Abstract
The aim of the study was to investigate the dynamics of venous blood glucose, insulin, and C-peptide in response to an intake of a mixed meal normalized to body mass in obese patients without and with type 2 diabetes mellitus. Venous blood samples were taken from seven healthy subjects, nine obese patients, and ten obese patients with type 2 diabetes mellitus (mean duration of diabetes 7 years) before and 30, 60, 90, 120, and 180 min after a mixed meal (6 kcal/kg of body mass); additionally, nine patients with obesity and type 2 diabetes mellitus and three healthy volunteers completed the hyperinsulinemic-euglycemic clamp test. In patient groups the energy content of food did not differ but was 1.8 times higher than in the control. An increase in glucose level 1 h after a meal was maximal in patients with type 2 diabetes, and an increase in insulin and C-peptide was higher in obese patients, which is related to impairment of insulin-dependent glucose uptake by tissues and of the rate of insulin secretion (dysfunction of beta-cells) in patients. At the same time, an increase in the total area under the curve “C-peptide–time” demonstrates that the maximal secretory response of beta-cells is comparable in obese patients without and with type 2 diabetes mellitus. The absolute blood glucose level 90 min after a meal closely correlated with the M-index—the marker of systemic sensitivity to insulin (rs = –0.82, p = 0.002). Our results characterize the features in the regulation of carbohydrate metabolism after intake of a mixed meal, normalized to body mass, in people with varying severity of metabolic disorders, and open up prospects for a wider application of this test in practice.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
In the Russian Federation, according to dispensary and registry records, the number of patients with diabetes mellitus is more than 3% of the population, of which 92.5% of patients have type 2 diabetes [1]. The average life expectancy of patients after the diagnosis of type 2 diabetes is 11.4 years. In the vast majority of cases, the main cause of insulin resistance and type 2 diabetes is obesity. One of the methods for assessing carbohydrate metabolism disorders is the oral glucose tolerance test (OGTT) with 75 mg of glucose or 82.5 g of glucose monohydrate dissolved in 250–300 mL of water. The dynamics of glucose and insulin levels in venous blood during OGTT is well characterized [2–4], which leads to the widespread use of this test in clinical practice for diagnosing prediabetes and type 2 diabetes [5]. However, OGTT does not mimic the absorption and digestion of complex foods, i.e., it does not mimic physiological postprandial metabolism, such as the mixed meal test.
In several studies, the dynamics of the content of glucose, insulin, and C-peptide were examined in a test with a standard dose (300–500 kcal) of mixed meal [3, 6, 7]. Obviously, standardizing the amount of glucose/meal taken complicates comparing and interpretation of results for people who differ significantly in body mass [8]. Usually obese people, compared to thin people, eat a significantly larger amount of food every day, so it is important to study the dynamics of blood glucose and insulin after a typical meal. To model a typical meal, a test with a mixed meal normalized to body mass or basal metabolic rate [9, 10], or unlimited access to food [11] is used. However, there are still no recommendations for interpreting the results of such tests.
Objective—To study the changes in the dynamics of blood glucose, insulin, and C-peptide in response to a typical meal in patients with “metabolically healthy obesity” and in patients with obesity and type 2 diabetes mellitus. To do this, the dynamics of the content of glucose, insulin, and C-peptide in venous blood after ingestion of a mixed meal normalized for body mass was compared in healthy lean volunteers, obese patients with or without type 2 diabetes mellitus. For some volunteers, these indicators were compared with the M-index determined in the hyperinsulinemic-euglycemic clamp test, the “gold standard” for assessing insulin resistance.
MATERIALS AND METHODS
The study involved seven healthy volunteers (H), nine obese patients (Ob) and ten patients with obesity and type 2 diabetes (Ob + T 2D; mean disease duration 7 (2.5–8.0) years (median and interquartile range). Characteristics of volunteers are presented in Table 1. Patients with Ob + T 2D were on hypoglycemic ther-apy; the treatment was stopped 1–2 days (sodium glucose cotransporter-2 inhibitors, dipeptidyl peptidase-4 inhibitors, sulfonylurea derivatives, biguanide [Metformin]) and/or 7 days (glucagon-like peptide-1 receptor agonist [Semaglutide]) before the mixed meal test. All volunteers completed an SF-12 questionnaire [12] for a subjective assessment of their physical capabilities.
