoga Group; (5) mafic-ultramafic intrusions; (6) metamorphic and magmatic rocks of the Svecofennian orogen. Mesoproterozoic: (7) rapakivi granite, Salmi massif. (8) Ruskeala marble deposit (studied area).
The age of the Sortavala metavolcanic rocks is controversial. Available age estimates vary from 1.96–1.99 Ga (U-Pb zircon dates on Sortavala metadacites and metagabbro dike considered as the conduit for the volcanics; Geologiya i petrologiya …, 2000) to 2.05–2.10 Ga (Sm-Nd whole-rock age of the volcanogenic rocks of the group; Matrenichev and Matrenichev, 2010). However, these publications do not provide the description of the used geochronological techniques and data on measured standard samples, which complicates their correct interpretation.
According to geological data, the sediments of the Sortavala Group (its larger upper part) are ascribed to the Ludicovian Superhorizon of the Fennoscandian Shield, which includes the Middle Paleoproterozoic volcanosedimentary rocks enriched in disseminated Corg and sulfides (Sokolov and Galdobina, 1982; Stratigrafiya …, 1984; Svetov and Sviridenko, 1992). At the Karelian Craton, the Ludicovian Corg-rich sediments cover the sediments of the Jatulian Subhorizon made up of red-colored stromatolitic carbonate and hematite-bearing arenite (Kratts, 1963; Stratigrafiya …, 1984). An important characteristics of the Jatulian carbonate rocks is their extremely high enrichment in 13С (Yudovich et al., 1990; Melezhik and Fallick, 1996; Karhu, 1993). This peculiarity is a marker of Jatulian carbonate rocks over the entire Fennoscandian shield, and coincides in time with a global positive carbon isotope excursion in the Paleoproterozoic (Galimov et al., 1975; Schidlowski et al., 1976; Melezhik, Fallick, 1996; Karhu, 1993; Melezhik et al., 2007, 2013a; Maheshwari et al., 2010, and others).
The Jatulian dolomites of the Tulomozero Formation in the Onega region are dated at 2.09 ± 0.07 Ga (Ovchinnikova et al., 2007), while the Ludicovi–Jatuli boundary is constrained at 2.06 Ga by U-Pb data on the Kuetsjarvi volcanics overlying the Jatulian dolomites in the Pechenga trough, the northern Fennoscandian shield (Melezhik et al., 2007, 2013a). The upper age boundary of the Ludicovi and the rocks of the Sortavala Group is constrained by ages of 1.99 ± 0.03 and 1.99 ± 0.06 Ga, which were obtained by Sm-Nd and Pb-Pb isochron methods for a sill comagmatic with Ludicovian lavas in the Onega Basin (Puchtel et al., 1998, 1999).
The rocks of the Sortavala and Ladoga groups were subjected to zonal regional andalusite-sillimanite facies metamorphism at 1.85–1.89 Ga (Gorokhov et al., 1970; Baltybaev et al., 2009). The metamorphic grade increased from the greenschist facies near the Karelian Craton margin to the high-temperature amphibolite facies near the Mejeri thrust bordering the Raahe-Ladoga Suture Zone from the south (Geologiy i petrologiya …, 2000). The outlines of the metamorphic zones are conformable to the craton margin, although have a more intricate configuration. Under the epidote-amphibolite and low to medium-temperature amphibolite facies, the mafic and intermediate volcanic rocks were transformed into amphibolites and amphibole schists; sandstones and graywackes, into biotite and garnet-biotite schists; mudstones, into garnet-biotite schists with aluminous minerals (staurolite, andalusite, cordierite, sillimanite, and muscovite), while limestones and dolostones were converted into calcite and dolomite marbles. In the southern part of the zonal complex, the schists grade into migmatized gneisses. The amphibolite-facies metamorphism and linked shear deformations led to the formation of granite gneiss domes surrounded by volcanosedimentary rocks of the Sortavala and Ladoga groups (Geologiya i petrologiya …, 2000).
The carbonate rocks of the Sortavala Group in the Northern Ladoga region are mainly represented by dolomite and calcite–dolomite, more rarely calcite marbles (Kitsul, 1963). They also could contain Ca-Mg silicates, mainly tremolite, diopside, or forsterite depending on the metamorphic grade. The Ruskeala marble deposit is located in the periclinal closure of the Ruskeala uplift, where the rocks of the Sortavala Group are extended as a wide band for approximately 13 km to the northwest from the Kirjavalahti dome. Ruskeala marbles are ascribed to the upper carbonate horizon (Kitsul, 1963; Karhu, 1993), which forms two lenses up to 600 m thick in the Ruskeala area (Metzger, 1925). The rocks of the Sortavala Group are boudined and folded, including isoclinal and sheath-like ones. Banding of the marbles is caused by the alternation of fine-grained gray (from light gray to almost black) and coarse-grained white bands. The former consists mainly of dolomite, and the latter are assentially calcitic. Their gray color is related to the admixture of disseminated carbonaceous matter. Some marbles contain small pale yellow or greenish lenses and veinlets consisting of tremolite, more rarely, diopside. The central parts of these lenses occasionally contain quartz, which has no contact with carbonate minerals.
ANALYTICAL TECHNIQUE
Marbles for isotope-geochemical and petrographic study were collected in the northeastern quarry of the Ruskeala deposit and include six samples of calcite marbles and six samples of dolomite marbles. The Ca and Mg contents in the carbonate component of the marbles were analyzed by wet chemical method, while Mn and Fe were determined by atomic absorption after dissolution of powdered samples in 1 N HCl (Table 1). The concentrations of chemical elements are given in wt % or ppm.
Samples for Rb-Sr isotope study were preliminarily treated with 0.1 N HCl and dissolved in 1 N HCl. Rb and Sr were extracted by ion-exchange method on a Dowex AG50Wx8 cationite (Gorokhov et al., 1998, 2016). The concentrations of these elements were determined by mass spectrometric isotope dilution technique using a mixed 87Rb-84Sr spike. The Rb and Sr isotope compositions were determined in static mode on the multi-collector Triton TI mass spectrometer. The average 87Sr/86Sr ratios in the NIST SRM-987 and USGS EN-1 standards normalized to 86Sr/88Sr = 0.1194 during measurements were, respectively, 0.710281 ± 0.000004 (2σav, n = 26) and 0.709211 ± 0.000005 (2σav, n = 20).
Carbonate rocks for С and О isotope analysis were digested in the orthophosphoric acid at 95°С and then analyzed using He-carrier gas on a Delta V+ (Thermo, Germany) mass spectrometer coupled with a GasBench II peripheral unit equipped with a PAL auto sampler (Dubinina et al., 2014). The accuracy of isotope analysis was ±0.1 and ±0.2‰ (2σ) for δ13С and δ18О values, respectively. The carbon and oxygen isotope compositions are given relative to the international V-PDB standard.
RESULTS
Chemical and Isotope Composition of Ruskeala Marbles
Various tints of Ruskeala marbles (white, light gray, dark gray, and greenish) are caused by the admixture of sedimentary carbonaceous matter and diverse metamorphic minerals. Structurally, the marbles are subdivided into banded and ornamented varieties. However, the major rock types are white calcite and dark gray dolomite marbles.
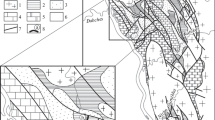
The calcite marble is devoid of carbonaceous admixture and consists mainly of coarse-grained calcite with crystal size up to 3 mm (Fig. 2a). Most of the studied samples have very low contents of Mg (0.2–0.8%), Mn (10–90 ppm), and Fe (160–640 ppm) at high Sr (850–2750 ppm). Only one sample containing small (up to 9%) amount of quartz and tremolite (Fig. 2b) has the elevated contents of Mg (up to 2.8%), Mn (up to 150 ppm), Fe (up to 1900 ppm), while the Sr concentration decreases to 405 ppm (Table 1) Measured 87Sr/86Sr isotope ratios are bracketed within 0.70483–0.70490, and initial 87Sr/86Sr (calculated assuming the marble age of 2.0 Ga) is 0.70482–0.70489. The δ13С values in most calcite marbles vary from 1.5 to 2.1‰, while δ18О from –10.9 to –8.1‰ (Table 1). The δ13С and δ18О values in the tremolite-bearing sample decrease to 0.1‰ and –12.2‰, respectively.
Microphotos of marble thin sections (crossed nicols): (a) coarse-grained calcite marble; (b) tremolite veinlet in fine-grained dolomite marble; (c) fine-grained dolomite marble with admixture of carbonaceous matter and silicate grains; (d) calcite–dolomite marble with small lenses and veinlets of calcite and silicate mineral (tremolite) in fine-grained dolomite mass.
The dolomite marble is mainly finely crystalline (Figs. 2c, 2d), more rarely medium-crystalline rock (Fugs. 2c, 2d). It contains an admixture of finely disseminated carbonaceous matter and scarce inclusions of silicate minerals. The latter, usually tremolite, could be disseminated in rock or restricted to thin veinlets (Fig. 2d). The content of non-carbonate admixture in the studied samples varies from 1.6 to 6.8%. The Mg/Ca ratio (0.45–0.52) in the Ruskeala dolomite marble is much lower than in stoichiometric dolomite (0.61), which suggests the presence of ca. 8–15% calcite. The dolomite marble has a relatively low content of Mn (70–110 ppm) and Fe (1600–3600 ppm) and high Sr (122–256 ppm). The high Sr content in these rocks is not typical of dolomites and explained by the presence of calcite. The measured and initial 87Sr/86Sr ratios are within 0.70465–0.70529 and 0.70463–0.70522, respectively. The δ13С values vary from 0.6 to 1.9‰, while δ18О from –13.2 to –10.2‰ (Table 1).
