Abstract
Twelve sambo wrestlers took part in the study. The spatio-temporal organization features of muscle synergies using principle components analysis (PCA) and by evaluating the auto and cross-correlation functions of skeletal muscle electromyograms when different periods of leg-grabbing throw performing was studied. It was shown than the electrical activity of large muscle synergies varies depending on the values of muscle efforts, typical for different periods of the performed movement. Skeletal muscle synergetic relationships are plastic in relation to their spatial and temporal organization, which provides reliable control of motor function in such conditions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
The problem of reducing the redundancy of the degrees of freedom of the neuromuscular apparatus in the control of voluntary motor activity remains an urgent issue of human physiology. One of the physiological mechanisms for solving this problem may consist in the unification of body segments, muscles, and central nervous structures into functional formations - motor synergies [1–3]. N.A. Bernstein enunciated the concept of “extensive muscle synergy,” i.e., the ability to perform highly coordinated movements of the whole body, involving many tens of muscles in coordinated work. The parameters of the coordination structure of motor actions, realized according to this principle, have signs of coordinated control at different levels of the motor system. At the kinematic level, such signs can be a combined change in the articular angles, the gradient of increase or decrease in the speed of movement of individual body segments, at the muscular level, a comparable change in the parameters of electroactivity of a number of skeletal muscles, and at the neuronal level, a change in the discharge patterns of motor neurons of individual motor units [4, 5].
To extract synergies, as a rule, data dimensionality reduction methods are used, such as the method of principal components, non-negative matrix decomposition, and others [6, 7]. These methods allow us to establish the spatial architecture of muscle activation, as well as its temporal pattern. Cross-correlation analysis of electromyograms (EMG) taken from a set of skeletal muscles at the same time allows to establish the degree of inphase of EMG activity and, accordingly, gives a basis for inclusion of a number of skeletal muscles to a functional formation termed a synergy. Cross-correlation analysis has been successfully used to study the processes of irradiation of excitation at the level of the spinal cord during prolonged muscle tension, as well as in individual phases of motor actions that differ in the prevailing mode of muscle contraction [8–10].
As a rule, when studying muscle synergies, locomotion becomes the object of research, while analyzing the synergistic interaction of the muscles of the lower extremities, and when performing voluntary movements, the EMG activity of the muscles of the upper extremities is most often studied. In terms of studying extensive functional synergies, of particular interest is a wide range of voluntary sports movements, which require the manifestation of large muscular efforts and high accuracy of movements. Such information can contribute to a better understanding of the structure of purposeful sports movements and can be used in the process of teaching and improving complex motor skills characteristic of a particular sport. However, insufficient attention is paid to the study of extensive muscle synergies during the performance of such movements. In this regard, the purpose of this work was to study the spatio-temporal organization of muscle synergies in different periods of complex coordination sports movement, differing in the amount of muscle efforts.
METHODS
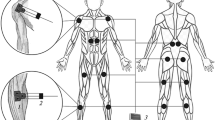
The study involved 12 athletes of high sports qualifications (aged 19–26 years), who go in for sambo wrestling. The research was carried out on the basis of the Research Institute of Sports and Recreational Physical Culture of the Velikie Luki State Academy of Physical Culture and Sports. The subjects performed a series of throws by “grabbing the legs.” The procedure for executing a throw is shown in Fig. 1. Each subject performed 10 throws with an interval of 30 s between them. The task was to execute the throw efficiently and with maximum speed. The analysis included only effective throws made without significant technical violations. The throws were performed without resistance, each wrestler performed throws on an opponent of his weight category. In total, the parameters of 120 performed throws were analyzed.
(a) The main EMG signals characterizing the activity of muscle synergies: a, synergy 1; b, synergy 2; c, synergy 3. Abscissa, time (ms); ordinate, the value of electroactivity (a.u.). The vertical dashed lines show the boundary interval of the periods of movement. (b) Skeletal muscle autocorrelation functions. Abscissa, time (ms); ordinate, the maximum coefficient of the cross-correlation function: a, GM R; b, TRAPS L, c, TRAPI L, d, ES L.
