Abstract
The results of studying the correlations between biochemical and immunological parameters in volunteers under conditions of 21-day “dry” immersion are presented. It is shown that, in comparison with the baseline period, at the final stage of the experiment, the number of statistically significant correlations (p ≤ 0.05, respectively, 27 and 51) between the parameters assessing the metabolic and immunological reactions of the body increased. The increase in the number of correlations was due mainly to an increase in the number of correlations between the indices of protein, carbohydrate, and lipid metabolism and indices characterizing the state of innate immunity. At the final stage of the experiment, the influence of protein and lipid metabolism on the cellular component of adaptive immunity increased. The revealed dynamics and the nature of correlations between biochemical and immunological indices indicate the metabolic regulation of the immune response under conditions of hypodynamic support unloading.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
A critical factor in space flight (SF) is a long-term limitation of motor loads, which may lead to the development of the so-called hypokinetic motor syndrome, which is characterized by changes in the state of the physiological systems of the body. Significant progress in understanding the mechanisms of adaptation processes that ensure the maintenance of homeostasis of body systems under conditions of a decrease in static and dynamic activity has become possible due to the development of ground-based model studies. One of these models is “dry” immersion (DI), which creates conditions for orthostatic and support unloading, hypokinesia, and redistribution of fluids in the body [1, 2]. Studies performed for many years using this model at the Institute of Biomedical Problems of the Russian Academy of Sciences (Moscow) made it possible to describe in detail the dynamics and degree of changes occurring in metabolism [3] and immune system [4, 5]. According to many studies, the common trigger mechanisms make metabolism and immunity the components of the same chain of homeostatic changes; therefore, immunological disorders are tightly related to biochemical disorders [6, 7]. However, to interpret the mechanisms of human adaptation to the influence of extreme factors (in particular, SF factors), correct studies of the functional unity of biochemical and immunological processes have not yet been performed.
In this regard, the objective of this study was to investigate the correlations between some biochemical and immunological parameters in healthy men under conditions of a 21-day DI.
MATERIALS AND METHODS
An experiment with a 21-day DI without prophylaxis involved ten apparently healthy men 24–32 years old whose participation in the experiment was approved by the expert medical commission. The experimental conditions were described in detail earlier [1].
The material of the study was venous blood taken on an empty stomach in the morning in the baseline period 7–14 days before the start of the experiment and on day 21 of exposure to DI conditions.
The correlations between the biochemical and immunological parameters were estimated using nonparametric Spearman’s rank correlation analysis. Using Statistica for Microsoft Windows version 10.0, we calculated Spearman’s rank correlation coefficient, which was then used to determine the actual degree of correlation between two quantitative series of biochemical and immunological parameters studied and to evaluate the strength of the established correlation between them. The significance of the results was estimated using the Wilcoxon signed-rank test.
RESULTS AND DISCUSSION
As shown earlier [3], at the end of the immersion exposure (on the 21st day of DI), the activity of creatine phosphokinase (CPK) and its muscle isoform (CPK-MM) in the blood serum of the participants in the experiment significantly (on average, 2.5 times) decreased. The content of calcium in the blood significantly (by 6%) increased. The indices of protein metabolism (total protein and albumin) remained at the same level, whereas the concentration of glucose decreased by 13%, which was beyond the physiological norm. Notably, the activity glutamate dehydrogenase (GDH) in blood serum drastically (more than 2.7 times) increased and significantly exceeded the upper limit of the physiological norm. Hypercholesterolemia, which manifested itself as an increase in cholesterol concentration by 17%, was also observed.
The study of immune homeostasis in volunteers also revealed a number of changes in the quantitative and functional characteristics of innate and adaptive immunity cells. For example, the analysis of the state of the system of signal pattern-recognition receptors of the Toll-like family of innate immunity cells, including the determination of the expression of TLR1, TLR2, TLR3, TLR4, TLR5, TLR6, TLR8, and TLR9 on peripheral blood leukocytes, made it possible to detect a significant increase in the absolute the content of granulocytes (Gr) expressing TLR3 and, conversely, a decrease in the content of monocytes (Mn) expressing TLR9 on the 21st day of the experiment. At the same time, according to the averaged data, the content of monocytes and granulocytes expressing other TLRs, as well as mean fluorescence intensity (MFI) of TLRs on monocytes and granulocytes, did not change significantly [8].