Estimation of the Metamorphic Conditions for Carbonate Rocks
Direct determination of P-T metamorphic conditions of the studied Ruskeala marble samples is impossible due to the absence of suitable mineral assemblages. However, the metamorphic conditions can be estimated from petrogenetic grids for marbles. Kitsul (1963) showed a change of mineral assemblages in marbles of the Northern Ladoga area with increasing metamorphic grade in zoned complex. The composition of most silicate-bearing marbles could be described in the context of the CaO–MgO–FeO–SiO2–H2O–CO2 (CFMASH–CO2) system. The low FeO content in Ruskeala marbles (<0.36% Fe) allows a simplified calculation in the context of the CaO–MgO–SiO2–H2O–CO2 (CMASH–CO2, Table 1) system, omitting Fe–Mg solid solutions. Marbles devoid of SiO2 or MgO are excluded from the consideration, since they are not suitable for metamorphic estimates. Marbles with high Al2O3 are not discussed, since they are rare in the Northern Ladoga metamorphic complex.
Published petrogenetic grids for silicate–carbonate rocks were calculated or obtained experimentally for contact metamorphic conditions at 0.5–2 kbar (Metz and Trommsdorff, 1968; Trommsdorff and Evans, 1977; Winkler, 1979; Eggert and Kerrick, 1981; Flowers and Helgeson, 1983; Masch and Heuss-Assbichler, 1991; Bucher and Frey, 1994; Luttge et al., 2004, and others). Since the pressure range does not correspond to the metamorphic conditions in the Northern Ladoga area, the published petrogenetic grids for our case seemed to be noninformative.
In this work, petrogenetic grids were calculated for 0.5–10 kbar and 400–800°С in the CMASH–CO2 system. To simplify calculations, solid solutions in the calcite–dolomite solvus system were excluded, because their mutual solubility under low and medium-temperature metamorphic conditions is insignificant. Petrogenetic grids were calculated using a winTWQ 2.34 software (Berman, 2007) and the JUN92 thermodynamic dataset (Berman, 1988). Calculation technique of these grids is considered in detail in (Kerrick, 1974; Trommsdorff and Connolly, 1990; Baker et al., 1991; Carmichael, 1991; Connolly and Trommsdorff, 1991). A choice of the dataset was caused by the presence of hydrous minerals, in particular, amphibole, in the marbles. Since the key variables during metamorphism of carbonate rocks are not only temperature and pressure, but also fluid composition, the petrogenetic grids were constructed with P versus Т for various \({{X}_{{{\text{C}}{{{\text{O}}}_{{\text{2}}}}}}}\) values (CO2 mole fraction) in a fluid (polybaric grids) and with T versus \({{X}_{{{\text{C}}{{{\text{O}}}_{{\text{2}}}}}}}\) for various total pressure (isobaric grids). The thermodynamic diagrams displayed results of calculation for mineral equilibria in the silicate–carbonate system CaO–MgO–SiO2–H2O–CO2 (CMASH–CO2) are shown in Figs. 3–6. Reactions between Ca–Mg silicate minerals (excluding solid solutions but with stoichiometric dolomite) in the CMASH–CO2 system at two independent variables are monovariant.
Polybaric P–T diagrams for silicate-carbonate rocks in the CMASH–CO2 system with excess of calcite and dolomite over silica and variable proportions of H2O and CO2 in a fluid. Numbers of mineral reactions in the diagrams: (1) 5Dol + 8Qtz + H2O = 3Cal + Tr + 7CO2 (2) 3Dol + 4Qtz + H2O = 3Cal + Tlc + 3CO2 (3) Dol + 2Qtz = Di + 2CO2 (4) 3Cal + 2Tlc = Dol + Tr + H2O + CO2 (5) 3Cal + Tr = Dol + 4Di + H2O + CO2 (6) 11Dol + Tr = 13Cal + 8Fo + H2O + 9CO2 (7) 3Dol + Di = 4Cal + 2Fo + 2CO2 (8) Dol = Cal + Per + CO2. Details of diagram calculations in this and next figures (Figs. 4–6) are described in text. (Dol) dolomite, (Cal) calcite, (Qtz) quartz, (Tr) tremolite, (Tlc) talc, (Di) diopside, (Fo) forsterite, (Per) periclase.
Isobaric T–\({{X}_{{{\text{C}}{{{\text{O}}}_{{\text{2}}}}}}}\) diagrams for silicate–carbonate rocks in the CMASH–CO2 system with excess of calcite and dolomite over silica. Numbers of mineral reactions in the diagrams are the same as in Fig. 3.
Polybaric P–T diagrams for silicate–carbonate rocks in the CMASH–CO2 system with excess of calcite and silica over dolomite and variable proportions of H2O and CO2 in a fluid. Numbers of mineral reactions in the diagrams: (1) 3Dol + 4Qtz + H2O = 3Cal + Tlc + 3CO2 (2) 5Dol + 8Qtz + H2O = 3Cal + Tr + 7CO2 (3) Dol + 2Qtz = Di + 2CO2 (4) 6Cal + 4Qtz + 5Tlc = 3Tr + 6CO2 + 2H2O (5) 3Cal + Tr + 2Qtz = 5Di + 3CO2 + H2O (6) Cal + Qtz = Wo + CO2.
Isobaric T–\({{X}_{{{\text{C}}{{{\text{O}}}_{{\text{2}}}}}}}\) diagrams for silicate–carbonate rocks in the CMASH–CO2 system with excess of calcite and silicate over dolomite. Numbers of mineral reactions in the diagrams are the same as in Fig. 5.
According to (Kitsul, 1963), a general sequence of mineral formation in marbles of the Northern Ladoga area with increasing metamorphic degree is as follows (mineral abbreviations after (Kretz, 1983)):
The major Ca–Mg silicate mineral in Ruskeala marbles is tremolite (Tr), while diopside (Di) is less common. These minerals are usually yellowish or yellowish-green, since they contain insignificant amount of iron. Diopside is wider developed in the southern part of the Ruskeala uplift. Forsterite (Fo) occurs only in the southernmost part of the zonal metamorphic complex. It is significant that the dolomite marbles of the Northern Ladoga area contain no talc, which is usually formed at the early stages of low-pressure contact-metamorphic transformation of silica (Qtz)-bearing calcite–dolomite (Cal–Dol) rocks (Mason, 1990).
In the divariant CMASH–CO2 system, diopside and tremolite could coexist in marbles only with one carbonate: either with dolomite without calcite or with calcite without dolomite on the monovariant equilibrium curves (Fig. 7). The simultaneous occurrence of diopside and tremolite with two carbonates (calcite and dolomite) is possible only at increasing variance of the system in the presence of divalent iron. In our case, due to the low iron content (<0.36%), divariant fields are extremely narrow and displacement of reaction lines could be ignored. Tremolite and diopside could coexist also in local carbonate domains containing only one carbonate. The simultaneous disequilibrium coexistence of several silicate minerals could be caused by incomplete mineral reactions.
Analysis of calculated phase diagrams (Figs. 3–6) shows that the sequence of mineral assemblages changing observed in the zoned Northern Ladoga metamorphic complex is in well consistence with modeling results for pressure over 3 kbar and mixed water–carbon dioxide fluid with \({{X}_{{{\text{C}}{{{\text{O}}}_{2}}}}}\) ~ 0.5–0.8. The water presence in the fluid is imperative to provide the tremolite stability in the carbonate rocks. At 3–5 kbar typical of the Northern Ladoga metamorphic complex, a transition from tremolite-bearing calcite dolomite to the diopside-bearing marbles at given proportions of water and carbon dioxide in the fluid occurs at 550–600°C, which corresponds to the staurolite subfacies of the amphibolite facies. These values are consistent with metapelite assemblages of the Ladoga Group in the Ruskeala area (Geologiya i petrologiya …, 2000).
Thus, carbonate rocks of the Sortavala Group in the Ruskeala area were metamorphosed under the medium-temperature low-pressure amphibolite facies, in the middle part of metamorphic zonality of the Northern Ladoga metamorphic complex.
DISCUSSION
Isotope-Geochemical Indicators of Transformation of Carbonate Sediments and Rocks
After precipitation, carbonate sediment is subjected to some irreversible alterations: early lithification, syngenetic (early) dolomitization, mechanical compaction, mineral stabilization, late diagenetic cementation, and dolomitization in burial environment, as well as the dissolution and cementation during interaction with meteoric waters (Moore, 1989). A set of carbonate transformations from sedimentation to metamorphism is referred to as “diagenesis” meaning “metamorphosis”. During diagenesis, sediment reaches fully equilibrated state, is converted in a rock, the composition of which is determined by initial material and/or potential interaction with external fluids. In these processes, Rb-Sr, C- and О-isotope systems of carbonate rock show different behavior, and only some of the aforementioned transformations in specific setting could lead to the disturbance of Rb-Sr and С-isotope systems of primary carbonate sediments.