During the execution of movements, EMG was recorded of 16 bilateral superficial muscles of the trunk, upper and lower extremities: m. tibialis anterior (TA), m. gastrocnemius (medial head) (GM), m. rectus femoris (RF), m. biceps femoris (BF), m. trapezius pars superior (TRAPS), m. trapezius pars inferior (TRAPI), m. erector spinae (at the T9 level of the vertebrae) (ES), and m. rectus abdominis (upper part) (RA). These muscles were selected for the study due to the limitations of the number of active channels of the biomonitor used, as well as based on the results of preliminary studies, in which the EMG of a number of muscles not considered in this work was recorded. To solve the problems of this study, we selected those muscles that demonstrated the highest EMG activity when performing a model movement. We used ab IU 6000 wireless 16-channel biomonitor (Mega Electronics, Finland), the EMG sampling rate was 2000 Hz. Bipolar disposable withdrawal electrodes with a diameter of 0.9 cm were used, the distance between the electrodes was 2 mm.
A video sequence of movements was recorded synchronously using the system 3D–Video analysis (Qualisys, Sweden) with a sampling rate of 100 frames per second. Based on the determination of the boundary moments, the obtained variational series were divided into two periods. The first period included movements from the beginning of the movement of the end anthropometric point of the right leg to its positioning on the support, the second—from the moment of completion of the first phase to the end of extension of the back of the standing leg in the knee joint (Fig. 1).
To extract muscle synergies, we used the principal component analysis. EMGs were preliminarily smoothed with an integration time of 0.002 s. The selection of the main components was carried out according to the Kaiser rule; the components were considered significant if they had eigenvalues of at least one and in aggregate describing at least 70% of variations in EMG activity. Since the significant components reflect the result of the simultaneous activation of a number of skeletal muscles, which is a characteristic feature of muscle synergies, the resulting components (hereinafter, the main EMG signals) were considered synergy. The EMG signals obtained in this way were averaged over the group of subjects, negative exponential smoothing was applied, and extrapolated in time relative to the reference point [11–13]. Analysis of auto- and cross-correlation functions of EMG was used to establish the composition of the extracted muscle synergies.
When analyzing autocorrelation functions, the rate of their decay was taken into account, i.e., the time from the origin to the first intersection of the function with the zero line, characterizing the presence or absence of periodic processes in the EMG. In cross-correlation functions, the maximum value of the function was analyzed, as well as the presence or absence of phase displacement in time (a shift of more than 5 ms was considered significant), characterizing the degree of their in-phase [8]. Signs of synergistic interaction were considered the presence of medium and high maximum values of the coefficients of cross-correlation functions—more than 0.5.
Mathematical and statistical data processing included the calculation of the arithmetic mean (M), the arithmetic mean error (m), median (Me). The coefficients of variation were calculated (V) for grouped data. The variation series included only the values bounded by the upper and lower quartiles. The V range of 0–30% were considered low; 30–60%, medium; 60% and above, high. To assess the significance of differences when comparing parameters in different periods, we used one-way analysis of variance (ANOVA) with post-hoc analysis using the Newman–Keuls test. The differences were considered significant at p < 0.05.
RESULTS
Before proceeding to the description of the spatiotemporal organization of extensive muscular synergies, it is necessary to clarify the features of the EMG activity of the studied muscles in the considered periods of the “leg grip” throw. It was found that in the first EMG period, the activity of the leg muscles was recorded in the range from 95.65 to 126.16 µV, of the thigh muscles—from 66.38 to 128.41 µV. The electrical activity of the trunk muscles ranged from 80.31 to 175.97 µV (Table 1). The largest amplitude was noted in TRAPI left and right side. It should be noted that in most cases the differences between the arithmetic mean and median were insignificant, only in three muscles (GM L; TRAPS L, R) the difference in values was more than 50 μV, in other muscles it did not exceed 23 μV. Variation of EMG-activity of the muscles of the right leg and muscles of the thigh of the left leg, TRAPI and RA the right side was assessed as average; the rest of the muscles showed high intragroup variability in the average EMG amplitude.
The second period of motor action fulfillment was characterized by reliably high values of the average EMG amplitude of almost all studied skeletal muscles, with the exception of GM left leg. The range of EMG activity values for the muscles of the lower leg of the left leg was from 138.26 to 197.13 µV; of the right leg, from 333.41 to 427.66 µV. The electrical activity of the trunk muscles was recorded in the range from 381.36 to 587.43 μV. Difference between M and Me more than 50 μV installed in GM left and right legs, TRAPI on the left and right side, RF of the right leg, RA and ES on the left side. The variability of the EMG activity of these muscles was assessed as high. The other muscles studied during this period showed average within-group variability. Thus, the EMG activity of most of the skeletal muscles under study in the second period of the model motor action was significantly higher than in the initial one. In addition, in the initial period of the movement, a higher variability of the average EMG amplitude of the recorded muscles was established. Since the differences in the values of the average EMG amplitude and its variability turned out to be significant, it was logical to assume that the organization of muscle synergies in different periods of movement should have its own specifics.
