Abstract
The article describes the clinical case of severe toxic polyneuropathy due to a confirmed exogenous intoxication with amphetamine in a 52-year-old man. The clinical picture included muscle weakness in several muscle groups (neck, back, arms, legs, and respiratory muscles), respiratory failure (requiring mechanical ventilation), rhabdomyolysis, mild nephropathy, and liver cytolysis. Differential diagnosis was made between myasthenia gravis, myopathy, and amyotrophic lateral sclerosis. A course of intensive therapy led to almost complete regression of signs and symptoms and improved the electroneuromyographic pattern. An immunochromatographic urine drug screen was performed in view of the rapid partial regression of signs and symptoms and results of a neurophysiological study; the patient tested positive for amphetamine. The clinical case demonstrates rare complications of amphetamine use, which should be kept in mind during a diagnostic search.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Severe toxic polyneuropathies due to narcotic abuse is a pressing problem of neurology and neurological resuscitation because of the prevalence of narcotic addiction. Narcotic addiction is a severe chronic disease due to abuse of various narcotic substances. Mental and physical dependence on the narcotic substance develops in the course of the disease. The incidence of mental and behavioral disorders due to narcotic abuse varying in periodicity is 372.3 per 100 000 persons according to data of 2015 from the Institute of Addiction Medicine [1]. Chronic intoxication damages the central (CNS) and peripheral nervous systems and visceral organs. The mean survival from the start of continuous narcotic abuse is 3–7 years [2]. Here we report a clinical case of severe toxic polyneuropathy with the development of respiratory failure and rhabdomyolysis due to exogenous amphetamine intoxication.
CLINICAL CASE
Patient Ch., 52, was admitted for emergency care to the Department of Anesthesiology and Resuscitation with Intensive Care Units (DARICU) of the Neurology Research Center in February 2017.
Life history. The patient considered himself healthy until the event. A diet was recommended because of overweight (154 kg) and a higher blood pressure (BP) by a primary care doctor. Narcotic abuse was denied. Oxymetazoline nasal drops had been used daily for more than 10 years (based on the drug labeling, cross reactions are possible in immunochromatographic urine tests for narcotics, but a cross reaction of oxymetazoline in an amphetamine test was not reported by the drug manufacturer [3, 4]).
Disease history. The patient had strictly followed a carbohydrate-free diet over 2016 and lost 40 kg. General weakness and fatigue were noted in spring 2016. In November, the patient developed weakness in the arms, legs, and back; had difficulty holding the head; and experienced dyspnea on exertion. In January 2017, weakness of neck muscles increased (the patient became unable to hold the head), dyspnea came to occur at rest, the patient could sleep in a sitting position only. The patient was seen by a neurologist in a private clinic, where amyotrophic lateral sclerosis was suspected based on needle electromyography findings; the patient was referred to the Neurology Research Center. The patient visited the Neurology Research Center and was hospitalized to the DARICU because of developing respiratory failure.
Condition at admission. The general condition was extremely serious. The patient was overnourished with a body mass index of 41. The skin was diffusely cyanotic. Breathing was independent and involved axillary muscles, respiration rate 38 breaths per minute. Auscultation showed vesicular breath sounds diminished in lower segments bilaterally; SpO2 86%; BP 130/80 mm Hg; HR 106 bpm. Oliguria; the urine was meat slops in color. The other organ systems were unremarkable.
Neurological status. The patient was fully conscious, emotionally labile, and anxious. Meningeal syndrome was absent. Cranial nerves were unremarkable. The muscle strength in the neck was low (a score of 2, the patient was unable to independently hold his head in a vertical position). Back muscles were weak (external assistance was required for sitting). The arms and legs were mildly paretic, mostly in distal regions. The vital capacity of the lungs was 32.7% of normal. Hypotrophy was observed for the thenar, hypothenar, and interosseous muscles of both hands, being greater in the left hand, and the shoulder girdle. Tendon and periosteal reflexes were lower in the arms and brisk in the legs. Fasciculations were widespread. Sensory defects were not detected. The patient could not walk independently because of pronounced general weakness.
Additional tests were performed on days 1 and 2 of hospitalization.
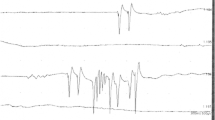
Electroneuromyography showed signs of generalized sensorimotor axonal neuropathy accompanied by ongoing denervation. Convincing signs of neuronal damage were not detected.