During diagenesis, recrystallization is caused by the partial dissolution and redeposition of initial sedimentary carbonate in pore space of sediment. Thereby, small crystals are more rapidly dissolved than large crystals, leading, respectively to the more rapid early diagenetic recrystallization of fine-grained sediments (carbonate ooze). During the early diagenetic recrystallization, metastable modifications of СаСО3 (aragonite and high-Mg calcite) or СаСО3 + MgCO3 (calcic dolomite or protodolomite) are converted into stable modifications: low-Mg calcite and dolomite. The crystal chemical peculiarities of the mentioned modifications result in principal differences of strontium concentrations in primary carbonate sediments. The strontium concentration in the present-day aragonite marine sediments is an order of magnitude higher than in magnesian calcites, while that in calcium dolomites is an order of magnitude lower. During the early diagenetic recrystallization, aragonite partially losses strontium, which saturates a pore space and is incorporated into newly formed diagenetic calcite. Similar loss occurs during transformation of high-Mg calcite into diagenetic calcite and dolomite. Thereby, the δ18О value of diagenetic dolomite is determined by oxygen isotope composition in dolomitizing fluid. If this fluid differs in the oxygen isotope composition from seawater and dolomitization temperature differs from that of sedimentation conditions, the δ18О value of dolomite will differ from that of primary sedimentary rock. In addition, it is possible that calcite, dolomite, and fluid do not reach isotope equilibrium during dolomitization (Faure, 1986). In spite of the partial strontium loss, strontium in the diagenetic carbonate phases forming in a closed pore space has the same isotope composition as primary phase. This general rule is also true for dolomitization: when the chemical equilibrium is attained between sediment and pore waters, the newly formed carbonate and host carbonate have the same strontium isotope composition. Thus, the diagenetic recrystallization should lead to a shift in oxygen isotope system whereas strontium isotope system of the rock remains unaltered.
The initial isotope-geochemical characteristics of carbonate rocks could subsequently shift during postsedimentary recrystallization depending on the composition of interacting solutions. The difference in chemical composition between potential late diagenetic solutions and seawater leads to the precipitation of secondary carbonate generations, which are usually enriched in Mn, Fe, and depleted in Sr compared to the initial marine sediments (Veizer et al., 1990; Gorokhov et al., 1995, 2016; Kuznetsov et al., 2006). Main suppliers of Mn and Fe and radiogenic 87Sr in late diagenetic fluids are silicate rocks, which are enriched in these elements compared to carbonate rocks. In most cases, diagenetic and metasomatic solutions could penetrate in carbonate reservoirs during burial and active tectonic settings. However, geological setting and large thickness of carbonate body (from a few tens to hundreds meters) at the Ruskeala deposit could restrict an influx of external fluids in the carbonate stratum, which would facilitate the rock isolation at all diagenetic stages.
A review of published trace element data on limestones and dolomites that preserved strontium isotope composition of Archean and Paleoproterozoic seawater shows that many samples of this age are significantly enriched in Mn and Fe compared to their recent and Neoproterozoic analogues (Fig. 8). However, Paleoproterozoic limestones have lower Mn and Fe contents than Archean limestones (Veizer et al., 1990; Farquhar et al., 2011). This difference is explained by the chemical composition of ocean, which in the Archean was notably depleted in free oxygen. For this reason, the polyvalent Mn and Fe were in a semi-reduced state and easily precipitated together with calcite as isomorphic admixtures. Nevertheless, Paleoproterozoic carbonates with the lowest Mn/Sr and Fe/Sr ratios in many instances have lower 87Sr/86Sr ratios (Kuznetsov et al., 2013).
Cross-plots of Mn vs Sr (а) and Fe vs Sr (b) contents in the metacarbonate rocks of the Ruskeala deposit compared to those for the present-day shallow-water marine sediments and Precambrian carbonate rocks and marbles that preserved the 87Sr/86Sr ratio of paleoocean. (А) modern aragonite sediments, (K) modern low-Mg calcites, (LA-AR) Archean limestones and calcites, (LA–PR) Paleoproterozoic limestones, (Dm-AR + PR) Archean and Paleoproterozoic dolomites. Dashed lines in figures (a) and (b) separate the fields of the least altered calcites for the Neoproterozoic carbonate rocks (Kuznetsov et al., 2003, 2014, 2018; Semikhatov et al., 2004, 2009). Blue circles are calcite marbles of Ruskeala, red boxes are dolomite marbles.
One of the geochemical indicators of diagenetic and metamorphic transformations of carbonate rocks is oxygen isotope composition, which depends on the recrystallization temperature of carbonate sediment, oxygen isotope composition of fluid, and water/mineral ratio. Since external fluids are usually depleted in 18О compared to seawater, the high temperatures of metamorphic processes provide enrichment of diagenetic and metamorphic carbonate generations in light 16О (Faure, 1986; Moore, 1989; Banner and Hanson, 1990; Boulvais et al., 1998; Dubinina et al., 2020, and others). According to numerous works, the δ18О value in the least altered Late Proterozoic marine carbonates is usually –6.5 ± 2.5‰ (Veizer and Hoefs, 1976; Pokrovsky and Vinogradov, 1991; Podkovyrov et al., 1998; Walter et al., 2000; Ray et al., 2003; Semikhatov et al., 2004, 2009; Pokrovsky et al., 2006, 2012; Kuznetsov et al., 2006; Melezhik et al., 2006, 2009, 2015; Frimmel, 2010; Khabarov and Varaksina, 2011; Sial et al., 2019, and others). However, the average δ18О values in the older Paleoproterozoic carbonates applied for the reconstruction of Sr- and C-isotope signatures of seawater are –8.1 ± 2.5‰ in dolomites and –10.2 ± 4‰ in limestones (Veizer and Hoefs, 1976; Veizer et al., 1990; Bekker et al., 2003a, 2003b; Melezhik et al., 2004; Frauenstein et al., 2009). This difference could be caused by the higher temperature of Paleoproterozoic seawater (Veizer et al., 1990), which led to the precipitation of carbonate sediments with δ18О values 2–3‰ lower than those of Neoproterozoic and Phanerozoic oceans (Fig. 9).
Isotope 87Sr/86Sr–δ18О (a) and δ13С–δ18О (b) diagrams for the metacarbonate rocks of the Ruskeala deposit. Fields of Precambrian shallow-marine carbonates and marbles that preserved 87Sr/86Sr paleoocean ratio are shown in the diagram for comparison. Symbols are the same as in Fig. 8.
Initial δ13С value in the carbonate sediments and rocks in the diagenesis zone remains practically unaltered due to the high carbon content in the mineral compared to the carbon concentration in diagenetic solutions (Moore, 1989; Banner and Hanson, 1990). Critical disturbances of initial C-isotope systems are usually assisted by external heterogeneous aqueous-salt and СО2-rich fluids. These fluids are supplied along regional permeable zones at metamorphic peak (Bushmin et al., 2020). The external fluid is either percolated in a dispersed state in a large rock volume, or is supplied as a focused flux in a narrow shear zone. The influx of external fluid leads to the disturbance of the geochemical system of the rocks, in which new silicate minerals are crystallized in thermodynamic equilibrium with environment (Ivanov and Bushmin, 2021). In closed system, carbonate rocks metamorphosed under the amphibolite and even granulite facies frequently well preserve the initial carbon isotope composition (Baker and Fallick, 1989; Melezhik et al., 2002, 2005, 2006). Nevertheless, a decrease of δ13С value in marbles could be caused by isotope exchange between carbonate minerals and light (δ13С –28 ± 3‰) organic carbon, which is formed after decomposition of organic matter at temperatures higher than 400°C, being converted in graphite. A shift of δ13С in the Proterozoic graphite–calcite marbles metamorphosed at 650–780°C during Grenville orogeny at the Canadian Shield reached 3–4‰ (Kitchen and Valley, 1995). Such scenario is possible in rocks with extremely high carbon content (10–25 mol %) in the calcite–graphite system. Therefore, the use of pure carbonate material devoid of organic matter (graphite) minimizes the metamorphic disturbance of the isotope-carbon system. The disturbance of the initial carbon isotope system is also possible during the partial decomposition of carbonates with equilibrium release of gaseous CO2 (so called decarbonization) and, respectively, fluid enrichment in 13C and 18O (Valley, 1986). However, this process leads not only to the formation of specific mineral assemblages, but also causes a consistent decrease of δ13C and δ18O values in a residual carbonate material, which allows the reliable recognition of decarbonization in altered rocks in the δ18О–δ13С coordinates.
Estimation of the Degree of the Isotope-Geochemical Preservation of Ruskeala Marbles
Ruskeala marbles contain admixture (up to 2%) of “dust-like graphite” equally distributed between carbonate crystals in fine-grained varieties (Kitsul, 1963). Disseminated carbonaceous matter is buried residual organic matter that avoided oxidation during diagenesis. Disseminated carbonaceous matter is widespread component in the Precambrian metacarbonate and metapelitic sedimentary rocks beginning from the Archean (Schidlowski et al., 1976; Veizer et al., 1990). The presence of biogenic decomposition products in Ruskeala marbles clearly points to a primary sedimentary origin of the metacarbonate rocks. Horizontal interbedding of calcite and carboniferous dolomite marbles suggests the preservation of relict primary layering in spite of strong deformations.