The study of one of the indices characterizing the functional state of TLRs—the basal production of cytokines involved in the regulation of homeostasis at the local and systemic levels—revealed a significant decrease in the levels of IL-1α, IL-1b, IL-6, and IL-12P70 and a pronounced downward trend in the levels of IL-8 and TNFα in cell culture supernatants obtained after 24-h incubation of CD14+ monocytes. The concentrations of cytokines IL-10, IL-12P40, and IFNα on day 21 of DI did not differ significantly from the baseline values; however, pronounced individual fluctuations both upwards and downwards were observed [9].
Evaluation of the influence of the factors of exposure to DI on the nature and degree of changes in the adaptive immunity of healthy volunteers made showed that, at the final stage of the experimental exposure, the level of B lymphocytes (CD19+ cells) did not differ significantly from the baseline values. At the same time, the absolute content of all studied subpopulations of T lymphocytes (CD3+ cells)—CD4+, CD8+, CD4+CD45RA+, CD4+CD45RO+, CD25+, and CD16+CD56+ cells—in the peripheral blood significantly (р < 0.05) increased [10]. Interestingly, in the study of the characteristics of basal production of a number of cytokines (IFNγ, IL-4, IL-6, IL-10, and TNFα) by CD3+ cells in vitro, different types of T-cell responses to simulated conditions were observed: no changes, decrease, and increase in the level of cytokines in cell culture supernatants. However, despite the substantial individual fluctuations, a general pattern—a decrease in the basal production of IL-5 and IL-13—was also observed.
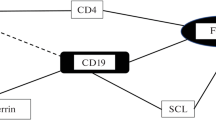
According to many studies, the development of the immune response is closely related to protein, carbohydrate, and lipid metabolism [11]. In this regard, in the investigation of the nature of the adaptive processes of the human body to the simulated gravity conditions, the determination of strong correlations (r ≥ 0.700, p < 0.05) between the studied indices of biochemical and immune status plays an important role (Fig. 1). The results of this study showed that the number of statistically significant (p ≤ 0.05) correlations between the parameters characterizing the metabolic and immunological reactions of the body (27 and 51, respectively) on the 21st day of exposure to DI increased as compared to the baseline period. In this case, the ratio between the inverse and direct correlations did not change significantly (1.25 in the baseline period and 1.12 on the 21st day of DI). It should also be noted that the increase in the number of correlations was due mainly to an increase in the number of correlations between the parameters of protein, carbohydrate, and lipid metabolism and indices characterizing the state of innate immunity: the number of such correlations was 18 (12 negative and 6 positive correlations) in the baseline period and 40 (22 negative and 18 positive correlations) on the 21st day of DI. This, apparently, can be explained by the fact that, as was shown on extensive experimental material, deterioration of environmental conditions and their abrupt change in populations and groups entail an increase in correlations between physiological parameters (indices of lipid metabolism, external respiration, enzyme activity, blood transport function, etc.) [12, 13].
Level of strong correlation (r ≥ 0.700, p < 0.05 and above) between the studied indices of biochemical and immune status in volunteers involved in the experiment with a 21-day dry immersion without prophylaxis: (a) innate immunity, (b) adaptive immunity. Designations: a, negative correlations; b, positive correlations.
It should be noted that a simultaneous change in the values of any parameters does not yet indicate their interdependence and may be random [14]. On the other hand, there are published data according to which protein-energy deficiency deficiency is accompanied by changes in the immune status. For example, correlations between the concentration of total protein and the number of lymphocytes in the peripheral blood, with a predominant decrease in the number of CD3+ T cells at a relatively stable content of B cells, were found. It is believed that, during protein starvation, the most pronounced changes in T cells are observed in the subpopulation of CD4+ lymphocytes. Against the background of a decrease in the total level of T cells in peripheral blood, a decrease in Th0 cells as a result of an increase in the number of Th2 cells was observed [15].