The study of volunteers was carried out in the morning (09:00) after an overnight fast. During the test, the volunteers were in the supine position; venous blood was taken from v. cephalica before and 30, 60, 90, 120 and 180 min after the mixed meal test (food supplement Resource 2.0 (Nestle Health Science, France), 3 mL or 6 kcal/kg body mass, protein/fat/carbohydrate ratio 3/3/7, calories 200 kcal/100 mL. Glycated hemoglobin (HbA1c) in whole blood was determined by high performance liquid chromatography on a D10 analyzer (BioRad, United States), the level of glucose in serum was determined on an Architect c8000 automatic analyzer (Abbott Diagnostics, United States); immunoreactive insulin and C-peptide were determined in blood serum using a Cobas 6000 electrochemiluminescent analyzer (Roche, Switzerland).
To assess the degree of insulin resistance a hyperinsulinemic-euglycemic clamp test was performed on a separate day in a subset of volunteers with type 2 diabetes (n = 9) and healthy controls (n = 3) after an overnight fast according to the classical method [13]. Metformin was discontinued 48 h before, and other hypoglycemic drugs 12 h before the clamp test. Recombinant human insulin (Actrapid NM, Novo Nordisk, Denmark) was administered intravenously at a rate of 1 mU/kg of body mass per minute (Perfusor Compact infusion system; B. Braun, Germany) until reaching the level of 100 mU/L. At the same time, 20% glucose was injected (Infusomat fmS volumetric pump; B. Braun, Germany) at a rate necessary to maintain a glucose level of 5.1–5.6 mmol/L. The content of glucose in venous blood was assessed every 5–10 min (One Touch Verio Pro+ glucometer, LifeScan, Switzerland). After reaching equilibrium between the rate of glucose administration and its uptake by tissues (120–180 min), this state was maintained for 30–40 min. The M-index was calculated as the average rate of glucose infusion at a steady state, normalized to body mass.
Data are presented as median and interquartile ranges. Differences between samples were assessed using one-way Kruskal-Wallis analysis of variance with correction for multiple comparisons (Dunn’s test) at a significance level of 0.05. The relationship between variables was assessed using the Spearman rank correlation coefficient (rs) at a significance level of 0.05.
RESULTS AND DISCUSSION
As expected, the body mass index, HOMA-IR index, baseline insulin and C-peptide levels were significantly elevated in both groups of patients. Glycated hemoglobin and baseline glucose levels were elevated in the Ob + T2D group relative to other groups. In addition, in the Ob + T2D group, relative to the control, the content of baseline triglycerides was increased, and the subjective assessment of physical capabilities was reduced (Table 1).
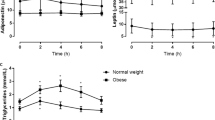
The calorie content of a meal in patients did not differ but was 1.8 times higher than in the control group (Table 1). The dynamics of biochemical parameters after a meal varied significantly between groups, while no pronounced differences were found between men and women (data not shown). In the Ob group the glucose level was increased by 2 times within 2 h after a meal. In the Ob + T2D group the postprandial glucose level was the highest, but it did not differ from the initial value due to the high initial level and large intragroup variation (Fig. 1). However, the increase in glucose for 1 h of the test, its maximum increase, and the increase in the area under the glucose-time curve for the test (iAUC) were the largest in the Ob + T2D group (Fig. 2), which indicates a pronounced insulin resistance and is consistent with the results in the OGTT [2] and mixed meal test with the standard dosage for all participants [3, 6].
The dynamics of glucose, insulin, and C-peptide in the blood after ingestion of a mixed meal (6 kcal/kg of body mass) in healthy volunteers (H), obese patients without (Ob) and with type 2 diabetes mellitus (Ob + T 2D). The median and interquartile range are presented; ×, ×× and ×××—difference from baseline p ≤ 0.05, ≤ 0.01 and ≤ 0.001, respectively.
The increase in blood glucose, insulin, and C-peptide after ingestion of a mixed meal (6 kcal/kg body mass) in healthy volunteers (H), obese patients without (Ob) and with type 2 diabetes mellitus (Ob + T 2D). Median, interquartile range and individual values are presented; *— difference from control, #—difference from Ob; one, two and three characters—p ≤ 0.05, ≤ 0.01 and ≤ 0.001, respectively.