One more important distinctive feature of Ruskeala metacarbonates is an extremely low content of silicate admixture. The absence of terrigenous admixture suggests that the carbonate sediments were accumulated at sizable distance from continental areas, large-scale uplifts, and zones of abundant flux of detrital material. In modern environment, such settings correspond to shelf seas at the transgressive stage and the high rate of carbonate accumulation.
The petrological studies of Ruskeala sedimentary carbonates showed that they underwent amphibolite-facies metamorphism under low pressures and medium temperatures (550–600°C). Recrystallization resulted in the formation of minor amount of typical metamorphic minerals, mainly tremolites formed by the interaction of calcium-magnesian carbonates with quartz likely of detrital origin. The absence of other mineral assemblages excludes an external input in the carbonate rock system. Moreover, high thickness of the Ruskeala marble sequence (up to 600 m) could prevent large-scale infiltration of external fluids within the studied part of the deposit. Thus, there are grounds to suggest that the recrystallization of sedimentary carbonates was not assisted by external fluids, but was mainly driven by elevated temperature and pressure.
The high Sr concentration in calcite marbles (up to 2790 ppm) in combination with low Mg content (<1%) testifies that an initial carbonate sediment consisted of aragonite. Dolomite marbles of the Ruskeala deposit comprise calcite admixture, which represents either a primary relict or a secondary diagenetic carbonate generation. The Sr concentration in dolomite marbles is much lower than in pure calcite marbles (122–256 ppm, respectively), but significantly exceeds that of Riphean dolomites (Fig. 8). The Mn (10–90 ppm) and Fe (160–640 ppm) contents in most samples of calcite marbles are comparable with those of the least altered Late Proterozoic limestones and even modern carbonate sediments (Fig. 10). Dolomite marbles have extremely low Mn content (70–110 ppm), whereas Fe content increases up to 1600–3600 ppm. The δ18О values in the most of calcite marbles vary within –10.9…–8.1‰, but decrease to ‒13.2…–10.2‰ in dolomite marbles and in a tremolite-bearing calcite marble (Table 1). The observed difference in δ18О value between calcites and dolomites contradicts the cogenetic precipitation of calcite and dolomite phases, since marine dolomites in equilibrium with limestones have higher δ18О (by 1–2‰) (Veizer and Hoefs, 1976). The elevated Fe content and lowered δ18О values in the dolomites and tremolite-bearing calcite sample indicate their more pervasive late diagenetic (metamorphic) recrystallization.
Variations of Sr vs. Mn (a) and Sr vs. Mg/Ca ratio (b) in the metacarbonate rocks of the Ruskeala deposit compared to the modern aragonite (A) and low-Mg calcite (K) sediments. Dashed lines in the figures outline the field of the least altered limestones (Kuznetsov et al., 2003, 2014, 2018; Semikhatov et al., 2004, 2009) and the “best” marbles (Melezhik et al., 2013b; Gorokhov et al., 2016), that preserved 87Sr/86Sr ratio of paleoocean. Arrows show a trend of late diagenetic recrystallization of carbonate rocks.
The study of calcite marbles in the Norwegian Caledonides showed that the rocks are able to preserve the Sr isotope composition of protosediment even under amphibolite-facies conditions (Melezhik et al., 2002, 2003, 2013b). It is recommended to use rocks with SiO2 and Al2O3 contents no more than 5 and 1%, respectively, for the reconstruction of the Sr isotope composition of sediment. Thereby, carbonate constituent of sample should have >1000 ppm Sr, <50 ppm Mn, >–8.6‰ δ18О, and Mg/Ca, Mn/Sr, and Rb/Sr ratios are ≤0.02, ≤0.02, and ≤0.0001, respectively (Melezhik et al., 2013b). These criteria are more strict than those (Mg/Ca < 0.024, Mn/Sr < 0.2, and Fe/Sr < 5) used for the Rb-Sr systems of the least altered non-metamorphosed limestones (Kuznetsov et al., 2014). Proposed critical δ18О = –8.6‰ for the Neoproterozoic “best” marbles seems to be slightly overestimated for Paleoproterozoic metasedimentary carbonate rocks. As mentioned above, δ18О values in Paleoproterozoic carbonate sediments could be 2–3‰ lower than in Neoproterozoic carbonates (Veizer et al., 1990). This is also supported by δ18О values in Ruskeala calcites with Mn/Sr < 0.1, which meet the criteria of the least altered limestones (Fig. 11). The δ18О values in such marbles vary within –8.1…–10.9‰.
Variations of Mn/Sr vs δ18О (a) and Mn/Sr vs 87Sr/86Sr (b) in the Ruskeala marbles compared to the field of the least altered limestones and “best” marbles that retained the 87Sr/86Sr ratio of paleoocean. For symbols, see Fig. 10.
The most of calcite marble samples of the Ruskeala deposit meet the isotope-geochemical criteria for the preservation of the Rb-Sr systems in the non-metamorphosed limestones and only three samples (K14-15, K14-18, and K14-20) correspond to the preservation criteria for the “best” marbles (Fig. 10). Unfortunately, some data required to estimate the preservation of the Rb-Sr system, in particular, SiO2 and Al2O3 contents in non-carbonate component of the marbles, are absent. However, in case of small amount of non-carbonate admixture in the mentioned samples (Table 1), the role of these data is insignificant. It is pertinent to mention that the initial 87Sr/86Sr ratios in all calcite marbles are well consistent with each other and fall within 0.70482–0.70489. Hence, the calcite marbles of the Ruskeala deposit can be used for determining the Sr isotope composition of sedimentary environment of the Ludicovian carbonate sediments.
The range of initial 87Sr/86Sr ratios (0.70463–0.70492) in the studied dolomite marbles is only slightly wider than that in calcite marbles (Table 1, Fig. 11) and practically coincides with it. Only one dolomite sample with relatively high fraction of silicate admixture (up to 7%) differs in the elevated 87Sr/86Sr (0.70529). These data are of interest due to the absence of geochemical criteria for the preservation of the Rb-Sr systems in dolomites. However, it should be noted that the values of such important geochemical parameter of the Ruskeala dolomites as the Mn/Sr ratio (0.28–0.75) are well consistent with those in the least altered Jatulian dolomites of the Tulomozero Formation of the Karelian Craton (0.3–1.5, Kuznetsov et al., 2010) and the Kuetsjarvi Formation of the Kola Craton (0.3–0.5, Kuznetsov et al., 2011). Similarly low Mn/Sr ratios (0.26–1.1) were noted in the least altered dolomites of the Min’yar Formation, which were used for the reconstruction of Sr isotope composition of the Neoproterozoic ocean (Kuznetsov et al., 2006). The Mn/Sr ratios in Ruskeala dolomites are also much lower than those of the Paleoproterozoic least altered dolomites of the Canadian Shield: 2.5 in the Nash Fork Formation (Bekker et al., 2003a) and 2.7 in the Alder Formation (Kuznetsov et al., 2003). The higher Mn/Sr ratios were observed in the Early Riphean dolomites of the Anabar shield, the δ13С and 87Sr/86Sr values in which were suitable for the chemostratigraphic considerations (Pokrovsky and Vinogradov, 1991; Gorokhov et al., 2018, 2019). Thus, the 87Sr/86Sr ratios in Ruskeala dolomite marbles with minor silicate admixture and low Mn/Sr ratio fit the general Sr-isotope chemostratigraphic database on Proterozoic oceans.
Lower 87Sr/86Sr ratios in some dolomite samples (0.70463–0.70492) compared to calcites (0.70482–0.70489) are explained by either sedimentological or diagenetic factors. In the former case, the calcareous and dolomitic sediments could represent different lithological units and, respectively, their 87Sr/86Sr ratios reflect small-size short-term variations in the paleobasin. The diagenetic (metamorphic) recrystallization follows from the absence of the oxygen isotope equilibrium expected during cogenetic calcite–dolomite precipitation. The recrystallization of dolomites could occur in the presence of fluid, which represented seawater that interacted with mafic volcanogenic matter of the Sortavala Group with low 87Sr/86Sr ratio of 0.703–0.704. The existence of such formation waters was described in the Mesozoic Alberta volcanosedimentary basin, Western Canada (Connolly et al., 1990).
Alteration of initial δ13С in Ruskeala marbles was likely controlled by metamorphic conditions and content of carbonaceous organic matter. Comparatively low temperatures (550–600°C) and low pressures at metamorphic peak did not lead to the formation of crystalline graphite in the Ruskeala marbles, how it happened in Grenville marbles of the Adirondack Central. Carbonaceous matter in fine-grained dolomite marbles preserved finely disseminated texture close to the primary sedimentary state. Moreover, the average content of organic carbon in the calcite and dolomite marbles of the Ruskeala deposit was no more than 1 mol %. Only several small units demonstrate an increase of carbon content in the dolomite–graphite system up to 8 mol % (Kitsul, 1963), which is much lower than that of the Grenville marbles of Adirondack (10–25 mol %, Kitchen and Valley, 1995). Thus, the initial δ13С values in pure (silicate-free) carbonate material with minor graphite admixture experienced only minimum disturbance.