In clinical studies, a correlation between the level of serum albumin and the number of peripheral blood lymphocytes was also established. A decrease in the albumin concentration was accompanied by a decrease in both the relative and absolute content of CD25+ cells. In addition, a decrease in the level of albumin led to a decrease in the proliferative activity of lymphocytes in response to T-cell mitogens and the synthesis of a number of cytokines (IL-1, IL-2, IFN, and MIF) [16]. Apparently, albumin can contribute to the immunological homeostasis by regulating the pathways leading to effective antigen presentation and subsequent immunological response to antigens by activated helper T cells [17, 18]. The study of the molecular mechanisms underlying the immunomodulatory properties of serum albumin showed that it caused considerable changes in the transcriptome of immune cells, especially in the genes for cytokines and type I interferons. However, albumin did not significantly affect the functions of leukocytes such as phagocytosis, efferocytosis, and the production of intracellular reactive oxygen species [19].
The study of the effect of protein metabolism indices on the immune system under the influence of simulated microgravity factors on a healthy person showed that the level of total protein in the baseline period was negatively correlated with the absolute content of TLR6- and TLR3-expressing granulocytes in the peripheral blood, as well as with the intensity of TLR4 expression on circulating granulocytes (r = ‒0.67, r = –0.86, r = –0.90, respectively) and with the basal production of cytokines such as IL-4 and TNFα (r = –0.83) by CD3+ cells. It was also shown that the level of albumin was negatively correlated with the absolute content of monocytes expressing TLR8 in peripheral blood (r = –0.82) (Table 1). Apparently, the revealed negative correlations might be associated with an increased protein intake under exposure to low-intensity nonspecific factors during the examination before the experiment. On the 21st day of DI, the effect of protein metabolism on the set of the studied parameters characterizing the expression of TLRs on cellular factors of innate immunity decreased. Only the correlation between the concentration of albumin and the intensity of TLR5 expression on granulocytes was found (r = –0.71). It should also be noted that, in this period of the study, no correlations between the protein metabolism indices and the level of baseline production of cytokines by CD14+ monocytes and CD3+ lymphocytes were found. At the same time, noteworthy is the positive correlation between the total protein concentration and the absolute content of CD8+ subpopulations of T cells in the peripheral blood, indicating an increasing effect of protein metabolism on the cellular component of adaptive immunity at the final stage of exposure to DI.
A number of articles discuss the state of indices of immunity and lipid metabolism. According to current data, lipids can interact with all components of the immune system, from the innate immunity to the T- and B-cell components. The results showing that fatty acids are able to induce or inhibit the activation of TLR2 and TLR4 were presented. For example, it was shown that the saturated fatty acids (SFAs) activate TLR4, whereas the polyunsaturated fatty acids (PUFAs) inhibit lipopolysaccharide-induced TLR4 activation [20, 21]. It was also found that medium-chain SFAs can affect the lipopeptide-induced activation of TLR2. It was shown that lauric acid potentiates TLR2 activation, whereas docosahexaenoic acid, conversely, inhibits it [22].
The results of studies confirming the differences in the state of adaptive immunity in individuals with hypo- and hyperlipidemia are of considerable interest [23, 24]. Against the background of a significant decrease in the total cholesterol content in blood, both the number of lymphocytes in the capillary network (primarily CD3+ T cells) and the production of interleukin-2 (IL-2) decrease when lymphocytes are stimulated with phytohemagglutinin (PHA). Cholesterol promotes activation, differentiation, and proliferation of both CD4+ and CD8+ T-cell subpopulations through suppression of liver X receptor β (LXRβ) and activation of the sterol regulatory element binding protein 2 (SREBP2) [25, 26]. M.F. Muldoon et al. found a statistically significant correlation between some of T-cell subpopulations (CD3+ and CD4+) and the level of triglycerides [24]. There are also data indicating that hypercholesterolemia leads to reprogramming of T cells (in particular, promotes the differentiation of T helper cells (Th) towards Th2 [27]. This shift towards Th2 under conditions of hypercholesterolemia may be partly due to attenuation of the production of proinflammatory (Th1–) cytokines CD8α–DC by induced by oxidized low-density lipoproteins [28]. Animal experiments showed that hypercholesterolemia leads to a steady increase in Treg in the spleen [29] and increased expression of IFNγ in CD8+CD28+ T cells in lymph nodes draining the aortic root [30]. It should be noted that higher fatty acids play the key role in the generation and functioning of Th17 and Foxp3+Treg cells [31, 32].