Standard repetitive nerve stimulation testing did not show signs of distorted neuromuscular transmission.
MRI of the neck segment of the vertebral column and spinal cord showed initial signs of osteoporosis.
Acid–base and gas composition of capillary blood (while breathing independently): pH 7.38, pCO2 68.6 mm Hg, pO2 72.7 mm Hg, FShunt 14.8%, cHCO3–(P) 39.4 mmol/L, ABE 10.9 mmol/L, SBE 13.8 mmol/L.
Blood count: hemoglobin 137 g/L, red blood cells 4.8 × 1012/L, color index 0.85, platelets 120 × 109/L, white blood cells 10 × 109/L, stab neutrophils 5%, segmented neutrophils 73%, lymphocytes 14%, eosinophils 2%, monocytes 6%, ESR 5 mm/h.
Blood test: bilirubin 20.57 μmol/L, glucose 4.8 mmol/L, creatinine 82 μmol/L, urea 9.04 mmol/L, alanine aminotransferase 54 units/L; aspartate aminotransferase 59 units/L, triglycerides 0.87 mmol/L, LDL cholesterol 1.01 mmol/L, HDL cholesterol 1.59 mmol/L, cholesterol 3.2 mM, atherogenicity coefficient 1.01, total protein 61 g/L, albumin 37 g/L, total creatine kinase 542 units/L (<195 units/L normally).
Antibodies to muscle-specific tyrosine kinase: 0.3 units/mL (0–0.4 units/mL normally).
Urinalysis: specific gravity 1020, deep yellow in color, not fully clear, protein 0.136 g/L, sugar absent, epithelial cells absent, red blood cells covered all fields of vision.
Urinary myoglobin: 5 ng/mL (<1 ng/mL normally).
ECG showed single supraventricular extrasystoles.
Esophagogastroduodenoscopy revealed an acute ulcer of the duodenal bulb with signs of past bleeding.
Chest computer tomography revealed bilateral low-lobe pneumonia.
The results of other tests (blood electrolyte composition; cerebrospinal fluid tests; blood antibodies against tick-borne encephalitis virus, West Nile virus, and Lyme borelliosis; total and free urinary carnitine; and porphobilinogen) were within the respective normal ranges.
Physical therapy evaluation revealed hypertension grade 2, cardiovascular complication risk grade 3, urinary stone disease, fatty hepatosis, and acute ulcer of the duodenal bulb.
Immunochromatographic test for narcotics in the urine was done on day 7 of hospitalization and revealed amphetamine traces.
Treatment included infusion, antibacterial, gastroprotective, bronchopulmonary, and symptomatic therapies; mechanical ventilation; nasogastric tube feeding; massage; and therapeutic exercise.
On admission to the DARICU, nasotracheal intubation was done and mechanical ventilation started because the patient was in a life-threatening condition and showed clinical and laboratory signs of respiratory failure. Positive changes in neurological and somatic status were observed during treatment on day 4 at the DARICU. Efferent treatments (plasmapheresis in particular) commonly used in toxic polyneurophathy were not administered because of the contraindications. In spite of this, muscle strength was fully restored in the neck, back, arms, and legs during therapy. By day 4 of hospitalization to the DARICU, the patient became capable of independently sitting on his bed and holding his head in a vertical position for a long time. The vital capacity of the lungs increased to 50%. Fasciculations decreased, rhabdomyolysis stopped (blood creatine kinase returned to normal, and myoglobin became undetectable in the urine), and nephropathy regressed. Electroneuromyography showed positive changes, including lower ongoing denervation activity and motor unit activity. The duodenal bulbar ulcer healed by day 7 at the DARICU; weaning was started on day 8; extubation was carried out on day 12; inflammatory changes in the lungs regressed by day 20. The patient was transferred to a clinical department of the Neurology Research Center to continue rehabilitation and then discharged to be followed up by a neurologist at the place of residence.
Various tests were performed to exclude primarily myasthenia, amyotrophic lateral sclerosis, and myopathies. Because axonal polyneuropathy and rhabdomyolysis with consequent nephropathy were observed, neurological signs quickly regressed in spite of the lack of pathogenetic therapy, while psychoemotional alterations increased (the patient was anxious, had a fear of death, was negative, and had paranoid thoughts), an immunochromatographic urine test for narcotic substances was performed and showed amphetamine traces, although the patient denied narcotic use.