A review of δ13С variations in the least altered Archean and Paleoproterozoic marine carbonate rocks shows that most of them fall in rather narrow range of 0 ± 2‰ (Fig. 9), except for the Paleoproterozoic “Lomagundi–Jatulian” stage (Veizer al., 1990; Yudovich et al., 1990; Karhu, 1993; Bekker et al., 2003a, 2003b; Melezhik et al., 2004, 2007, 2013a; Kuznetsov et al., 2019). This suggests that the δ13С values in the Ruskeala unaltered carbonate rocks of the Sortavala Group could be truly within +0.6…+2.1‰.
Geochemical and Chemostratigraphic Significance of Obtained Isotope Data
Ruskeala marbles are metamorphosed carbonate sediments, which compose the upper part of the Ludicovian Sortavala Group in the Northern Ladoga area (Kratts, 1963; Kitsul, 1963).
The Sr content in calcite marble sample from the Ruskeala quarry was first determined by isotope dilution method (see Gerling and Shukolyukov, 1957, sample 2). The Sr content in this sample was 0.17%, which is consistent with the average Sr concentration of 1530 ppm in calcite marbles obtained in this work (Table 1) and indicates a high quality of analytical investigation in the mid-20th century. The 87Sr/86Sr in sample 2 from (Gerling and Shukolyukov, 1957) recalculated with regard for the fractionation effect on mass spectrometer MC-2M and measured errors are 0.7034–0.7063 (Fig. 12a). These data were used for the first Russian attempt to reconstruct the Sr isotope composition in the Early Precambrian ocean while studying the Archean–Proterozoic carbonate rocks. Unfortunately, the precision of mass spectrometers of that time was poor to determine the Sr isotope composition in Ruskeala marble with lower analytical error. In opinion of the authors of this pioneering work, a quite high 87Sr/86Sr ratio in marble suggests that the rocks were “contaminated” by extraneous strontium. This conclusion gave rise to temporal disappointment in capability of marble for isotope chemostratigraphy. However, it was established few decades later that the obtained value is consistent with 87Sr/86Sr range in the Paleoproterozoic carbonate rocks (Veizer and Compston, 1976).
Variations of 87Sr/86Sr and δ13C in the least altered metasedimentary carbonate rocks of the Sortavala Group against the secular trends of 87Sr/86Sr and δ13C in the Paleoproterozoic ocean (Veizer al., 1990; Karhu, 1993; Gorokhov et al., 1998; Bekker et al., 2003a, 2003b; Kuznetsov et al., 2003, 2010, 2011, 2018, 2019; Melezhik et al., 2004, 2007, 2013a), formations: (1) Gamohaan, Transvaal Supergroup; (2) Gandarella, Minas Group; (3) Temryuk, Central Azov Group, (4) Duitschland, Pretoria Group; (5) Kona, Chocolay Group; (6) Alder, Kaniapiskau Supergroup; (7) Nash Fork, Snow Pass Supergroup; (8) Tulomozero, Jatuli; (9) Fecho do Funil, Minas Group; (10) Kuestjarvi, Pechenga Group; (11) Zaonega, Ludikovi; (12) Cowles Lake Coronation Supergroup; (13) Utsingi, Pethei Group; (14) Duck Creek, Wyloo Group; (15) Albanel, Mistassini Group; (16) McArthur Group. Calcite marbles of Ruskeala (Sortavala Group) are shown by blue circles, dolomite marbles, by red boxes; calcites, by gray circles, and dolomites, by gray boxes.
Comprehensive high-precision isotope-geochemical and petrological study of Ruskeala marbles performed in this work made it possible to reveal few samples suitable for the reconstruction of Sr and C-isotope signatures of the Paleoproterozoic ocean. The initial 87Sr/86Sr ratios in marine carbonate sediments of the Sortavala Group are constrained within 0.70482–0.70489 and did not fall beyond a narrow range of 0.70463–0.70492 (Fig. 12a). It should be noted that the Ruskeala marble is the third geological object within the Fennoscandian Shield, which included unaltered carbonate rocks suitable for reconstructing the strontium isotope composition in the Paleoproterozoic ocean. Two other points characterized the Juatuli Subhorizon in the Northern Ladoga area (0.70343–0.70442, Tulomozero Formation, Gorokhov et al., 1998; Kuznetsov et al., 2010) and Pechenga Trough (0.70407–0.70431, Kuetsjarvi Formation, Melezhik et al., 2004; Kuznetsov et al., 2011). Only one value of 87Sr/86Sr ratio available for calcite nodule from the Zaonega volcanogenic-terrigenous formation of the Karelian Craton allowed us to constrain the upper limit of this ratio (0.70534) in the Ludicovian paleobasin (Kuznetsov et al., 2012).
The 87Sr/86Sr values obtained for the Ludicovi Subhorizon in the Northern Ladoga area continue the curve of 87Sr/86Sr variations in Paleoproterozoic ocean, which varied from 0.70302 to 0.70495 during the 2.20–2.06 Ga Lomagundi-Jatulian stage (Gorokhov et al., 1998; Bekker et al., 2003a, 2003b; Kuznetsov et al., 2010, 2018). In the terminal Jatulian (2.06–2.09 Ga), the 87Sr/86Sr ratio in the ocean reached minimum of 0.70343 and then rapidly increased up to 0.70431 (Fig. 12a). With allowance for our new data on the Sortavala carbonates, this ratio increased in the Ludicovi (1.99–2.06 Ga) up to 0.70463–0.70492. A drastic growth of the 87Sr/86Sr ratio in the ocean was caused by the attenuation of rifting after the Kenorland supercontinent breakup at the end of the Early Paleoproterozoic and likely an increase of volume (“maturity”) of continental crust, as well as by the intensification of continental weathering at ca. 2 Ga due to the growth of free oxygen proportion in Earth’s atmosphere at 2.32 Ga (Bekker et al., 2004; Melezhik et al., 2013a). It is pertinent to mention that the Ludicovi became a crucial boundary, after which 87Sr/86Sr value did not decrease below 0.70460. Thus, this boundary marks a new episode of the crustal growth, which produced crust that differed in composition from the Late Archean and Early Paleoproterozoic crust.
The δ13С values in the most of Ruskeala marbles vary from +0.6 to +2.1‰ (Fig. 12b), thus falling within the range published previously for metacarbonate rocks of this horizon (from +1.0 to +2.3‰, Karhu, 1993). These data confirm a significant decrease of δ13Сcarb in the Ludicovian marine sediments at 2.06–1.88 Ga stage, which followed the epoch of a global Lomagundi-Jatulian positive δ13Сcarb excursion (Schidlowski et al., 1976; Melezhik, Fallick, 1996; Melezhik et al., 2007, 2013a).
CONCLUSIONS
The marbles of the Ruskeala deposit are the unique geological object, which can be used for studying the metamorphism of sedimentary carbonate rocks and reconstructing the initial isotope-geochemical signatures of the Paleoproterozoic ocean. The transformation of the Ruskeala sedimentary carbonate rocks into marbles depended on the initial mineral composition of carbonate sediment, sedimentary setting and burial environment, presence of silicate admixture, and rock permeability for external fluid.
Carbonate rocks of the Sortavala Group were metamorphosed under staurolite (medium-temperature) subfacies of amphibolite facies. The calculated pressure of 3–5 kbar and temperature of 550–600°C for tremolite–diopside-bearing calcite–dolomite marbles are consistent with metamorphic conditions of metapelites of the Ladoga Group in the Ruskeala area.
The isotope-geochemical characteristics of calcite and some dolomite marbles correspond to the geochemical criteria for the preservation of Rb-Sr and С‑isotope systems of non-metamorphosed sedimentary carbonate rocks. This fact and mainly isochemical metamorphism reveal that Ruskeala marbles are suitable proxy to obtain the chemostratigraphic signatures for carbonate sediments in ancient ocean.
Geochemical features of metacarbonate rocks of the Sortavala Group (Ruskeala deposit) suggest that the initial carbonate sediment is made up of aragonite (protolith of calcite marbles) and high-Mg calcite (protolith of dolomite marbles). Carbonate was accumulated in wide paleobasin, far away from continental area, at comparatively high sedimentation and burial rate.
The isotope-geochemical study of Ruskeala marbles (upper horizon of the Sortavala Group) allowed us to estimate the δ13С values and to obtain the first Sr isotope data on the Svecofennian ocean, which surrounded the Karelian Craton from the southwest (in the present-day coordinates). The δ13С value in 1.9–2.0 Ga ocean was +1.5 ± 1‰, at 87Sr/86Sr of 0.70463–0.70492. On a global scale, the obtained Sr-isotope data demonstrate an increase of radiogenic 87Sr proportion in ocean at ca. 2 Ga after the Kenorland Supercontinent breakup and intensification of weathering. Based on δ13С data, the Ludicovi defines the beginning of C-isotope stasis in ocean that continued for about one billion years up to the end of the Mesoproterozoic.
REFERENCES
Baker, A.J. and Fallick, A.E., Evidence for CO2 infiltration in granulite-facies marbles from Lofoten–Vesteralen, Norway, Earth Planet. Sci. Lett., 1988, vol. 91, nos. 1–2, pp. 132–140.
Baker, J., Holland, T., and Powell, R., Isograds in internally buffered systems without solid solutions: principles and examples, Contrib. Mineral. Petrol., 1991, vol. 106, no. 2, pp. 170–182.