This study showed that, in the pre-experimental period, the parameters of lipid metabolism correlated only with the indices characterizing the TLR expression on the cellular factors of innate immunity (Table 1). We identified strong correlations of the cholesterol level with the TLR1 and TLR8 expression intensity on granulocytes (r = –0.86 and r = 0.79, respectively), the triglyceride level with the TLR2 expression intensity on granulocytes (r = –0.79), and the phospholipid level with the TLR3 and TLR4 expression intensity on monocytes (r = 0.90 and r = ‒0.90, respectively). On the 21st day of DI, the influence of these lipid metabolism indices on the indices of the TLR system of innate immunity cells was retained. This fact seems quite natural, because adaptation to stress factors, along with the activation of TLRs by endogenous ligands (allarmins), can also be accompanied by the ligand-independent TLR activation with the involvement of lipids (cholesterol [33] and exogenous and endogenous fatty acids [34]). The observation indicating that, at the final stage of long-term immersion exposure, an increase in the serum cholesterol concentration had a positive effect on the state of the T-cell component of adaptive immunity is of interest. This is evidenced, in particular, by the strong correlation between the level of cholesterol and the absolute content of T cells with the CD8+ phenotype (r = 0.81) (Table 1).
It was shown that glucose, products of its metabolism, and enzymes involved in glycolysis can perform a signaling function, thereby affecting metabolism and expression regulation in activated T cells. It is known that glucose is the main energy source that provides T cells with ATP, substrates, and NADPH reducing equivalents, which are required for biosynthesis [35]. For example, glucose intermediates can be involved in the pentose phosphate pathway (glucose 6-phosphate), the serine pathway (3-phosphoglycerate), and the fatty acid synthesis (acetyl-CoA) and serve as precursors for the formation of nucleotides, proteins, and lipids [36]. The glucose-initiated signaling pathway leading to inhibition of glycogen synthase kinase-3 (GSK-3) [37] prevents cell death by stabilizing the antiapoptotic protein Mcl-1 of the Bcl-2 family [38]. Impaired glucose uptake negatively affects many aspects of the T-cell functioning, including changes in both proliferation and cytokine production [39, 40].
Data on the role of carbohydrate metabolism in the innate immunity functioning are fairly scarce and contradictory. It is known from the literature that a number of neutrophil functions such as mobilization ability, phagocytosis, production of superoxide anion radicals, and formation of neutrophil extracellular traps, are inhibited during hyperglycemia. Elevated blood glucose levels affect the expression of Toll-like receptors on cellular factors of innate immunity, promoting their decrease [41, 42]. However, the results of the studies of the effect of various glucose concentrations on the cytokine-producing ability indicate both a negative [43] and a positive [41] correlation between the blood glucose concentration and the ability of immune cells to produce cytokines.
Table 1 shows that, in the baseline period, there were no statistically significant correlations between the blood glucose content and almost all studied immunological indices characterizing the TLR system of innate immunity and the cellular factors of adaptive immunity. Only one strong correlation of the glucose level with the intensity of TLR2 expression on peripheral blood granulocytes was revealed (r = –0.74). A different picture was observed on the 21st day of DI exposure: the glucose concentration was directly correlated with the absolute content of monocytes expressing TLR1 and TLR4 in the peripheral blood (r = 0.69 and r = 0.68, respectively), as well as showed a strong negative correlation with the basal production of cytokines IL-12P70, IL-12P40, and IFNα by CD14+-Mn (r = –0.79, r = –0.84, and r = –0.85, respectively).
Within the framework of complex metabolic networks associated with the immunity functioning, a number of enzymes play the key role in the regulation of immunological reactions. In some cases, these enzymes control the flow along the pathways necessary to meet the specific energy or metabolic needs of the immune response. In other cases, the key enzymes control the concentrations of immunoreactive metabolites that are immediately involved in signaling [44]. There are published data according to which the level of CK in the blood serum reflects the state of the immune response, including the innate and adaptive immune responses [45, 46].