Thus, exogenous intoxication with amphetamine was the most likely cause of axonal polyneuropathy, rhabdomyolysis with mild nephropathy, and severe respiratory failure partly due to bilateral lower-lobe pneumonia in the patient.
DISCUSSION
The above clinical case shows the amphetamine abuse complications that have been described in the literature, but rarely occur together: axonal polyneuropathy, respiratory failure, rhabdomyolysis, nephropathy, and liver cytolysis.
Amphetamine is a synthetic narcotic substance that stimulates the CNS and cause mental dependence. There are data that amphetamine was synthesized at Berlin University in 1877, but American chemist and pharmacologist G. Alles is thought to be the first to synthesize the substance in many works [5]. Its synthesis received little attention at that time. Discussion of amphetamine began when the pharmaceutical company Smith, Kline, and French Company started producing a Benzedrine nasal inhaler in 1932. The inhaler was designed to treat bronchial asthma and rhinitis and contained 250 mg of racemic methamphetamine [6]. Amphetamine use became widespread during World War II because troops were supplied with amphetamine tablets to boost morale in Germany and Japan. In the 1950s and 1960s, received broad application in the United States and Europe. Amphetamine was among the drugs that doctors most commonly prescribed as a psychostimulant and a means to reduce weight. Amphetamine was administered to treat asthma, rhinitis, narcolepsy, high somnolence, depression, and pediatric attention deficit syndrome [7, 8]. Amphetamine was used to improve the recovery of motor functions after stroke [6, 9]. Although single cases of psychoses were observed in patients on amphetamine as early as the 1930s, British psychiatrist Connell [10, 11] was the first to associate psychosis with amphetamine in 1958. Now amphetamine is prohibited for use in Russia according to the RF Regulation no. 486 “On Amendments to Certain Governmental Acts of the Russian Federation Concerning Circulation of Narcotic Drugs, Psychotropic Substances, and Their Precursors” dated June 30, 2010.
Amphetamine use cause a variable complex of motor, sensory, and vegetative defects; respiratory failure; and behavioral changes [12]. Its clinical effects are due to stimulation of central and peripheral adrenergic receptors. Main side effects most broadly described for amphetamine in the literature usually occur after intravenous administration of high doses and are related to its stimulatory effect on the CNS (decreased somnolence, higher physical activity, hyperthermia, lower appetite and rapid weight loss, stroke, and cerebral vasculitis) and sympathetic effect on the cardiovascular system (tachycardia, arrhythmia, elevated BP, myocardial ischemia, cardiomyopathy, myocarditis, and sudden death). It is known that a direct stimulatory effect of amphetamine on the CNS may cause neurogenic lung edema and that high amphetamine doses inhibit the respiration center.
Long-term amphetamine use causes changes to the dopaminergic system of the brain. Emotional, cognitive, and motor disorders develop as a result. Depression, aggressive episodes, hallucinations, and paranoid behavior are characteristic signs. Risk of Parkinson’s disease and parkinsonism in amphetamine users is almost three times higher than in healthy people [13, 14].
Axonal polyneuropathy, respiratory failure, kidney failure, rhabdomyolysis, and cytolysis have already been reported in the literature. However, the reports are few.
The pathogenesis of polyneuropathy in amphetamine users is still poorly understood. Damage to nerves is thought to result not only from the toxic effect of the drug, but also from the metabolic changes that develop in the body. There is an opinion that mononeuropathy or polyneuropathy are complications of amphetamine-induced angiitis [15, 16]. Impurity of amphetamine synthesized illegally is also likely to contribute to polyneuropathy.
Large-scale multicenter studies have not been performed to demonstrate a direct effect of amphetamine and its derivative on lung tissue. Chest pain and dyspnea are the most common complaints in methamphetamine abuse by smoking, but the actual incidence of respiratory complaints is actually unknown for smoking and other routes of methamphetamine administration. Kidney failure usually develops as a result of rhabdomyolysis due to malignant hyperthermia [6].
Our observation illustrates the multiplicity of clinical signs of amphetamine use. Although narcotic abuse was denied by the patient, long-term amphetamine use at low doses was most likely responsible for the patient’s clinical picture without signs of acute intoxication. Trace, rather than higher, amphetamine concentrations were detected in the patient’s urine because the test was performed rather late. Amphetamine withdrawal led to incomplete regression of the signs, as characteristic of exogenous intoxication.