Baltybaev, Sh.K., Levchenkov, O.A., and Levsk, L.K., Svekofennskii poyas Fennoskandii: prostranstvenno-vremennaya korrelyatsiya ranneproterozoiskikh endogennykh protsessov (Svecofennian Belt of Fennoscandin: Spatiotemporal Correlation of Paleoproterozoic Endogenous Processes), St. Petersburg: Nauka, 2009.
Banner, J.L. and Hanson, G.N., Calculation of simultaneous isotopic and trace element variations during water–rock interaction with applications to carbonate diagenesis, Geochim. Cosmochim. Acta, 1990, vol. 54, no. 11, pp. 3123–3137.
Bekker, A., Karhu, J.A., Eriksson, K.A., and Kaufman, A.J., Chemostratigraphy of the Paleoproterozoic carbonate successions of the Wyoming Craton: tectonic forcing of biogeochemical change?, Precambrian Res., 2003a, vol. 120, nos. 3–4, pp. 279–325.
Bekker, A., Sial, A.N., Karhu, J.A., et al., Chemostratigraphy of carbonates from Minas Supergroup, Quadrilatero Ferifero (iron quadrangle), Brazil: a stratigraphic record of Early Proterozoic atmospheric, biogeochemical and climatic change, Am. J. Sci., 2003b, vol. 330, no. 10, pp. 865–904.
Bekker, A., Holland, H.D., Wang, P.-L., et al., Dating the rise of atmospheric oxygen, Nature, 2004, vol. 427, pp. 117–120.
Berman, R.G., Internally–consistent thermodynamic data for minerals in the system Na2O–K2O–CaO–MgO–FeO–Fe2O3–Al2O3–SiO2–TiO2–H2O–CO2, J. Petrol., 1988, vol. 29, no. 2, pp. 445–522.
Berman, R.G., WinTWQ (version 2.3): a software package for performing internally-consistent thermobarometric calculations, Geol. Surv. Canada, 2007.
Bolhar, R., Hofmann, A., Woodhead, J., et al., Pb- and Nd-isotope systematics of stromatolitic limestones from the 2.7 Ga Ngezi Group of the Belingwe greenstone belt: constraints on timing of deposition and provenance, Precambrian Res., 2002, vol. 114, nos. 3–4, pp. 277–294.
Boulvais, P., Fourcade, S., Gruau, G., et al., Persistence of pre-metamorphic c and o isotopic signatures in marbles subject to Pan-African granulite-facies metamorphism and U-Th mineralization (Tranomaro, southeast Madagascar), Chem. Geol., 1998, vol. 150, pp. 247–262.
Bucher, K. and Frey, M., Petrogenesis of Metamorphic Rocks, Berlin–Heidelberg: Springer–Verlag, 1994.
Bushmin, S.A. and Glebovitsky, V.A., Scheme of mineral facies of metamorphic rocks and its application to Fennoscandian shield with representative sites of orogenic gold mineralization, Trans. Karelian Res. Centre RAS. Precambrian Geol. Ser., 2016, no. 2, pp. 3–27.
Bushmin, S.A., E.A. Vapnik, M.V. Ivanov, Yu.M. Lebedeva, and E.V. Savva, Fluids in high–pressure granulites, Petrology, 2020, vol. 28, no. 1, pp. 1–16.
Carmichael, D.M., Univariant mixed-volatile reactions; pressure–temperature phase diagrams and reaction isograds, Can. Mineral., 1991, vol. 29, no. 4, pp. 741–754.
Connolly, C.A., Walter, L.M., Baadsgaard, H., and Longstaffe, F.J., Origin and evolution of formation waters, Alberta Basin, Western Canada sedimentary basin. II. Isotope systematics and water mixing, Appl. Geochem., 1990, vol. 5, pp. 397–413.
Connolly, J.A.D. and Trommsdorff, V., Petrogenetic grids for metacarbonate rocks: pressure–temperature phase-diagram projection for mixed-volatile systems, Contrib. Mineral. Petrol., 1991, vol. 108, nos. 1–2, pp. 93–105.
Demidov, N.F. and Kratts, K.O., Rhythmic properties of the Ladoga schist sequence in Southwestern Karelia, Izv. Karel’sk. Kol’sk. Fil. AN SSSR, 1958, no. 5, pp. 3–9.
Dubinina, E.O., Chugaev, A.V., Ikonnikova, T.A., et al., Sources and fluid regime of quartz–carbonate veins at the Sukhoi Log gold deposit, Baikal–Patom Highland, Petrology, 2014, vol. 22, no. 4, pp. 329–358.
Dubinina, E.O., Kramchaninov, A.Yu., Silantyev, S.A., and Bortnikov, N.S., Influence of the precipitation rate on the isotope (δ18O, δ13C and δ88Sr) composition of carbonate chimneys of the Lost City hydrothermal field (30° N, Mid–Atlantic Ridge), Petrology, 2020, vol. 28, no. 4, pp. 374–388.
Dufour, M.S., Kol’tsov, A.B., Zolotarev, A.A., and Kuznetsov, A.B., Corundum-bearing metasomatic rocks in the Central Pamirs, Petrology, 2007, vol. 15, no. 2, pp. 151–167.
Eggert, R.G. and Kerrick, D.M., Metamorphic equilibria in the siliceous dolomite system: 6 kbar experimental data and geologic implications, Geochim. Cosmochim. Acta, 1981, vol. 45, no. 7, pp. 1039–1049.
Farquhar, J., Zerkle, A.L., and Bekker, A., Geological constraints on the origin of oxygenic photosynthesis, Photosynth. Res., 2011, vol. 107, no. 1, pp. 11–36.
Faure, G., Principles of Isotope Geology, 2nd ed, New York: Wiley and Sons, 1986.
Flowers, G.C. and Helgeson, H.C., Equilibrium and mass transfer during progressive metamorphism of siliceous dolomites, Am. J. Sci., 1983, vol. 283, no. 3, pp. 230–286.
Frauenstein, F., Veizer, J., Beukes, N., et al., Transvaal Supergroup carbonates: implications for paleoproterozoic δ18O and δ13C records, Precambrian Res., 2009, vol. 175, nos. 1/4, pp. 149–160.
Frimmel, H.E., On the reliability of stable carbon isotopes for Neoproterozoic chemostratigraphic correlation, Precambrian Res., 2010, vol. 182, no. 4, pp. 239–253.
Galimov, E.M., Migdisov, A.A., and Ronov, A.B., Isotope variations of carbonate and organic carbon in sedimentary rocks in the Earth’s evolution, Geokhimiya, 1975, no. 3, pp. 323–342.
Gast, P.W., Abundance of 87Sr during geologic time, Geol. Soc. Am. Bull., 1955, vol. 66, no. 11, pp. 1149–1453.
Geologiya i petrologiya svekofennid Priladozh’ya (Geology and Petrology of Svecofennides of the Ladoga Area), Glebovitskii, V.A., Eds., St. Petersburg: St. Petersburg. Univ., 2000.
Gerling, E.K. and Shukolyukov, Yu.A., Determination of absolute age from 87Sr/86Sr ratio in sedimentary rocks, Geokhimiya, 1957, no. 3, pp. 187–190.
Ghent, E.D. and O’Neil, J.R., Late Precambrian marbles of unusual carbon–isotope composition, southeastern British Columbia, Can. J. Earth Sci., 1985, vol. 22, no. 3, pp. 324–329.
Gorokhov, I.M., Varshavskaya, E.S., Kutyavin, E.P., and Lobach–Zhuchenko, S.B., Preliminary Rb–Dr geochronology of the North Ladoga region, Soviet Karelia, Eclogae Geol. Helv, 1970, vol. 63, no. 1, pp. 95–104.
Gorokhov, I.M., Semikhatov, M.A., Baskakov, A.V., et al., Strontium isotope composition of Riphean, Vendian, and Lower Cambrian carbonate rocks of Siberia, Stratigraphy. Geol. Correlation, 1995, vol. 3, no. 1, 3–33.
Gorokhov, I.M., Kuznetsov, A.B., Melezhik, V.A., et al., Sr isotopic composition in the Upper Jatulian (Early Paleoproterozoic) dolomites of the Tulomozero Formation, Southeastern Karelia, Dokl. Earth Sci., 1998, vol. 360, no. 4, pp. 609–612.
Gorokhov, I.M., Kuznetsov, A.B., Ovchinnikova, G.V., et al., Pb-Sr–O–C isotope compositions of metacarbonate rocks of the Derbina Formation (East Sayan): chemostratigraphic and geochronological significance, Stratigraphy. Geol. Correlation, 2016, vol. 24, no. 1, pp. 1–18.
Gorokhov, I.M., Kuznetsov, A.B., Konstantinova, G.V., et al., Carbonate rocks from the Riphean–Vendian boundary deposits of the Anabar Uplift: isotope (87Sr/86Sr, δ13C, δ18O) systematics and chemostratigraphic consequences, Dokl. Earth Sci., 2018, vol. 482, no. 2, pp. 1306–1310.
Gorokhov, I.M., Kuznetsov, A.B., Semikhatov, M.A., et al., Early Riphean Billyakh Group of the Anabar Uplift, North Siberia: C–O isotopic geochemistry and Pb-Pb age of dolomites, Stratigraphy. Geol. Correlation, 2019, vol. 27, no. 5, pp. 514–528.
Ivanov, M.V. and Bushmin, S.A., Thermodynamic model of the H2O–CO2–NaCl fluid system at middle and lower crustal P–T parameters, Petrology, 2021, vol. 29, no. 1, pp. 90–103.