The results of the correlation analysis of biochemical and immunological parameters in the volunteers exposed to a 21-day DI revealed significant correlations between the CPK and CPK-MM levels in blood serum and the intensity of TLR9 expression on monocytes (r = –0.90 and r = –0.94, respectively), as well as the absolute content of CD19+ cells (r = 0.79), CD3+ cells (r = 0.87 and r = 0.88, respectively), and CD3+CD8+ cells (r = 0.97 and r = 0.95, respectively) in the peripheral blood before the experiment. After a 3-week exposure to DI, the character and number of correlations changed significantly (Table 1). For example, the correlations between the CPK and CPK-MM levels with the intensity of TLR9 expression on monocytes disappeared. At the same time, correlations between the CPK and CPK-MM levels and the absolute content of monocytes expressing TLR1 (r = –0.91 and r = –0.90, respectively), TLR2 (r = –0.70 and r = –0.65, respectively), TLR4 (r = –0.78 and r = –0.73, respectively) in the peripheral blood were found. In addition, during this period, a statistically significant correlation of CPK and CPK-MM with the basal production of cytokines IL-12P70 (r = 0.81), IL-12P40 (r = 0.84), and IFNα (r = 0.85) by CD14+-Mn in the volunteers was found. On the 21st day of exposure to DI, a negative correlation between the serum level of CPK and the absolute content of CD3+ lymphocytes (r = –0.66) and CD3+ lymphocytes with the CD4+CD45R0+ phenotype (r = –0.64) in the peripheral blood was observed. A peculiar character of the correlations between CPK and CPK-MM with the basal production of cytokines by T cells was noted: a positive correlation with IFNγ and TNFα (r = 0.90) but a negative correlation with IL-13 (r = –0.90).
If the energy balance is disturbed (e.g., in the case of glucose deficiency in the blood or tissues), the function of a reserve energy substrate that makes it possible to provide most organs with additional energy can be performed by β-hydroxybutyrate (β-HB). The observations indicate that β-HB can inhibit the activation of the NLRP3 inflammasome [47], which is an important sensor of innate immunity and is activated by a wide range of signals of pathogenic, endogenous, and environmental origin [48]. In this regard, an important result of the study is the finding of strong positive correlations of the level β-hydroxybutyrate with mean fluorescence intensity (MFI) of TLR1 on monocytes of peripheral blood and the basal production of IL-12P70 and IFNα by CD14+ cells on the 21st day of exposure to DI (Table 1). It can be assumed that, under long-term immersion exposure, the cellular factors of innate immunity use ketones as an energy substrate. However, it should be noted that, at the final stage of the experimental exposure, negative correlations between the level of β-hydroxybutyrate in blood serum and the TLR8 expression on monocytes and granulocytes were observed.
It is known that calcium plays an important role in ensuring the efficiency of functioning of the immune system. It was established that the level of Ca2+ in the cytoplasm and organelles of T cells significantly affects their metabolism, proliferation, and differentiation, as well as the secretion of antibodies and cytokines by them [49]. The main sources of Ca2+ influx into T cells after antigen receptor stimulation are calcium channels (CRACs). Moderate inhibition of the Ca2+ influx through these channels was shown to inhibit Th1 and Th17 cell functions, whereas follicular T helper cells (Tfh), regulatory T cells (Treg), and CD8+ T cells remain relatively resistant to inhibition of CRAC function [50]. We analyzed the correlations between the calcium content in the blood serum of the subjects and the studied parameters of the immune status during their long-term exposure to DI. It was found that, in the baseline period, the calcium concentration in the blood serum showed a statistically significant negative correlation with the indices characterizing the level of innate and adaptive immunity cells in the peripheral blood (the content of monocytes expressing TLR4 and T cells with the CD4+CD45RO+ phenotype). On the 21st day of the experiment, the calcium concentration in the blood serum was significantly negatively correlated with the indices reflecting the functional state of immunocompetent cells (the intensity of TLR4 expression on granulocytes and the basal production of IL-12P40 by CD14+ monocytes and IL-10 by CD3+ lymphocytes). However, by the end of the 21-day immersion exposure, the total calcium concentration in blood showed a strong positive correlation with the number of TLR4-expressing granulocytes and the intensity of TLR8 expression on granulocytes.
CONCLUSIONS
The results presented in this paper show that the exposure of a healthy person to dry immersion for 21 days is associated with the appearance of changes in the correlations of a number of biochemical and immunological parameters. The peculiarities of the metabolic bases of the functioning of the innate and adaptive components of the immune system during long-term limitation of motor activity are worth noting. They manifest themselves as an increase in the number of positive and negative correlations of the levels of total protein, albumin, carbohydrate and lipid substrates, and metabolites primarily with the indices characterizing the state of the system of signal pattern-recognition receptors of the TLR family of the innate immunity cells rather than with the indices characterizing the state of the T-cell component of the adaptive immunity. Further accumulation of data on the metabolic regulation of the immune response, their systematization, and careful analysis will expand the understanding of adaptive processes in the human body under exposure to extreme environmental factors. This will contribute to the development of tools based on a simultaneous effect on metabolic and immunological processes in the body for the prevention and correction of health disorders not only in space mission crew members but also in people living under adverse environmental conditions.
