Abstract
The effect of 20-minute transcutaneous electrical spinal cord stimulation (tESCS) on the severity of nonreciprocal and recurrent inhibition of spinal α-motoneurons in humans at rest and during a weak muscular effort was studied. It was found that during the entire time of exposure to tESCS at rest, the nonreciprocal and recurrent inhibition of the α-motoneurons of the synergist muscle (m. soleus) weakened, inverting to nonreciprocal and recurrent facilitation. Nonreciprocal facilitation of the soleus α-motorneurons was maintained throughout the entire after effect and recurrent facilitation was inverted to recurrent inhibition, which increased up to 20 min after the end of stimulation. The retention of a weak muscular effort during spinal cord stimulation was accompanied by an increase in nonreciprocal and recurrent inhibition of α-motoneurons of the synergist muscle. This post-activation effect lasted up to 20 min after electrical stimulation of the spinal cord. The activity of recurrent inhibition was more pronounced during spinal cord stimulation when performing a weak voluntary effort, and the post-activation effect was manifested by similar changes in the severity of recurrent and nonreciprocal inhibition: their enhancement occurred within 10 min and weakening at 20 min after the end of stimulation to background values. The reflex mechanisms of descending supraspinal and ascending peripheral influences on the functional activity of nonreciprocal and recurrent inhibition in the system of lower leg synergistic muscles in humans based on the effects of tESCS are discussed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
The inhibitory systems of the spinal cord play an essential role in human motor activities [1, 2]. The main inhibitory interactions in the antagonist muscle system are presynaptic and reciprocal inhibition. Inhibitory interactions of synergistic muscles of the spinal level are known as nonreciprocal inhibition or Ib inhibition, which is achieved along Ib afferents from the Golgi tendon organs via inhibitory Ib interneurons to the α-motoneurons of the own muscle and/or the synergistic muscle. The functional role of nonreciprocal inhibition is timely protection of the skeletal muscle from its excessive tension and to coordinate the activities of different muscle groups [1–5].
Recurrent postsynaptic inhibition is another inhibitory mechanism at the spinal level in the system of synergistic muscles, which regulates the activity of nonreciprocal inhibition through Renshaw cells [1]. Recurrent inhibition plays the role of negative feedback, thereby limiting the frequency of α-motoneuron discharges and restricting the development of excessive muscular effort [2, 6–9].
In the last decade, a large number of experimental studies of the use of noninvasive transcutaneous electrical spinal cord stimulation (tESCS) have been published [10–13]. tESCS is based on the cutaneous electrical activation of spinal circuitry using electrodes applied to segments of the lower thoracic and/or lumbosacral vertebrae. Its innovative feature is the use of painless stimulation modes [10, 11]. At low spinal cord stimulation intensity, low-threshold afferent fibers are activated and motor axons are also involved in the process to a certain extent. An increase in stimulation intensity involves a greater number of motor axons, which leads to a decrease in the latency of the elicited motor response and an occlusive effect of afferent pathways [10, 11]. These data are consistent with the previous results obtained in experiments using transcutaneous [14] and epidural [15, 16] spinal cord stimulation.
The post-activation effects of 20-min electrical spinal cord stimulation on the manifestation of presynaptic and reciprocal inhibitory interactions in the system of antagonist muscles in healthy subjects have been shown [17]. Previously unknown consistent patterns of the effect of 20-min electrical spinal cord stimulation on increasing muscle strength [13] and modulation of nonreciprocal inhibition of spinal α-motoneurons, which provides optimal functioning in maintaining skeletal muscle tension [18], have been established. At the same time, there is no information in the available literature about the effect of tESCS on the functional activity of inhibitory mechanisms in the system of human synergistic muscles.
The aim of our research was to study the effect of 20-min electrical spinal cord stimulation on the manifestation of nonreciprocal and recurrent inhibition of spinal α-motoneurons in humans and the possible physiological mechanisms of these manifestations.
MATERIALS AND METHODS
The study involved 18 healthy men whose ages varied between 27 and 35 years. tESCS (Neiro-MVP-8 stimulator, Neirosoft, Russia) was performed using an active electrode with a diameter of 2.5 cm at the level of the thoracic T11–T12 vertebrae in the supine position for 20 min [10, 11, 19]. Indifferent rectangular 5 × 10.2 cm2 electrodes were placed bilaterally over the iliac crests. Stimulus intensity during the first 10 min was within 30 mA to subsequently attain 40 mA. The duration of a single stimulus was 0.5 ms; the stimulus repetition rate was 10 Hz [13].