Postprandial values of insulin and C-peptide were higher in patients than in controls (Fig. 1). At the same time, the rate of increase in insulin and C-peptide per hour after a meal was increased relative to the control only in the Ob group (Fig. 2a), which is associated with a compensatory increase in insulin secretion during the development of insulin resistance in obese patients. The absence of differences in this parameter in the Ob + T2D group from the control attributed to β-cell dysfunction caused by long-term type 2 diabetes mellitus [14]. However, the maximum increase in insulin and C-peptide and increase in area under the C-peptide-time curve, a semi-quantitative marker of the secreting activity of β-cells, were comparable between groups of patients and were greater than in the control (Figs. 2b and 2c). This response in patients with type 2 diabetes mellitus differs from what is usually observed during OGTT [2, 4] or in a test with standard dose of mixed meal [3, 6], which shows a smaller increase in these parameters in patients with type 2 diabetes relative to patients with obesity.
The difference from the tests with standard dose of glucose/mixed meal seems to be due to the fact that in our study, patients received more food, which caused a significant increase in blood glucose and insulin secretion in the Ob + T2D group. The difference from OGTT may be due to stronger stimulation of insulin production by mixed meal [6], which is partly related to greater secretion of incretins [3]. It should be noted that in the Ob + T2D group there were patients in whom the increase in the area under the curves “insulin/C-peptide-time” was significantly lower than the average value (Fig. 2c); it turned out that these patients had the longest duration of type 2 diabetes mellitus in the group (11 and 14 years versus 7 years on average for the group).
An analysis of all the data revealed the strong correlations between the M-index and the glucose content (rs = –0.82…–0.69; p = 0.002–0.02) and with the area under the glucose-time curve (AUC; rs = –0.78… –0.76; p = 0.004–0.01) at 30, 60, 90, 120 and 180 min. It is important to note that a decrease in the M-index (insulin sensitivity) is closely associated with an increase in blood glucose 90 min after a meal (Fig. 3); at the same time, with an M-index value of less than 3 (severe insulin resistance), the glucose content varies significantly and can reach very high concentrations (>17 mmol/L). The strong relationship between these parameters indicates that the test with a mixed meal normalized to body mass can be used to indirectly assess systemic insulin resistance.
The relationship between blood glucose levels 90 min after ingestion of a mixed meal (6 kcal/kg body mass) and M-index is an indicator of systemic of insulin sensitivity. Gradation of insulin resistance according to the M-index: ≤2, severe, >2–4, medium; >4–6, mild, >6, no insulin resistance (vertical dotted lines).
CONCLUSIONS
The test with a normalized intake of mixed meal reveals significant disturbances in the dynamics of glucose, insulin and C-peptide in the blood in patients with obesity and type 2 diabetes mellitus. The increase in glucose and C-peptide 1 h after a meal distinguishes obese patients without and with type 2 diabetes mellitus well, which reflects disturbances in both insulin-dependent glucose uptake by tissues and in the rate of insulin secretion. At the same time, the increase in the area under curve of the “C-peptide-time” for the test indirectly shows that the maximum secretory response of β-cells is comparable in patients with obesity without and with type 2 diabetes mellitus (average duration of diabetes is 7 years). At the same time, patients with the longest experience of type 2 diabetes mellitus demonstrate a significantly lower increase in this parameter, which indirectly indicates a pronounced dysfunction of β-cells. The absolute level of blood glucose 90 min after a meal is closely correlated with the M-index, the marker of systemic sensitivity to insulin. Our findings, and that the mixed meal test has good reliability (reproducibility) [15, 16] and weakly depends on differences in the protein-carbohydrate composition of various commercial food mixtures [15], opens up prospects for a wider use of this test in practice.
REFERENCES
Dedov, I.I., Shestakova, M.V., Vikulova, O.K., et al., Epidemiological characteristics of diabetes mellitus in the Russian Federation: clinical and statistical analysis according to the Federal Diabetes Register data as of January 01, 2021, Sakh. Diabet, 2021, vol. 24, no. 3, p. 204.
Wang, Q., Jokelainen, J., Auvinen, J., et al., Insulin resistance and systemic metabolic changes in oral glucose tolerance test in 5340 individuals: an interventional study 2, BMC Med., 2019, vol. 17, no. 1, p. 217.
Shankar, S.S., Vella, A., Raymond, R.H., et al., Standardized mixed-meal tolerance and arginine stimulation tests provide reproducible and complementary measures of beta-cell function: results from the Foundation for the National Institutes of Health Biomarkers Consortium Investigative Series, Diabetes Care, 2016, vol. 39, no. 9, p. 1602.
Tura, A., Morbiducci, U., Sbrignadello, S., et al., Shape of glucose, insulin, C-peptide curves during a 3‑h oral glucose tolerance test: any relationship with the degree of glucose tolerance? Am. J. Physiol.: Regul. In-tegr. Comp. Physiol., 2011, vol. 300, no. 4, p. R941.