REFERENCES
Tomilovskaya, E.S., Rukavishnikov, I.V., Amirova, L.E., et al., 21-day dry immersion: design and primary results, Aviakosm. Ekol. Med., 2020, vol. 54, no. 4, p. 5.
Navasiolava, N.M., Custaud, M.A., Tomilovskaya, E.S., et al., Long-term dry immersion: review and prospects, Eur. J. Appl. Physiol., 2011, vol. 111, no. 7, p. 1235.
Markin, A.A., Zhuravleva, O.A., Kuzichkin, D.S., et al., Investigation of metabolic reactions in human subjects during 21-day dry immersion, Aviakosm. Ekol. Med., 2020, vol. 54, no. 4, p. 88.
Berendeeva, T.A., Rykova, M.P., Antropova, E.N., et al., State of the immune system after seven-day “dry” immersion in human, Hum. Physiol., 2011, vol. 37, no. 7, p. 840.
Ponomarev, S.A., Rykova, M.P., Antropova, E.N., et al., Human innate immunity under the conditions of five-day dry immersion, Hum. Physiol., 2013, vol. 39, no. 7, p. 780.
Hotamisligil, G.S., Foundations of immunometabolism and implications for metabolic health and disease, Immunity, 2017, vol. 47, no. 3, p. 406.
Zmora, N., Bashiardes, S., Levy, M., and Elinav, E., The role of the immune system in metabolic health and disease, Cell Metab., 2017, vol. 25, no. 3, p. 506.
Ponomarev, S.A., Shulguina, S.M., Kalinin, S.A., et al., State of the system of recognition and signaling toll-like receptors of human monocytes and granulocytes during 21-day dry immersion without countermeasures, Aviakosm. Ekol. Med., 2019, vol. 53, no. 2, p. 36.
Ponomarev, S.A., Rykova, M.P., Antropova, E.N., et al., Cytokine profile in volunteers during a 21-day dry immersion without countermeasures, Hum. Physiol., 2020, vol. 46, no. 2, p. 175. https://doi.org/10.1134/S0362119720020139
Kutko, O.V., Rykova, M.P., Antropova, E.N., et al., Effect of 21-day dry immersion on the production of T-lymphocytes cytokines involved in the regulation of bone metabolism, Hum. Physiol., 2020, vol. 46, no. 7, p. 787. https://doi.org/10.1134/S0362119720070099
Romantsova, T.I. and Sych, Yu.P., Immunometabolism and metainflammation in obesity, Ozhirenie Metab., 2019, vol. 16, no. 4, p. 3.
Grzhibovskij, A.M., Statistics application in therapy: critical analysis of the publication, Byull. Smolensk. Gos. Med. Univ., 2000. №. 2, p. 22.
Gorban, A.N., Karlin, I.V., Ilg, P., and Ottinger, H.C., Corrections and enhancements of quasi-equilibrium states, J. Non-Newtonian Fluid Mech., 2001, vol. 96, nos. 1—2, p. 203.
Zemskov, A.M., Zemskov, V.M., Vornovsky, V.A., and Novikova, L.A., Biochemical constituent of immunopathology, Immunopathol., Allergol., Infectol., 2000, no. 4, p. 37.
Vologzahanin, D.A., Kalinina, N.M., Sosyukin, A.E., et al., Metabolic basis of immune deficiency in trauma, Ross. Biomed. Zh., 2005, vol. 6, p. 597.
Vinnik, Y.S., Cherdancev, D.V., Markelova, N.M., et al., The role of cytokines and nitroxidergic system in development of immunological disturbances during pancreanecrosis, Sib. Med. J. (Irkutsk), 2004, vol. 45, no. 4, p. 18.
Aubin, E., Roberge, C., Lemieux, R., and Bazin, R., Immunomodulatory effects of therapeutic preparations of human albumin, Vox Sang., 2011, vol. 101, no. 2, p. 131.