Based on the purpose of the work, the main EMG signals of the studied skeletal muscles in different periods of the studied movement were analyzed. According to the results of the analysis of the main components of the entire movement, three synergies were revealed (Fig. 2). The first synergy was characterized by a gradual increase in the EMG-activity of the muscles included in its structure, then a gradual decrease in it was recorded during the transition from the first period to the second. The duration of the activity of the first synergy turned out to be comparable to the duration of the considered period and amounted to 636 ms. In the second period of motor action, an increase in the EMG-activity of synergy 1 and a gradual decrease towards the end of active actions were similarly observed, the duration of electroactivity in this period was 564 ms. Thus, the activity of the first established synergy had a wavy character, and in the second period a slightly lower value of EMG activity was recorded than in the first. It should also be noted that in each considered period of movement, the amplitude of the EMG activity peaks of synergy 1 was greater than the amplitude of the peaks of synergies 2 and 3 (Fig. 2).
Muscle synergy 2 was characterized by an increase in activity, and a subsequent decrease to the initial level, in the time interval from 300 to 500 ms. During the transition to the second period of movement, the activity of the muscles included in the synergy gradually increased and then was at a higher level than in the first period. The activity of the third synergy 3 showed approximately the same dynamics as the second synergy. It was possible to determine which muscles are part of the revealed synergies in each considered period of the model motor action. The analysis of the parameters of the autocorrelation functions made it possible to classify the studied skeletal muscles into certain groups or synergies.
It was found that the time until the first intersection of the autocorrelation function with the zero line TA, GM and RF on the left side in the initial period of movement was 101–113 ms; in the period of the end of the movement, 26–50 ms. In both studied periods, the named muscles had similar parameters of autocorrelation functions. Along with the named muscles, the presence of the same pattern in EMG can be noted RA on both sides of the body. Analyzing the autocorrelation functions of EMG of other muscles in the first period, we can distinguish its similar parameters as follows: GM on the right, TRAPI and TRAPS on the left, ES on the left. In the second period, characteristic EMG patterns of the following muscles were established: TA on the right, TRAPI on the left and right sides, TRAPS on the right and ES on the left side. Since autocorrelation functions reflect only the presence or absence of a regular component in the EMG signals, it allows one to identify muscles that have similar EMG patterns with regular fluctuations in activity, or irregular, but such analysis does not allow establishing the temporal organization of muscle synergies.
To study the temporal structure of extensive muscle synergies, we analyzed the cross-correlation functions of each pair of skeletal muscles, regardless of the type of their interaction. On the whole, the parameters of 240 cross-correlation functions were analyzed in two periods of the “leg grab” throw. A greater number of medium and high correlations were found in the initial period of movement in the absence of a shift in cross-correlation functions. In the initial period, statistically significant relationships between EMG activity were found in a number of skeletal muscles, e.g., TA on the right, BF on the right, TRAPI and TRAPS on the left side. Their cross-correlation functions are shown in Figs. 3a–3c. Significant connections have also been established RA on the right side with RF on the left, TA on the right, with TRAPS on the left; ES on the left and RA the left side of the body. Relationships were also revealed for other skeletal muscles in this period, but they were not as extensive, e.g., between TA, RF of the left leg and TRAPI on the right side of the body. TRAPI and RA on the right side had the largest number of significant relationships with other studied muscles in the initial period of movement.
Cross-correlation functions of some examined skeletal muscles and their EMGs in different periods of execution of the throw “by grabbing the legs”. Initial period: (a) TA L (a) and TRAPI L (b); (b) BF R (a) and TRAPI L (b); (c) TRAPS L (a) and TRAPI L (b). Completion period: (d) TRAPS L (a) and ES L (b), (e) TRAPS R (a) and ES L (b); (f) TRAPI R (a) and ES L (b).