Draznin, B., Aroda, V.R., Bakris, G., et al., 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes-2022, Diabetes Care, 2022, vol. 45, no. 1, p. S17.
Rijkelijkhuizen, J.M., Girman, C.J., Mari, A., et al., Classical and model-based estimates of beta-cell function during a mixed meal vs. an OGTT in a population-based cohort, Diabetes Res., Clin. Pract., 2009, vol. 83, no. 2, p. 280.
van Bussel, I.P.G., Fazelzadeh, P., Frost, G.S., et al., Measuring phenotypic flexibility by transcriptome time-course analyses during challenge tests before and after energy restriction, FASEB J., 2019, vol. 33, no. 9, p. 10280.
Vorotnikov, A.V., Popov, D.V., and Makhnovskii, P.A., Signaling and gene expression in skeletal muscles in type 2 diabetes: current results and OMICS perspectives, Biochemistry (Moscow). 2022, vol. 87, no. 9, p. 1021. https://doi.org/10.1134/S0006297922090139
McQuaid, S.E., Hodson, L., Neville, M.J., et al., Downregulation of adipose tissue fatty acid trafficking in obesity: a driver for ectopic fat deposition? Diabetes, 2011, vol. 60, no. 1, p. 47.
Bastarrachea, R.A., Laviada-Molina, H.A., Nava-Gonzalez, E.J., et al., Deep multi-OMICs and multi-tissue characterization in a pre- and postprandial state in human volunteers: the GEMM family study research design, Genes (Basel), 2018, vol. 9, no. 11, p. 532.
Small, L., Brandon, A. E., Parker, B. L., Desh-pande, V., Samsudeen, A. F., et al., Reduced insulin action in muscle of high fat diet rats over the diurnal cycle is not associated with defective insulin signaling, Mol. Metab., 2019, vol. 25, p. 107. https://doi.org/10.1016/j.molmet.2019.04.006
Ware, J., Jr., Kosinski, M., and Keller, S.D., A 12-Item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity, Med. Care, 1996, vol. 34, no. 3, p. 220.
DeFronzo, R.A., Tobin, J.D., and Andres, R., Glucose clamp technique: a method for quantifying insulin secretion and resistance, Am. J. Physiol., 1979, vol. 237, no. 3, p. E214.
Cerf, M.E., Beta cell dysfunction and insulin resistance, Front. Endocrinol. (Lausanne), 2013, vol. 4, p. 37.
Kossler, T., Bobrov, P., Strassburger, K., et al., Impact of mixed meal tolerance test composition on measures of beta-cell function in type 2 diabetes, Nutr. Metab. (London), 2021, vol. 18, no. 1, p. 47.
Paglialunga, S., Guerrero, A., Roessig, J.M., et al., Adding to the spectrum of insulin sensitive populations for mixed meal tolerance test glucose reliability assessment, J. Diabetes Metab. Disord., 2016, vol. 15, p. 57.
ACKNOWLEDGMENTS
The authors are grateful to Ph.D. I.A. Sklyanik (Institute of Diabetes, State Scientific Center of the Russian Federation, Federal State Budgetary Institution “NMITs of Endocrinology,” Ministry of Health of Russia, Moscow) for his help in working with patients.
Funding
The study was supported by the Russian Science Foundation (grant no. 21-75-10 146).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All studies were conducted in accordance with the principles of biomedical ethics as formulated in the 1964 Declaration of Helsinki and its subsequent updates and approved by the Biomedical Ethics Committee of the Institute of Biomedical Problems of the Russian Academy of Sciences (Moscow), No. 613 of March 29, 2022 and the Local Ethics Committee of the National Medical Research Center for Endocrinology (Moscow), No. 4 of February 14, 2022.
Each participant in the study provided a voluntary written informed consent signed by him after explaining to him the potential risks and benefits, as well as the nature of the upcoming study.
CONFLICT OF INTEREST
The authors of this work declare that they have no conflicts of interest.
Additional information
Publisher’s Note.
Pleiades Publishing remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Lednev, E.M., Gavrilova, A.O., Vepkhvadze, T.F. et al. Disturbances in Dynamics of Glucose, Insulin, and C-Peptide in Blood after a Normalized Intake of a Mixed Meal in Obesity and Type 2 Diabetes Mellitus. Hum Physiol 49, 668–674 (2023). https://doi.org/10.1134/S0362119723600297
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0362119723600297