Chen, Z., Shao, Y., Yao, H., et al., Preoperative albumin to globulin ratio predicts survival in clear cell renal cell carcinoma patients, Oncotarget, 2017, vol. 8, no. 29, p. 48291.
Casulleras, M., Flores-Costa, R., Duran-Güell, M., et al., Albumin internalizes and inhibits endosomal TLR signaling in leukocytes from patients with decompensated cirrhosis, Sci. Transl. Med., 2020, vol. 12, no. 566, p. eaax5135.
Lee, J.Y., Ye, J., Gao, Z., et al., Reciprocal modulation of toll-like receptor-4 signaling pathways involving MyD88 and phosphatidylinositol 3-kinase/AKT by saturated and polyunsaturated fatty acids, J. Biol. Chem., 2003, vol. 278, no. 39, p. 37041.
Zhu, Y.J., Wang, C., Song, G., et al., Toll-like receptor-2 and -4 are associated with hyperlipidemia, Mol. Med. Rep., 2015, vol. 12, no. 6, p. 8241.
Lee, J.Y., Zhao, L., Youn, H.S., Weatherill, A.R., et al., Saturated fatty acid activates but polyunsaturated fatty acid inhibits toll-like receptor 2 dimerized with toll-like receptor 6 or 1, J. Biol. Chem., 2004, vol. 279, no. 17, p. 16971.
Moreno, L.A., Sarria, A., Lazaro, A., et al., Lymphocyte T subset counts in children with hypercholesterolemia receiving dietary therapy, Ann. Nutr. Metab., 1998, vol. 42, no. 5, p. 261.
Muldoon, M.F., Marsland, A., Flory, J.D., et al., Immune system differences in men with hypo-or hypercholesterolemia, Clin. Immunol. Immunopathol., 1997, vol. 84, no. 2, p. 145.
Fessler, M.B., The intracellular cholesterol landscape: dynamic integrator of the immune response, Trends Immunol., 2016, vol. 37, no. 12, p. 819.
Kidani, Y., Elsaesser, H., Hock, M.B., et al., Sterol regulatory element-binding proteins are essential for the metabolic programming of effector T cells and adaptive immunity, Nat. Immunol., 2013, vol. 14, no. 5, p. 489.
Robertson, A.K., Zhou, X., Strandvik, B., and Hansson, G.K., Severe hypercholesterolaemia leads to strong th2 responses to an exogenous antigen, Scand. J. Immunol., 2004, vol. 59, no. 3, p. 285.
Shamshiev, A.T., Ampenberger, F., Ernst, B., et al., Dyslipidemia inhibits toll-like receptor-induced activation of CD8alpha-negative dendritic cells and protective th1 type immunity, J. Exp. Med., 2007, vol. 204, no. 2, p. 441.
Maganto-Garcia, E., Tarrio, M.L., Grabie, N., et al., Dynamic changes in regulatory t cells are linked to levels of diet-induced hypercholesterolemia, Circulation, 2011, vol. 124, no. 2, p. 185.
Kolbus, D., Ramos, O.H., Berg, K.E., et al., CD8+ T cell activation predominate early immune responses to hypercholesterolemia in Apoe-/-mice, BMC Immunol., 2010, vol. 11, no. 1, p. 1.
Cluxton, D., Petrasca, A., Moran, B., and Fletcher, J.M., Differential regulation of human Treg and Th17 cells by fatty acid synthesis and glycolysis, Front. Immunol., 2019, vol. 10, p. 115.
Lochner, M., Berod, L., and Sparwasser, T., Fatty acid metabolism in the regulation of T cell function, Trends Immunol., 2015, vol. 36, no. 2, p. 81.
Varshney, P., Yadav, V., and Saini, N., Lipid rafts in immune signalling: current progress and future perspective, Immunology, 2016, vol. 149, no. 1, p. 13.
Glass, C.K. and Olefsky, J.M., Inflammation and lipid signaling in the etiology of insulin resistance, Cell Metab., 2012, vol. 15, no. 5, p. 635.
Palmer, C.S., Ostrowski, M., Balderson, B., et al., Glucose metabolism regulates T cell activation, differentiation, and functions, Front. Immunol., 2015, vol. 6, p. 1.
Zubatkina, O.V., T-cell metabolic reprogramming, Vestn. Ural. Med. Akad. Nauki, 2019, vol. 16, no. 3, p. 365.