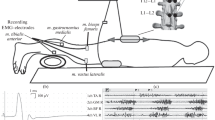
The method for recording nonreciprocal and recurrent inhibition of homonymous spinal cord α-motoneurons. The conditioning (n. common peroneal) and testing stimuli (n. tibialis) were applied to each subject at an interstimulus interval of 6 and 20 ms (Fig. 1). A short-latency conditioning stimulus activates Ib afferents 6 ms before the testing stimulus, thereby repressing the soleus testing H-response and causing functional changes in the activity of nonreciprocal inhibition at the spinal level [20, 21]. A long-latency conditioning stimulus, 20 ms before the test stimulus, activates Renshaw cells via the extending motor axon collaterals of the gastrocnemius medialis and soleus α-motoneurons, which, in turn, decreases the excitability of α-motoneurons of the corresponding muscles [5, 22]. The control H-reflex was used to determine the strength of nonreciprocal and recurrent inhibition, which was calculated with the following formula: Amplitude of the testing H-response/Amplitude of the control H-response × 100. The severity of nonreciprocal and recurrent inhibition was assessed by the highest percentage of repression of the testing H-reflex. The strength of the control and testing stimuli applied to the n. tibialis was 15% of the intensity causing the maximum amplitude of soleus H-response, and the conditioning stimulation applied to the common peroneal nerve constituted 95% of the stimulus value causing the maximum amplitude of gastrocnemius medialis M-response. The H-reflex and M‑response amplitudes were recorded on a mini-electromyograph with the Myo software (ANO IMR Vozvrashchenie, Russia); conditioning stimulation of Ib afferents, efferent fibers and testing stimulation of Ia afferents were performed; the EMG activity of synergistic muscles (m. soleus, m. gastrocnemius medialis) was recorded with surface skin electrodes 9 mm in diameter: the active electrode was fixed in the projection of the muscle motor point; the reference electrode was shifted at a distance of 2 cm to the tendon [2].
A diagram of the methodology for assessing nonreciprocal and recurrent inhibition of homonymous soleus α-motoneurons. Mn GM, m. gastrocnemius medialis motoneurons; Mn SOL, m. soleus motoneurons; In Ib, inhibitory Ib interneurons; RC, inhibitory Renshaw cells; NI, nonreciprocal inhibition; RI, recurrent inhibition.
The method for recording voluntary muscle contraction. During the execution of a weak muscular effort (5% of the maximum voluntary contraction (MVC)), the subjects lay comfortably on their backs; their right foot was rigidly fixed using adjustable straps on a dynamometer platform (Biodex Multi-Joint System Pro-3, United States, 2006). At the beginning of each experiment, the subjects performed the MVC of the lower leg muscles (isometric type of contraction). They were then asked to make a static effort equal to 5% of the MVC and retain it for 20 min. A weak muscle contraction was monitored by the subject visually on a computer monitor. The choice of a weak MVC is explained by the fact that the subjects could retain this muscle tension during tESCS for 20 min.
Experimental conditions. The amplitudes of the soleus testing H-responses (nonreciprocal and recurrent inhibition) were recorded: (1) at rest before exposure to prolonged tESCS, at 5, 10, and 20 min of stimulation, and at 5, 10, and 20 min of electrical aftereffect and (2) when maintaining a weak isometric contraction (5% of MVC) before long-term tESCS, during stimulation, at 5, 10, and 20 min while retaining 5% of MVC, and after stimulation, at 5, 10, and 20 min without retaining 5% of MVC. Control H‑reflexes of the m. soleus were recorded in a state of relative muscle rest.
Statistical data were analyzed using the Statistica v.12.5 Build 192.7 software (StatSoft, United States). Statistically significant differences in the studied parameters were identified using parametric (one-way analysis of variance with post-hoc Newman-Keuls analysis) and nonparametric methods (Kruskal-Wallis Anova analysis of variance). The normal distribution of samples was determined using the Shapiro-Wilk’s W test. The critical value of the level of statistical significance when testing null hypotheses was taken to be 5% (p = 0.05).