In the second studied period of movement, where the manifestation of muscle efforts was significantly higher than in the first, the parameters of cross-correlation functions were analyzed. A distinctive feature was that here the number of medium and high coefficients, in the absence of function bias, turned out to be significantly less than in the previous period. So, we can note the relationship of EMG activity ES on the left side with EMG activity TRAPI on both sides of the body, and TRAPS on the right side (Figs. 3d–3f). We also found a relationship between EMG activity of RA on the left side and EMG activity of TRAPS and ES on the left side.
Analysis of cross-correlation functions with a shift towards negative values in the studied periods of movement revealed the following patterns. In the initial period, a greater number of relationships were found with ES right side. The muscles of the thigh of the left leg were interconnected with it and GM of the right leg. Relationships were found with the rectus abdominis muscle of the right side of the body TA on the left, TRAPS on the right and ES on the left side. In the period of manifestation of great muscular efforts, such synergistic relationships are established between ES on the the left side, and the muscles of the thigh on the right, as well BF of the left leg.
In the presence of a shift in the maximum of cross-correlation functions in the positive direction in the first period, extensive relationships were established BF of the right leg with TRAPS on the right side, as well as with the muscles of the trunk (Table 2). The peculiarity of the interrelationships of muscular activity in the second period was their significantly smaller number than in the period of the beginning of the motor action fulfillment. One can note the electroactivity of the rectus abdominis muscle on the left side and the characteristic patterns of EMG-activity of the muscles of the thigh of the right lower limb and the muscle that straightens the spine of the left side. The maximum values of their coefficients of cross-correlation functions turned out to be average here.
DISCUSSION
The research results showed significant differences in the values of skeletal muscle electroactivity during the execution of the throw “by grabbing the legs” in different periods of its implementation. The average EMG amplitude of the majority of skeletal muscles in the final period of movement was significantly higher than in the initial one, and the higher EMG activity values were characterized by less variability. We can assume that the observed variability of skeletal muscle electroactivity in the initial period of movement is more significant, since it is known that different control mechanisms are used at different levels of the central nervous system in movements or its individual phases that have different target significance [1, 12, 13]. Probably, the final period of the studied movement has a resulting value, and therefore the range of acceptable variability of EMG activity of skeletal muscles involved in movement turned out to be lower than in the initial one.
In the present study, three muscle synergies were established, the activity of which had characteristic features, manifested in an increase in the electrical activity of the main EMG signals at the beginning of movement and its decrease at the border of the transition between the recorded periods of movement. In this case, by the relative magnitude of the electroactivity of synergy, one can find out which muscles are its components. For example, according to the results of the analysis of the main components, it was found that one of the muscle synergies includes the muscles with the largest values of the average EMG amplitude. In the initial period of movement, these were the lower leg muscles of the left lower limb, the biceps femoris muscle, the upper and lower bundles of the trapezius muscles of the left and right sides. The same number of synergies were identified Frere and Hug in the study of some specialized gymnastic, difficult to coordinate movements [14]. A similar amount of synergy was extracted from the EMG muscles involved in bench presses by highly qualified athletes [15]. However, in works where locomotor movements were the object of research, from 4 to 5 muscle synergies were recorded [6, 16]. Thus, for the effective fulfillment of motional tasks associated with the manifestation of significant muscular efforts and high coordination accuracy, a more significant limitation of the degrees of freedom of the muscular apparatus is required than is necessary for locomotor activity. It is possible that such features of the organization of muscle synergies are a mechanism that reduces the possibility of injury, the likelihood of which, when performing complex sports movements, is quite high.
In the initial period of the studied movement, a large variability of the composition of muscle synergies was established, which manifests itself in extensive relationships between the EMG activity of the trunk muscles with the muscles of the thigh and lower leg, and such relationships are also found in the bilateral muscles. In the final period of the throw with the “capture of the legs,” the composition of synergies turned out to be less varied than in the initial period. The relationship of EMG activity here was mainly concentrated on the muscles of the trunk. Our data are in some aspects consistent with the results by Shaharudin [17]. The authors have shown the flexibility of the composition of muscle synergies when performing stroke movements on various simulators. The number of extracted synergies from EMG muscles in such conditions turned out to be the same (three); however, the authors observed a different variability in the composition of the muscles of each established synergy, which is explained by the strategy of the central nervous system aimed at the effective implementation of complex coordination movement. A similar number of synergies was found in studies where the object of study was a complex gymnastic element - large turns on the bar [14]. This work shows the variability of one of the three established muscle synergies, while the other two turned out to be similar in different athletes, which confirms the existence of a single neuromuscular strategy for controlling a complex specialized sports movement. Three synergies were identified by a group of authors in the study of power bench press performed by athletes of different sports qualifications [15]. The study did not find differences in the number of muscle synergies in different phases of movement, differing in the predominant mode of muscle contractions. However, a large variability in the composition of muscle synergies was found among the group of highly skilled powerlifters in the concentric phase, compared with less qualified ones.