Hermida, M.A., Dinesh, K.J., and Leslie, N.R., GSK3 and its integration with the PI3K/Act/mTOR signaling network, Adv. Biol. Regul., 2017, vol. 65, p. 5.
Carrington, E.M., Tarlinton, D.M., and Gray, D.H., The life and death of immune cell types: the role of BCL-2 antiapoptotic molecules, Immunol. Cell Biol., 2017, vol. 95, no. 10, p. 870.
Fox, C.J., Hammerman, P.S., and Thompson, C.B., Fuel feeds function: energy metabolism and the T-cell response, Nat. Rev. Immunol., 2005, vol. 5, no. 11, p. 844.
Michalek, R.D. and Rathmell, J.C., The metabolic life and times of a T-cell, Immunol. Rev., 2010, vol. 236, p. 190.
Jafar, N., Edriss, H., and Nugent, K., The effect of short-term hyperglycemia on the innate immune system, Am. J. Med. Sci., 2016, vol. 351, no. 2, p. 201.
Dasu, M.R., Park, S., Devaraj, S., and Jialal, I., Pioglitazone inhibits toll-like receptor expression and activity in human monocytes and db/db mice, Endocrinology, 2009, vol. 150, no. 8, p. 3457.
Wijsman, C.A., Mooijaart, S.P., Westendorp, R.G., and Maier, A.B., Responsiveness of the innate immune system and glucose concentrations in the oldest old, Age (Dordrecht), 2012, vol. 34, no. 4, p. 983.
Godfrey, W.H. and Kornberg, M.D., The role of metabolic enzymes in the regulation of inflammation, Metabolites, 2020, vol. 10, no. 11, p. 426.
Khan, H.A., Alhomida, A.S., Sobki, S.H., et al., Blood cell counts and their correlation with creatine kinase and C-reactive protein in patients with acute myocardial infarction, Int. J. Clin. Exp. Med., 2012, vol. 5, no. 1, p. 50.
Wang, J.J., Hu, Z., and Chen, J.Y., Characteristics of abnormal serum creatine kinase-MB levels in children with COVID-19, World J. Pediatr., 2021, vol. 17, no. 3, p. 326.
Goldberg, E.L., Asher, J.L., Molony, R.D., et al., β‑Hydroxybutyrate deactivates neutrophil NLRP3 inflammasome to relieve gout flares, Cell Rep., 2017, vol. 18, no. 9, p. 2077.
Sutterwala, F.S., Haasken, S., and Cassel, S.L., Mechanism of NLRP3 inflammasome activation, Ann. N.Y. Acad. Sci., 2014, vol. 1319, no. 1, p. 82.
Trebak, M. and Kinet, J.-P., Calcium signaling in T cells, Nat. Rev. Immunol., 2019, vol. 19, no. 3, p. 154.
Vaeth, M., Kahlfuss, S., and Feske, S., CRAC channels and calcium signaling in T cell-mediated immunity// Trends Immunol., 2020, vol. 41, no. 10, p. 878.
Funding
This study was performed within the framework of the Basic Research Program of the Russian Academy of Sciences (theme no. 65.1) and was supported by the Russian Science Foundation (project no. 18-75-10 086-P).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest. The authors declare the absence of obvious and potential conflicts of interest related to the publication of this article.
Statement of compliance with standards of research involving humans as subjects. All studies were carried out in accordance with the principles of biomedical ethics formulated in the Declaration of Helsinki of 1964 and its subsequent updates. The research program was approved at the meeting of the Academic Council (protocol no. 6 dated June 27, 2018) and by the Commission on Biomedical Ethics of the Institute of Biomedical Problems of the Russian Academy of Sciences (Moscow) (protocol dated September 30, 2018). Each participant in the study provided a signed voluntary written informed consent after explaining the potential risks and benefits, as well as the nature of the upcoming study.
Additional information
Translated by M. Batrukova
Rights and permissions
About this article
Cite this article
Ponomarev, S.A., Zhuravleva, O.A., Rykova, M.P. et al. Correlations between Biochemical and Immunological Parameters in Volunteers under Conditions of 21-Day “Dry” Immersion. Hum Physiol 48, 724–731 (2022). https://doi.org/10.1134/S0362119722600278
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0362119722600278