RESULTS
The effect of tESCS on the severity of nonreciprocal inhibition of α-motoneurons of synergistic leg muscles in a state of relative muscular rest. A 20-min tESCS at rest facilitates the soleus testing H-response, reducing the severity of nonreciprocal inhibition in relation to the state before the spinal cord stimulation (Table 1). This was manifested by an increase in the soleus testing H‑response amplitude from 5 to 20 min of exposure to electrical stimulation (p = 0.000; Kruscal-Wallis Anova) in relation to the background values. During the 20-min exposure of spinal cord to stimulation, the level of nonreciprocal facilitation remained constant. Nonparametric analysis of variance did not reveal significant differences in the soleus testing H-response amplitudes between the time intervals of exposure to tESCS (p = 1.000; Kruscal-Wallis Anova). The effect of facilitation of the soleus testing H-responses was observed up to 20 min after the end of tESCS in relation to the background (p = 0.000; Kruscal-Wallis Anova).
The effect of tESCS on the severity of nonreciprocal inhibition of α-motoneurons of the synergistic leg muscles while maintaining a weak muscular effort. When the voluntary effort was maintained at 5% of the individual maximum without tESCS, a slight weakening of nonreciprocal inhibition (p = 0.056; Newman-Keuls) was noted compared with the resting state (background) (Table 2). Nonreciprocal inhibition increased at 5 (p = 0.022; Newman-Keuls), 10 (p = 0.021; Newman-Keuls), and 20 (p = 0.015; Newman-Keuls) min under the influence of tESCS in combination with voluntary retention of 5% of MVC. During spinal cord stimulation at 5, 10, and 20 min in combination with voluntary skeletal muscle tension, the severity of nonreciprocal inhibition was constant (p = 1.000; Newman-Keuls). An increase in nonreciprocal inhibition was observed at 5 (p = 0.011; Newman-Keuls) and 10 (p = 0.017; Newman-Keuls) min after the end of the effect of stimulation on the spinal cord; at 20 min its gradual weakening was noted, which reached background values (before spinal cord stimulation (background): p = 0.955; before spinal cord stimulation + 5% of MVC: p = 0.121, Newman-Keuls).
The effect of tESCS on the severity of recurrent inhibition of α-motoneurons of the synergistic lower leg muscles in the state of relative muscular rest. tESCS most significantly influenced the soleus testing H-response amplitude: in this case, the recurrent inhibition of α‑motoneurons was inverted to its facilitation in relation to the background (Table 3; p = 0.000; Newman-Keuls). The method of parametric analysis of variance revealed a statistically significant increase in the recurrent facilitation of m. soleus α-motoneurons at 5 min of spinal cord stimulation compared with 10 (p = 0.038; Newman-Keuls) and 20 min (p = 0.002; Newman-Keuls). Spinal cord stimulation of 20 min duration produced a recurrent inhibitory effect at the spinal level after exposure (Table 3). The increase in recurrent inhibition proceeded up to 20 min of the tESCS aftereffect (p = 0.010; Newman-Keuls).
The effect of tESCS on the severity of recurrent inhibition of α-motoneurons of the synergistic lower leg muscles while maintaining a weak muscular effort. The performance of a voluntary muscular effort of 5% of the individual maximum in the absence of tESCS slightly weakened recurrent inhibition (p = 0.597; Newman-Keuls) compared with the resting state (background) (Table 4). Under the influence of an electrical effect on the spinal cord in combination with a voluntary muscle tension of 5% of MVC, recurrent inhibition was significantly increased at 5 (p = 0.000; Newman-Keuls), 10 (p = 0.000; Newman-Keuls), and 20 (p = 0.000; Newman-Keuls) min. A statistically significant increase in recurrent inhibition was revealed at 5 min of stimulation compared to 20 min (p = 0.000; Newman-Keuls). After end of the stimulation effect on the spinal cord, an increase in the inhibitory effect was noted at 5 (p = 0.011; Newman-Keuls) min followed by a decrease at 20 (p = 0.017; Newman-Keuls) min to the background values (before spinal cord stimulation (background): p = 0.528; before spinal cord stimulation + 5% of MVC: p = 0.836, Newman-Keuls).