Karhu, J.A., Paleoproterozoic evolution of the carbon isotope ratios of sedimentary carbonates in the Fennoscandian shield, Geol. Sur. Finland Bull., 1993, no. 371, pp. 1–87.
Kerrick, D.M., Review of metamorphic mixed-volatile (H2O–CO2) equilibria, Am. Mineral., 1974, vol. 59, nos. 7–8, pp. 729–762.
Khabarov, E.M., and Varaksina, I.V., The structure and depositional environments of Mesoproterozoic petroliferous carbonate complexes in the western Siberian craton, Russ. Geol. Geophys., 2001, vol. 52, no. 8, pp. 923–944.
Kitchen, N.E. and Valley, J.W., Carbon isotopic thermometry in marbles of the Adirondack mountains, New York, J. Metamorph. Geol., 1995, vol. 13, no. 5, pp. 577–594.
Kitsul, V.I., Petrologiya karbonatnykh porod ladozhskoi formatsii (Petrology of Carbonate Rocks of the Ladoga Formation), Moscow: AN SSSR, 1963.
Kratts, K.O., Geologiya karelid Karelii (Geology of the Karelian Karelides), Moscow–Leningrad: AN SSSR, 1963.
Kretz, R., Symbols of rock–forming minerals, Am. Mineral., 1983, vol. 68, nos. 1–2, pp. 277–279.
Kuznetsov, A.B., Melezhik, V.A., Gorokhov, I.M., et al., Sr isotope composition in Paleoproterozoic carbonates extremely enriched in 13C: Kaniapiskau Supergroup, the Labrador Trough of the Canadian Shield, Stratigraphy. Geol. Correlation, 2003, vol. 11, no. 3, pp. 209–219.
Kuznetsov, A.B., Semikhatov, M.A., Maslov, A.V., et al., New data on Sr- and C-isotopic chemostratigraphy of the Upper Riphean type section (Southern Urals), Stratigraphy. Geol. Correlation, 2006, vol. 14, no. 6, pp. 602–628.
Kuznetsov, A.B., Melezhik, V.A., Gorokhov, I.M., et al., Sr isotopic composition of Paleoproterozoic 13C–rich carbonate rocks: the Tulomozero Formation, SE Fennoscandian Shield, Precambrian Res., 2010, vol. 182, no. 4, pp. 300–312.
Kuznetsov A.B., Gorokhov I.M., Ovchinnikova G.V., et al., Rb-Sr and U-Pb systematics of metasedimentary carbonate rocks: the Paleoproterozoic Kuetsjarvi Formation of the Pechenga greenstone belt, Kola Peninsula, Lithol. Miner. Resour., 2011, vol. 46, no. 2, pp. 151–164.
Kuznetsov, A.B., Gorokhov, I.M., Melezhik, V.A., et al. Strontium isotope composition of the Lower Proterozoic carbonate concretions: the Zaonega Formation, Southeast Karelia, Lithol. Miner. Resour., 2012, vol. 45, no. 4, pp. 319–333.
Kuznetsov, A.B., Gorokhov, I.M., and Melezhik, V.A., Sr isotopes in sedimentary carbonates, Reading the Archive of Earth’s Oxygenation. Volume 3: Global Events and the Fennoscandian Arctic Russia—Drilling Early Earth Project, Melezhik, V.A., Kump, L.R., Fallick, A.E., et al., Eds., Heidelberg: Springer, 2013, pp. 1459–1467. https://doi.org/10.1007/978-3-642-29670-3
Kuznetsov, A.B., Semikhatov, M.A., and Gorokhov, I.M., The Sr isotope chemostratigraphy as a tool for solving stratigraphic problems of the Upper Proterozoic (Riphean and Vendian), Stratigraphy. Geol. Correlation, 2014, vol. 22, no. 6, pp. 553–575.
Kuznetsov, A.B., Semikhatov, M.A., and Gorokhov, I.M., Strontium isotope stratigraphy: principles and state of the art, Stratigraphy. Geol. Correlation, 2018, vol. 26, no. 4, pp. 367–386.
Kuznetsov, A.B., Lobach–Zhuchenko, S.B., Kaulina, T.V., and Konstantinova, G.V., Paleoproterozoic age of carbonates and trondhjemites of the Central Azov Group: Sr isotope chemostratigraphy and U–Pb geochronology, Dokl. Earth Sci., 2019, vol. 484, no. 2, pp. 142–145.
Luttge, A., Bolton, E.W., and Rye, D.M., A kinetic model of metamorphism: an application to siliceous dolomites, Contrib. Mineral. Petrol., 2004, vol. 146, no. 5, pp. 546–565.
Maheshwari, A., Sial, A.N., Gaucher, C., et al., Global nature of the paleoproterozoic lomagundi carbon isotope excursion: a review of occurrences in Brazil, India, and Uruguay, Precambrian Res., 2010, vol. 182, no. 4, pp. 274–299.
Masch, L. and Heuss-Assbichler, S., Decarbonation reactions in siliceous dolomites and impure limestones, Equilibrium and Kinetics in Contact Metamorphism, Voll, G., Töpel, J., Pattison, D.R.M., and Seifen. F., Eds., Berlin–Heidelberg: Springer-Verlag, 1991.
Mason, R., Petrology of the Metamorphic Rocks, London: Unwin Hyman, 1990, p. 2.
Matrenichev, A.V. and Matrenichev, V.A., Petrology of the Ludicovian volcanism of the Onega structure and Raahe–Ladoga zone. Baltic Shield. Sb. IGGD RAN (A Collection of Papers of the Institute of Precambrian Geology and Geochronology, RAS) Abushkevich, V.S, Alfimova, N.A, Eds., St. Petersburg: Politekhn. Univ., 2010, pp. 223–255.
Melezhik, V.A. and Fallick, A.E., A widespread positive δ13Ccarb anomaly at around 2.33–2.06 Ga on the Fennoscandian shield: a paradox?, Terra Nova, 1996, vol. 8, no. 2, pp. 141–157.
Melezhik, V.A., Roberts, D., Gorokhov, I.M., et al., Isotopic evidence for a complex Neoproterozoic to Silurian rock assemblage in the north-central Norwegian Caledonides, Precambrian Res., 2002, vol. 114, nos. 1/2, pp. 55–86.
Melezhik, V.A., Zwaan, B.K., Motuza, G., et al., New insights into the geology of high-grade Caledonian marbles based on isotope chemostratigraphy, Norwegian J. Geol., 2003, vol. 83, pp. 209–242.
Melezhik, V.A., Fallick, A.E., and Kuznetsov, A.B., Palaeoproterozoic, rift-related, 13C-rich, lacustrine carbonates, NW Russia. Part II: global isotopic signal recorded in the lacustrine dolostone, Trans. R. Soc. Edinburgh Earth Sci., 2004, vol. 95, nos. 3/4, pp. 423–444.
Melezhik, V.A., Roberts, D., Fallick, A.E., et al., Geochemical preservation potential of high–grade calcite marble versus dolomite marble: implication for isotope chemostratigraphy, Chem. Geol., 2005, vol. 216, nos. 3–4, pp. 203–224.
Melezhik, V.A., Kuznetsov, A.B., Fallick, A.E., et al., Depositional environments and an apparent age for the Geci meta-limestones: constraints on geological history of northern Mozambique, Precambrian Res., 2006, vol. 148, nos. 1/2, pp. 19–31.
Melezhik, V.A., Huhma, H., Condon, D.J., et al., Temporal constraints on the Paleoproterozoic Lomagundi–Jatuli carbon isotopic event, Geology, 2007, vol. 35, no. 7, pp. 655–658.
Melezhik, V.A., Pokrovsky, B.G., Fallick, A.E., et al., Constraints on 87Sr/86Sr of late Ediacaran seawater: insights from Siberian high-Sr limestones, J. Geol. Soc. London, 2009, vol. 166, pp. 183–191.
Melezhik, V.A., Fallick, A.E., Martin, A.P., et al., The Palaeoproterozoic perturbation of the global carbon cycle: the Lomagundi–Jatuli isotopic event, Reading the Archive of Earth’s Oxygenation. Global Events and the Fennoscandian Arctic Russia, Drilling Early Earth Project (FAR–DEEP), Berlin–Heidelberg: Springer-Verlag, 2013a, vol. 3, pp. 1111–1150.
Melezhik, V.A., Roberts, D., Gjelle, S., et al., Isotope chemostratigraphy of high-grade marbles in the Rognan area, north-central Norwegian Caledonides: a new geological map, and tectonostratigraphic and palaeogeographic implications, Norwegian J. Geol., 2013b, vol. 93, no. 3, pp. 107–150.
Melezhik, V.A., Ihlen, P.M., Kuznetsov, A.B., et al., Pre-Sturtian (800–730 Ma) depositional age of carbonates in sedimentary sequences hosting stratiform iron ores in the uppermost allochthon of the Norwegian Caledonides: a chemostratigraphic approach, Precambrian Res., 2015, vol. 261, pp. 272–299.
Metz, P. and Trommsdorff, V., On phase equilibria in metamorphosed siliceous dolomites, Contrib. Mineral. Petrol., 1968, vol. 18, no. 4, pp. 305–309.