Analysis of the EMG dependences recorded in our study of muscles showed the presence of maximum coefficients of cross-correlation functions with their displacement, both in the positive and in the negative direction. This indicates that the synergistic relationships of skeletal muscles have a temporary organization, namely, the activity of one muscle in the structure of synergy can be delayed or precede the activity of another. At the same time, EMG activity of muscles retains the characteristic features that determine their relationship to synergy. It is known that synergies are organized at the cortical, subcortical and spinal levels, each of which contributes to their structure, but the leading role in the management of muscle synergies belongs to the structures of the cerebellum [18]. During motor activity, the brain corrects the work of the spinal mechanisms on the basis of signals coming from the receptors of the executive motor apparatus; signals about the work of the central generators are also sent to the brain [19]. The output signals of such generators are synergy programs that determine their structural and temporal organization [2, 20]. These parameters of the motor output are the “code” of the motor program that controls the activation of motoneuronal pools of various segments of the spinal cord that control the activity of skeletal muscles [2, 21]. In this case, the motor program determines the sequence and intensity of control signals in the central nervous system for the successful solution of the realized motor task.
It was established that there were always fewer active muscle synergies in the period of motor action, where more significant muscle tensions were recorded (the final period). This organization of muscle synergies can be carried out using mechanisms for regulating the strength of muscle contractions of the central nervous system, including the mechanism for synchronizing the activity of individual motor units (MUs) in time. The essence of such control lies in the summation of the contractions of individual units, due to which the strength of muscle contraction increases. The synchronization of the MU activity affects the rate of contraction and is important in the initial period of any movement. As a rule, when performing fast movements, the synchronization of the activity of the DE is more pronounced at its beginning than in the final period. This is due to the need to overcome a significant external load at the beginning of a motor action. The high frequency of impulses and the activity of a large number of motor neurons increases the likelihood of coincidence of the contractile cycles of different MUs at the beginning of movement [22]. Thus, the formation of a greater number of active muscular synergies in the initial period of a complex coordinating motor action is probably due to the need to perform fast and precise movements aimed at overcoming a significant load.
CONCLUSIONS
Thus, the established consistent patterns of variability of skeletal muscles electroactivity involved in the implementation of a complex coordinating motor action in different periods of its implementation are associated with their target significance. The electroactivity of the extensive synergies changes depending on the magnitude of the muscular efforts that are characteristic for different periods of the performed movement. The synergistic relationships of skeletal muscles demonstrate the plasticity of their spatial and temporal organization. The greater number of active muscular synergies in the initial period of the “leg grip” throw is probably due to the need to perform fast and precise movements aimed at overcoming a significant load, which ensures reliable control of the motor function. When studying extensive muscle synergies, it is advisable to use a set of techniques (principal component analysis, analysis of auto- and cross-correlation functions), which makes it possible to establish their spatial and temporal organization.
REFERENCES
Bernshtein, N.A., Ocherki po fiziologii dvizhenii i fiziologii aktivnosti (The Physiology of Movement and Activity), Moscow: Meditsina, 1966.
Latash, M.L. and Zatsiorsky, V.M., Biomechanics and Motor Control: Defining Central Concepts, New York: Academic, 2016.
Nicholls, J., Wallace, B., Fuchs, P., and Martin, A., From Neuron to Brain, Sunderland, MA: Sinauer, 2001.
Person, R.S., Spinal’nye mekhanizmy upravleniya myshechnym sokrashcheniem (Spinal Mechanisms of Muscle Contraction Control), Moscow: Nauka, 1985.
Rathelot, J. and Strick, P., Muscle representation in the macaque motor cortex: an anatomical perspective, Proc. Natl. Acad. Sci. U.S.A., 2006, vol. 103, no. 21, p. 8257.
Ivanenko, Y.P., Cappellini, G., Dominici, N., et al., Coordination of locomotion with voluntary movements in humans, J. Neurosci., 2005, vol. 25, no. 31, p. 7238.