Comparative analysis of the effect of tESCS on the manifestation of nonreciprocal and recurrent inhibition of α-motoneurons of the synergistic lower leg muscles in a state of relative muscular rest. The data in Fig. 2 indicate that before spinal cord stimulation the levels of nonreciprocal and recurrent inhibition of soleus α‑motoneurons were constant (p = 0.784; Newman-Keuls). Electrical spinal cord stimulation at 20 min of exposure caused more marked nonreciprocal facilitation compared to recurrent facilitation (p = 0.012; Newman-Keuls). Upon completion of electrical spinal cord stimulation, the recurrent facilitation was inverted to recurrent inhibition from 5 to 20 min of the aftereffect, and nonreciprocal facilitation was constant as in the case of electrical stimulation of the spinal cord. At 20 min (p = 0.011; Newman-Keuls), the aftereffect of electrical stimulation of the spinal cord showed the highest level of recurrent inhibition (Fig. 2).
Comparative analysis of the effect of tESCS on the manifestation of nonreciprocal and recurrent inhibition of α-motoneurons of the synergistic lower leg muscles while maintaining a weak muscular effort. The comparative analysis of the testing H-reflex amplitudes showed that the effect of 20-min electrical spinal cord stimulation was manifested by the intensification of the inhibitory processes of the synergistic lower leg muscles against the background of maintaining a weak muscular effort of 5% of MVC (Fig. 3). As seen from Fig. 3, during stimulation of the spinal cord at 5 (p = 0.013; Newman-Keuls) and 10 (p = 0.012; Newman-Keuls) min, the greatest severity of recurrent inhibition was observed relative to nonreciprocal inhibition. The aftereffect of tESCS at 5 and 10 min increased recurrent and nonreciprocal inhibition and at 20 min decreased their activity to the background values.
The amplitude of the soleus testing H-reflex from the control reflex before transcutaneous electrical spinal cord stimulation (tESCS) at rest (background) and with retention of 5% of MVC, during tESCS in combination with retention of 5% of MVC, and after exposure to tESCS at rest, %. (a) before tESCS; (b) during tESCS in combination with retention of 5% of MVC; (c) after tESCS.
DISCUSSION
The results of the study of the effect of electrical spinal cord stimulation on the functional activity of spinal inhibition in the system of synergistic lower leg muscles in humans showed that during a 20-minute stimulation of the spinal cord in a state of relative muscle rest, the nonreciprocal and recurrent inhibition of α-motoneurons of the synergistic muscle decreased (nonreciprocal and recurrent facilitation) (Tables 1, 3). Nonreciprocal facilitation of the synergistic α-motoneurons persisted for no less than 20 min after electrical spinal cord stimulation, and recurrent facilitation was inverted to recurrent inhibition, which increased up to 20 min of the aftereffect. During a study of the effects of tESCS on the manifestation of presynaptic and reciprocal inhibitory interactions in the system of antagonist muscles in healthy subjects, Yamaguchi et al. [17] found that after 20-min electrical stimulation of the spinal cord, reciprocal inhibition increased within 15 min of the aftereffect, and presynaptic inhibition did not differ from the baseline level for 30 min of the aftereffect. The authors suggest that the long-term effect of noninvasive electrical stimulation on the spinal cord induces short-term plastic changes in inhibitory Ia interneurons of the reciprocal inhibition system [17].
The authors who developed the noninvasive method of tESCS suggest that when electrical stimulation is applied to the spinal cord group Ia and group Ib afferents, group II afferents, excitatory and inhibitory spinal interneurons, which control poly- and oligosynaptic reflexes, as well as the pyramidal, reticulospinal, and sympathetic tracts, are sequentially involved [10, 11]. Based on the statements of these authors, it may be suggested that on exposure to 20-min tESCS (1) at rest, ascending peripheral influences from Ia (2), (3), Ib (4) afferents on α-motoneurons and efferent α-motoneuron axon collaterals (5), as well as excitatory supraspinal inputs (cortico- (6), vestibulo- (7), reticulospinal (8)) on the corresponding motoneurons, are sequentially involved, which leads to an increase in nonreciprocal and recurrent facilitating influences on the motor nuclei of the lower leg synergists (m. soleus and m. gastrocnemius) (Fig. 4).
A putative model of an inhibitory interneuronal circuit of the synergistic lower leg muscles mediated by ascending and descending influences on spinal motoneurons during and after 20-minute transcutaneous electrical spinal cord stimulation (tESCS) in combination with weak muscle tension. 1, tESCS; 2, 3, 4, 5, ascending peripheral influences from Ia and Ib afferents and efferent α-motoneuron axon collaterals; 6, 7, 8, descending supraspinal influences from the cortico-, vestibulo- and reticulospinal tracts; 9, nonreciprocal (Ib) inhibition; 10, recurrent inhibition via the Renshaw cell; 11, Ia presynaptic inhibition; 12, 5% of MVC (maximum voluntary contraction); CST, corticospinal tract; VST, vestibulospinal tract; RST, reticulospinal tract; NI, nonreciprocal inhibition of α-motoneurons; RI, recurrent inhibition of α-motoneurons; PI, presynaptic Ia inhibition.