Metzger, A.A.Th., Die kalksteinlagerstatten von Ruskeala in Ostfinnland, Bull. Comm. Geol. Finlande, 1925, no. 74.
Moore, C.H., Carbonate Diagenesis and Porosity. Developments in Sedimentology, Amsterdam: Elsevier, 1989, vol. 46.
Ovchinnikova, G.V., Kuznetsov, A.B., Melezhik, V.A., et al., Pb–Pb age of Jatulian carbonate rocks: The Tulomozero Formation of southeast Karelia, Stratigraphy. Geol. Correlation, 2007, vol. 15, no. 4, pp. 359–372.
Podkovyrov, V.N., Semikhatov, M.A., Kuznetsov, A.B., et al. Carbonate carbon isotopic composition in the Upper Riphean stratotype, the Karatau Group, Southern Urals, Stratigraphy. Geol. Correlation, 1998, vol. 6, no. 4, pp. 319–335.
Pokrovsky, B.G. and Vinogradov, V.I., Strontiu, oxygen, and carbon isotope composition of the Upper Precambrian carbonates of the western slope of the Anabar Uplift (Kotuikan River), Dokl. AN SSSR, 1991, vol. 320, no. 5, pp. 1245–1250.
Pokrovsky, B.G., Melezhik, V.A., and Buyakaite, M.I., Carbon, Oxygen, Strontium, and Sulfur isotopic compositions in Late Precambrian rocks of the Patom Complex, Central Siberia: Communication 2. Nature of carbonates with ultralow and ultrahigh δ13C values, Lithol. Miner. Resour., 2006, vol. 41, no. 5, pp. 576–587.
Pokrovsky, B.G., Buyakaite, M.I., and Kokin, O.V., Geochemistry of C, O, and Sr isotopes and chemostratigraphy of Neoproterozoic rocks in the northern Yenisei Ridge, Lithol. Miner. Resour., 2012, vol. 47, no. 2, pp. 177–200.
Puchtel, I.S., Arndt, N.T., Hofmann, A.W., et al., Petrology of mafic lavas within the Onega Plateau, Central Karelia: evidence for 2.0 Ga plume-related continental crustal growth in the Baltic Shield, Contrib. Mineral. Petrol., 1998, vol. 130, no. 2, pp. 134–153.
Puchtel, I.S., Brügmann, G.E., and Hofmann, A.W., Precise Re-Os mineral isochron and Pb-Nd-Os isotope systematics of a mafic–ultramafic sill in the 2.0 Ga Onega Plateau (Baltic Shield), Earth Planet. Sci. Lett., 1999, vol. 170, no. 4, pp. 447–461.
Ray, J.S., Veizer, J., and Davis, W.J., C, O, Sr, and Pb isotope systematics of carbonate sequences of the Vindhyan Supergroup, India: age, diagenesis, correlations and implications for global events, Precambrian Res., 2003, vol. 121, nos. 1/2, pp. 103–140.
Satish-Kumar, M., Miyamoto, T., Hermann, J., et al., Pre–metamorphic carbon, oxygen and strontium isotope signature of high-grade marbles from the Lutzow–Holm Complex, East Antarctica: apparent age constraints of carbonate deposition, Geol. Soc. Spec. Publ. London, 2008, vol. 308, pp. 147–164.
Schidlowski, M., Eichmann, R., and Junge, C.E., Carbon isotope geochemistry of the Precambrian Lomagundi carbonate province, Rhodesia, Geochim. Cosmochim. Acta, 1976, vol. 40, no. 4, pp. 449–455.
Scholle, P.A. and Arthur, M.A., Carbon isotopic fluctuations in cretaceous pelagic limestones: potential stratigraphic and petroleum exploration tool, Amer. Assoc. Petrol. Geol. Bull., 1980, vol. 64, pp. 67–87.
Semikhatov, M.A., Kuznetsov, A.B., Podkovyrov, V.N., et al., The Yudoma Group of stratotype area: C–isotope chemostratigraphic correlations and Yudomian–Vendian relation, Stratigraphy. Geol. Correlation, 2004, vol. 12, no. 5, pp. 435–459.
Semikhatov, M.A., Kuznetsov, A.B., Maslov, A.V., et al. Stratotype of the Lower Riphean, the Burzyan Group of the Southern Urals: lithostratigraphy, paleontology, geochronology, Sr– and C–isotopic characteristics of its carbonate rocks, Stratigraphy. Geol. Correlation, 2009, vol. 17, no. 6, pp. 574–601.
Shuldiner, V.I., Kozyreva, I.V., and Baltybaev, Sh.K., Geochronology and formation subdivisions of the Lower Precambrian in the northwestern Ladoga Region, Stratigraphy. Geol. Correlation, 1996, vol. 4, no. 3, pp. 220–230.
Sial, A.N., Gaucher, C., Ramkumar, M., and Ferreira, V.P., Chemostratigraphy as a formal stratigraphic method, Chemostratigraphy across Major Chronological Boundaries, American Geophys. Union. Geophys. Monogr., 2019, vol. 240, pp. 3–25.
Sokolov, V.A. and Galdobina, L.P., Ludicovian as a new stratigraphic subdivision of the Paleoproterozoic, Dokl. AN SSSR, 1982, vol. 267, no. 1, pp. 187–190.
Spooner, E.T.C., The strontium isotopic composition of seawater, and seawater–oceanic crust interaction, Earth Planet. Sci. Lett., 1976, vol. 31, no. 1, pp. 167–174.
Stratigrafiya dokembriya KASSR (arkhei, nizhnii proterozoi) (Precambrian Stratigraphy of the Karelian ASSR (Archean, Paleoproterozoic), Petrozavodsk: KFAN SSSR, 1984.
Svetov, A.P. and Sviridenko, L.P., Stratigrafiya dokembriya Karelii. Sortaval’skaya seriya svekokarelid Priladozh’ya (Precambrian Stratigraphy of Karelia. Sortavala Group of the Svecokarelides of the Ladoga Region), Petrozavodsk: Karel’skii nauchnyi tsentr, 1992. 152 s.
Trommsdorff, V. and Evans, B.W., Antigorite–ophicarbonates: phase relations in a portion of the system CaO–MgO–SiO2–H2O–CO2, Contrib. Mineral. Petrol., 1977, vol. 60, no. 1, pp. 39–56.
Trommsdorff, V. and Connolly, J.A.D., Constraints on phase diagram topology for the system CaO–MgO–SiO2–CO2–H2O, Contrib. Mineral. Petrol., 1990, vol. 104, no. 1, pp. 1–7.
Valley, J.W., Stable isotope geochemistry of metamorphic rocks, Stable isotopes in High Temperature Geological Processes, Valley, J.W. Taylor, H.P. O’Neil. J.R., Eds., Rev. Mineral. Geochem. Mineral. Soc. Am., 1986, vol. 16, pp. 445–490.
Veizer, J. and Compston, W., 87Sr/86Sr in Precambrian carbonates as an index of crustal evolution, Geochim. Cosmochim. Acta, 1976, vol. 40, no. 8, pp. 905–914.
Veizer, J. and Hoefs, J., The nature of 18O/16O and 13C/12C secular trends in sedimentary carbonate rocks, Geochim. Cosmochim. Acta, 1976, vol. 40, no. 11, pp. 1387–1395.
Veizer, J., Holser, W.T., and Wilgus, C.K., Correlation of 13C/12C and 34S/32S secular variations, Geochim. Cosmochim. Acta, 1980, vol. 44, pp. 579–587.
Veizer, J., Clayton, R.N., and Hinton, R.W., Geochemistry of Precambrian carbonates: 3–shelf seas and non–marine environments of the Archean, Geochim. Cosmochim. Acta, 1990, vol. 54, no. 10, pp. 2717–2729.
Walter, M.R., Veeres, J.J., Calver, C.R., et al., Dating the 840–544 Ma Neoproterozoic interval by isotopes of strontium, carbon and sulfur in seawater and some interpretative models, Precambrian Res., 2000, vol. 100, no. 1, pp. 371–433.
Wickman, F.E., Isotope ratios – a clue to the age of certain marine sediments, J. Geol., 1948, vol. 56, no. 1, pp. 61–66.
Winkler, H.G.F., Petrogenesis of Metamorphic Rocks, New York: Springer-Verlag, 5th ed, 1979.
Yudovich, Ya.E., Makarikhin, V.V., Medvedev, P.V., and Sukhanov, N.V., Carbon isotope anomalies in carbonates of the Karelian Complex, Geokhimiya, 1990, no. 7, pp. 972–978.
ACKNOWLEDGMENTS
We are grateful to B.G. Pokrovsky for valuable comments that significantly improved the manuscript.
Funding
This work was supported by the Russian Science Foundation (Geochemistry and Sr isotopes in Carbonate Rocks, RSF no. 18-17-00247). Petrological study was carried out in the framework of the State Task (NIR no. 0132-2019-0013).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated by M. Bogina
Rights and permissions
About this article
Cite this article
Kuznetsov, A.B., Gorokhov, I.M., Azimov, P.Y. et al. Sr- and C-Chemostratigraphy Potential of the Paleoproterozoic Sedimentary Carbonates under Medium-Temperature Metamorphism: the Ruskeala Marble, Karelia. Petrology 29, 175–194 (2021). https://doi.org/10.1134/S0869591121010033
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0869591121010033