Tresch, M.C., Cheung, V.C., and D’Avella, A., Matrix factorization algorithms for the identification of muscle synergies: evaluation on simulated and experimental data sets, J. Neurosci., 2006, vol. 95, no. 4, p. 2199.
Person, R.S., Elektromiografiya v issledovaniyakh cheloveka (Electromyography in Human Researches), Moscow: Nauka, 1969.
Shelyakin, A.M., Preobrazhenskaya, I.G., and Bogdanov, O.V., Cross-correlation analysis of the bioelectrical activity of antagonist muscles in the study of random motor activity in a person with motor disorders, Ross. Fiziol. Zh. im. I.M. Sechenova, 1997, vol. 83, no. 9, p. 88.
Wren, T.A.L., Do, K.P., Rethlefsen, S.A., et al., Cross-correlation as a method for comparing dynamic electromyography signals during gait, J. Biomech., 2006, vol. 39, no. 14, p. 2714.
Latash, M.L., Scholz, J.P., and Schoner, G., Motor control strategies revealed in the structure of motor variability, Exercise Sport Sci. Rev., 2002, vol. 30, no. 1, p. 26.
Latash, M.L., Structured variability as a key feature of biological processes, Vopr. Psikhol., 2016, no. 3, p. 120.
Moiseev, S.A. and Pukhov, A.M., The role of functional synergies in the control of the spatio-temporal structure of human precision movements (by the example of archery), Zh. Med.-Biol. Issled., 2019, vol. 7, no. 4, p. 410.
Frère, J. and Hug, F., Between-subject variability of muscle synergies during a complex motor skill, Front. Comput. Neurosci., 2012, vol. 6, p. 99.
Kristiansen, M., Samani, A., Madelein, P., et al., Effects of 5 weeks of bench press training on muscle synergies: a randomized controlled study, J. Strength Cond. Res., 2016, vol. 30, no. 7, p. 1948.
Kibushi, B., Hagio, S., Moritani, T., and Kouzaki, M., Speed-dependent modulation of muscle activity based on muscle synergies during treadmill walking, Front. Hum. Neurosci., 2018, vol. 12, no. 4, p. 4.
Shaharudin, S., Zanotto, D., and Agrawal, S., Muscle synergies of untrained subjects during 6 min maximal rowing on slides and fixed ergometer, J. Sports Sci. Med., 2014, vol. 13, no. 4, p. 793.
McMorland, A.J., Runnalls, K.D., and Byblow, W.D., A neuroanatomical framework for upper limb synergies after stroke, Front. Hum. Neurosci., 2015, vol. 9, p. 82.
Orlovsky, G.N., Deliagina, T.G., and Grillner, S., Neuronal Control of Locomotion: From Mollusc to Man, Oxford: Oxford Univ. Press, 1999.
Gerasimenko, Y., Sayenko, D., Gad, P., et al., Electrical spinal stimulation, and imagining of lower limb movements to modulate brain-spinal connectomes that control locomotor-like behavior, Front. Physiol., 2018, vol. 9, p. 1196.
Bizzi, E. and Cheung, V.C., The neural origin of muscle synergies, Front. Comput. Neurosci., 2013, vol. 7, no. 51, p. 51.
Gurfinkel’, V.S. and Levik, Yu.S., Skeletnaya myshtsa: struktura i funktsiya (Skeletal Muscle: Structure and Function), Moscow: Nauka, 1985.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
COMPLIANCE WITH ETHICAL STANDARDS
All experiments were carried out in compliance with the requirements and principles of biomedical ethics formulated in the 1964 Declaration of Helsinki and approved by the Bioethical Committee of the Velikie Luki State Academy of Physical Culture and Sports (Velikiye Luki).
CONFLICT OF INTEREST
The authors declare no obvious and potential conflicts of interest related to the publication of this article.
INFORMED CONSENT
Each participant provided a voluntary written informed consent to participate in the research, signed by him/her after explanation of the potential risks, as well as the nature of the upcoming research.
Rights and permissions
About this article
Cite this article
Moiseev, S.A., Gorodnichev, R.M. Characteristics of Synergetic Interaction of Skeletal Muscles during the Performance of a Complicated Coordination Motor Task. Hum Physiol 47, 42–50 (2021). https://doi.org/10.1134/S0362119720060079
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0362119720060079