The results of our own study have shown that under conditions of maintaining an effort of 5% of MVC, nonreciprocal and recurrent inhibition of the soleus α-motoneurons before the action of electrical stimulation on the spinal cord was weaker than in the state of relative muscular rest (Tables 2, 4; Fig. 3). Similar results describing the weakening of nonreciprocal inhibition of soleus α-motoneurons when a moderate static effort was made were presented in [23] by E. Pierrot-Deseilligny et al. [23] and in [24, 25] by A.A. Chelnokov et al. With a moderate static effort, presynaptic inhibition, which actively regulates the excess afferent influx to α-motoneurons of the lower leg agonist and antagonist muscles, disinhibiting nonreciprocal and reciprocal inhibitory influences on them and affording normal human motor activity, is most pronounced [24].
Our own research results indicate that against the background of a 20-min tESCS and a weak muscular effort, recurrent inhibition of soleus α-motoneurons is more pronounced compared with nonreciprocal inhibition, which persisted for 10 min of exposure of the spinal cord to stimulation (Fig. 3). The postactivation effect of a 20-minute tESCS was characterized by an increase in the functional activity of nonreciprocal and recurrent inhibition at 5 and 10 min and by the decline in their manifestation to background values at 20 min. No differences in the strength of these inhibitory processes in the system of synergistic muscles were observed (Fig. 3).
From the currently available data, it is known that corticospinal fibers poly- and oligosynaptically converge onto inhibitory Ia and Ib interneurons, spinal cord Renshaw cells coordinating afferent inputs to the motor centers of homonymous and heteronymous α-motoneurons through the inhibitory spinal systems (presynaptic, reciprocal, nonreciprocal, and recurrent inhibition) [2, 5, 26, 27]. Descending lateral and ventral corticospinal pathways are glutamatergic, which excite α-motoneurons mono- and polysynaptically and γ-motoneurons polysynaptically [28, 29]. However, the lateral vestibulospinal pathways exert polysynaptic facilitating influences on the extensor α‑motoneurons and inhibitory influences on the flexor α-motoneurons of the lower and upper limbs. At the same time, the lateral reticulospinal pathways polysynaptically inhibit the extensor α-motoneurons and facilitate the flexor motoneurons [30]. It is believed that the rubrospinal tract has an excitatory effect on the motor centers of skeletal muscles [31].
The diagram in Fig. 4 suggests that a 20-minute tESCS (1) in combination with a weak static effort (12) and its post-activation effect additionally activates the excitatory corticospinal pathways (6) and peripheral influences of Ib afferents from Golgi receptors (4) and efferent α-motoneuron axon collaterals (5) enhancing the functional activity of inhibitory Ib interneurons of nonreciprocal inhibition (9) and Renshaw cells of recurrent inhibition (10). The manifestation of nonreciprocal inhibition of the lower leg synergist α-motoneurons is regulated by the mechanisms of recurrent inhibition through the Renshaw cell (10) and presynaptic inhibition mediated by Ia afferents to the corresponding interneurons (11) [2, 24, 25, 32] (Fig. 4). It is more likely that against the background of a 20-minute electrical spinal cord stimulation (1) combined with a weak muscular effort (12) and after its action the descending vestibulo- (7) and reticulospinal (8) pathways exert excitatory influences on the motor centers of synergistic muscles affording the coordinated work of all spinal inhibitory systems. This idea agrees with the study of the features of manifestation of presynaptic inhibition of homonymous Ia afferents and reciprocal inhibition of soleus α-motoneurons at rest upon activation of the reticulo- and vestibulospinal pathways in response to transcranial magnetic stimulation of the cerebellum [33].