Amphetamine polyneuropathy with respiratory failure and rhabdomyolysis has not been described in the literature in detail. Multicenter studies of the condition are lacking, and pathogeneitc aspects of toxic polyneuropathy should be better understood to improve its clinical outcome. However, possible complications of amphetamine use are necessary to know for each doctor who treats patients with neurology disorders.
REFERENCES
The Main Indicators of the Narcological Service in the Russian Federation in 2014—2015: Statistical Sourcebook, Moscow, 2016.
Fedorov, G.V., Counteraction to illegal circulation of narcotic drugs, psychotropic substances and their precursors among minors and youth, Vestn. Polotsk. Gos. Univ., Ser. D: Ekonomic. Yuridic. Nauki, 2009, no. 10, p. 211.
Shanin, I.A., Khan, O.Yu., and Petukhov, A.E., Detection of amphetamines in urine using immunochromatographic test strips, Sud.-Med. Ekspert., 2012, vol. 55, no. 4, p. 33.
Beck, O., Kraft, M. Moeller, M.R., et al., Frontline immunochromatographic device for on-site urine testing of amphetamines: laboratory validation using authentic specimens, Ann. Clin. Biochem., 2000, vol. 37, no. 2, p. 199. https://doi.org/10.1258/0004563001899005
Connell, P.H., Clinical manifestations and treatment of amphetamine type of dependence, JAMA, 1966, vol. 196, p. 718.
Albertson, T.E., Walby, W.F., and Derlet, R.W., Stimulant-induced pulmonary toxicity, Chest, 1995, vol. 108, p. 1140.
Pothos, E.N., Creese, I., and Hoebell, B.G., Restricted eating with weight loss selectively decreases extracellular dopamine in the nucleus accumbens and alters dopamine response to amphetamine, morphine, and food intake, J. Neurosci., 1995, vol. 75, no. 10, p. 6640.
Koren’, E.V. and Kupriyanova, T.A., Hyperkinetic Disorders (ADHD): Clinical Recommendations, Moscow; 2015.
Crisostomo, E.A., Duncan, P.W., Propst, M., et al., Evidence that amphetamine with physical therapy promotes recovery of motor function in stroke patients, Ann. Neurol., 1988. https://doi.org/10.1002/ana.410230117
Connell, P.H., Amphetamine psychosis, Br. Med. J., 1957, vol. 1, p. 582.
Parkes, J.D. and Fenton, G.W., Levo(–) amphetamine and dextro(+) amphetamine in the treatment of narcolepsy, J. Neurol. Neurosurg. Psychiatry, 1973, vol. 36, p. 1076.
Albertson, T.E., Derlet, R.W., and Van Hoozen, B.E., Methamphetamine and the expanding complications of amphetamines, West. J. Med., 1999, vol. 170, p. 214.
Garwood, E.R., Bekele, W., McCulloch, C.E., and Christine, C.W., Amphetamine exposure is elevated in Parkinson’s disease, Neurotoxicology, 2006, vol. 27, p. 1003. https://doi.org/10.1016/j.neuro.2006.03.015
Curtin, K., Fleckenstein, A.E., Robison, R.J., et al., Methamphetamine/amphetamine abuse and risk of Parkinson’s disease in Utah: a population-based assessment, Drug Alcohol Depend., 2015, vol. 146, p. 30. https://doi.org/10.1016/j.drugalcdep.2014.10.027
Stafford, C.R., Bogdanoff, B.M., Green, L., and Spector, H.B., Mononeuropathy multiplex as a complication of amphetamine angiitis, Neurology, 1975, vol. 25, p. 570.
Citron, B.P., Halpern, M., McCarron, M., et al., Necrotizing angiitis associated with drug abuse, N. Engl. J. Med., 1970, vol. 283, p. 1003. https://doi.org/10.1056/NEJM197011052831901
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interests. The authors declare that they have no conflict of interest.
This article does not contain any studies involving human subjects performed by any of the authors.
Additional information
Translated by T. Tkacheva
Rights and permissions
About this article
Cite this article
Ryabinkina, Y.V., Zakharova, M.N., Polishchuk, R.V. et al. Critical Neurological Conditions: Severe Toxic Polyneuropathy with the Development of Respiratory Failure and Rhabdomyolysis. Hum Physiol 48, 952–955 (2022). https://doi.org/10.1134/S0362119722080114
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0362119722080114