CONCLUSIONS
The data we obtained bridge the gap in scientific knowledge about the mechanisms of functioning of the spinal inhibitory systems of the synergistic muscles of the lower leg, which are influenced by transcutaneous electrical spinal cord stimulation. tESCS modulates nonreciprocal and recurrent inhibition of spinal α-motoneurons in a state of relative muscular rest and while maintaining a weak muscle effort. Exposure of the spinal cord to electrical stimulation at rest weakens the functional activity of the inhibitory spinal neuronal structures of the synergistic muscles and, in contrast, increases it when a weak muscle tension is maintained, with recurrent inhibition being the most pronounced. The fundamental data from of this kind of research can find practical application in the correction of segmental disorders in patients with neuromotor diseases and injuries.
REFERENCES
Bikmullina, R.Kh., Rozental’, A.N., and Pleshchinskii, I.N., Inhibitory systems of the spinal cord in the control of interactions of functionally coupled muscles, Hum. Physiol., 2007, vol. 33, no. 1, p. 105.
Chelnokov, A.A. and Gorodnichev, R.M., Zakonomernosti formirovaniya spinal’nogo tormozheniya u cheloveka (Pattern of Development of Spinal Inhibition in a Man), Moscow: INFRA-M, 2020.
Hunt, C.C. and Kuffler, S.W., Stretch receptor discharges during muscle contraction, J. Physiol., 1951, vol. 113, p. 298.
Haase, J., Cleveland, S., and Ross, H.G., Problems of postsynaptic autogenous and recurrent inhibition in the mammalian spinal cord, Rev. Physiol. Biochem. Pharmacol., 1975, vol. 73, p. 74.
Pierrot-Deseilligny, E. and Burke, D., The Circuitry of the Human Spinal Cord: Spinal and Corticospinal Mechanisms of Movement, Cambridge: Cambridge Univ. Press, 2012.
Windhorst, U., Muscle proprioceptive feedback and spinal networks, Brain Res. Bull., 2007, vol. 73, nos. 4–6, p. 155.
Kudina, L.P. and Piotkevich, M., Analysis of recurrent inhibition innervating fast muscles in humans, Neiroinformatika, 2006, part 1, p. 137.
Obeidat, A.Z., New insights into the spinal recurrent inhibitory pathway normally and after motoneuron regeneration, PhD Thesis, Dayton, OH: Wright State Univ., 2013.
Barrué-Belou, S., Marque, P., and Duclay, J., Supraspinal control of recurrent inhibition during anisometric contractions, Med. Sci. Sports Exercise, 2019, vol. 51, no. 11, p. 2357.
Gerasimenko, Y., Gorodnichev, R., Machueva, E., et al., Novel and direct access to the human locomotor spinal circuitry, J. Neurosci., 2010, vol. 30, no. 10, p. 3700.
Gorodnichev, R.M., Pivovarova, E.A., Puhov, A., et al., Transcutaneous electrical stimulation of the spinal cord: a noninvasive tool for the activation of stepping pattern generators in humans, Hum. Physiol., 2012, vol. 38, no. 2, p. 158.
Yafarova, G.G., Militskova, A.D., Shul’man, A.A., et al., The effect of transcranial magnetic stimulation on the responses of the leg muscles caused by transcutaneous electrical stimulation of the spinal cord, Prakt. Med., 2017, no. 8 (109), p. 201.
Roshchina, L.V. and Chelnokov, A.A., The effect of transcutaneous electrical spinal cord stimulation on the functional state of the human motor system, Teor. Prakt. Fiz. Kul’t., 2020, no. 4 (982), p. 30.
Minassian, K., Persy, I., Rattay, F., et al., Posterior root-muscle reflexes elicited by transcutaneous stimulation of the human lumbosacral cord, Muscle Nerve, 2007, vol. 35, no. 3, p. 327.
Dimitrijevic, M., Gerasimenko, Yu., and Pinter, M., Evidence for a spinal central pattern generator in humans, Ann. N.Y. Acad. Sci., 1998, vol. 860, p. 360.
Harkema, S., Gerasimenko, Y., Hodes, J., et al., Epidural stimulation of the lumbosacral spinal cord enables voluntary movement, standing, and assisted stepping in a paraplegic human, Lancet, 2011, vol. 377, no. 9781, p. 1938.
Yamaguchi, T., Fujiwara, T., Takahara, T., et al., The effects of transcutaneous spinal cord stimulation on spinal reciprocal inhibition in healthy persons, Clin. Neurophysiol., 2017, vol. 128, no. 3, p. 115.
Roshchina, L.V., Gladchenko, D.A., Pivovarova, E.A., and Chelnokov, A.A., Effect of long-term electrical spinal cord stimulation on expression of non-reciprocal inhibition α-motoneurons of human skeletal muscles, Vestn. Ross. Univ. Druzhby Nar., Ser.: Med., 2019, vol. 23, no. 4, p. 390.
Gerasimenko, Yu., Kozlovskaya, I., and Edgerton, V.R., Sensorimotor regulation of movements: novel strategies for the recovery of mobility, Hum. Physiol., 2016, vol. 42, no. 1, p. 90.
Pierrot-Deseilligny, E., Katz, R., and Morin, C., Evidence for IB inhibition in human subjects, Brain Res., 1979, vol. 166, no. 1, p. 176.
Chelnokov, A.A. and Gorodnichev, R.M., Age-related features in the formation of spinal inhibition of skeletal muscles in males, Hum. Physiol., 2015, vol. 41, no. 6, p. 644.
Rossi, A., Zalaffi, A., and Decchi, B., Heteronymous recurrent inhibition from gastrocnemius muscle to soleus motoneurones in humans, Neurosci. Lett., 1994, vol. 169, nos. 1–2, p. 141.
Pierrot-Deseilligny, E., Morin, C., Bergego, C., and Tankov, N., Pattern of group I fibre projections from ankle flexor and extensor muscle in man, Exp. Brain Res., 1981, vol. 42, nos. 3–4, p. 337.
Chelnokov, A.A. and Buchatskaya, I.N., Functional features spinal inhibition during voluntary motor activity, Teor. Prakt. Fiz. Kul’t., 2015, no. 6, p. 11.
Chelnokov, A.A., Gladchenko, D.A., Fedorov, S.A., and Gorodnichev, R.M., Age-related parameters of spinal inhibition of skeletal muscles in regulation of voluntary movements in men, Hum. Physiol., 2017, vol. 43, no. 1, p. 38.
Knikou, M., The H-reflex as a probe: pathways and pitfalls, J. Neurosci. Methods, 2008, vol. 171, no. 1, p. 1.
Kubota, S., Uehara, K., Morishita, T., et al., Inter-individual variation in reciprocal Ia inhibition is dependent on the descending volleys delivered from corticospinal neurons to Ia interneurons, J. Electromyogr. Kinesiol., 2014, vol. 24, no. 1, p. 46.
Rhoades, R.A. and Bell, D.R., Medical Physiology: Principles for Clinical Medicine, Philadelphia: Lippincott Williams and Wilkins, 2012, 4th ed.
Korolev, A.A., Functional anatomy of descending motor systems in the normal state and during spastic paresis, Fundam. Issled., 2013, no. 3, p. 92.
Chez, C., The control of movement/posture. Voluntary movement, in Principles of Neural Science, New York: McGraw-Hill, 1999, p. 553.
Fujito, Y. and Aoki, M., Monosynaptic rubrospinal projections to distal forelimb motoneurons in the cat, Exp. Brain Res., 1995, vol. 105, no. 2, p. 181.
Rossi, A. and Decchi, B., Changes in Ib heteronymous inhibition to soleus motoneurones during cutaneous and muscle nociceptive stimulation in humans, Brain Res., 1997, vol. 774, p. 55.
Matsugi, A., Mori, N., Uehara, S., et al., Effect of cerebellar transcranial magnetic stimulation on soleus Ia presynaptic and reciprocal inhibition, Neuroreport, 2015, vol. 26, no. 3, p. 139.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All procedures performed in studies involving human participants were in accordance with the biomedical ethics principles formulated in the 1964 Helsinki Declaration and its later amendments and approved by the local bioethical committee of the Velikie Luki State Academy of Physical Education and Sports (Velikie Luki).
Conflict of interests. The authors declare that they have no conflict of interest.
Informed consent. Each study participant provided a signed voluntary written informed consent after explanation of the potential risks and benefits, as well as the nature of the upcoming study, to him.
Additional information
Translated by E. Babchenko
Rights and permissions
About this article
Cite this article
Chelnokov, A.A., Roshchina, L.V., Gladchenko, D.A. et al. The Effect of Transcutaneous Electrical Spinal Cord Stimulation on the Functional Activity of Spinal Inhibition in the System of Synergistic Muscles of the Lower Leg in Humans. Hum Physiol 48, 121–133 (2022). https://doi.org/10.1134/S0362119722020037
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0362119722020037