Abstract
The aging phenomenon involves complex interrelated mechanisms at different levels of physiological processes, resulting in a decreased ability to maintain homeostasis of the organism due to organ dysfunctions. The chronic inflammation, which is progressing at the tissue level, operates as a driver of many age-related diseases. Nowadays, researchers consider cell aging (senescence) as one of the aging key hallmarks, manifested as qualitative and quantitative changes in the cellular composition and intercellular communication in tissues. This review highlights the modern concepts of aging at the cellular level. Particular attention is given to the mesenchymal stromal/stem cells (MSCs), which are involved in tissue homeostasis maintenance. Age-related MSC function alterations in tissue niches including immunomodulatory activity, hematopoiesis, and paracrine regulation are discussed. In addition, the approaches to MSC modification in vitro to attenuate the negative effects of aging are considered.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
FROM AGING TO CELL SENESCENCE
Increased life expectancy and rapid increase in the number of elderly people necessitates improving the quality of their life and prolonging its productive period, which requires a deep understanding of the fundamental mechanisms of aging in order to find the ways of their regulation and improve approaches in the treatment of age-related pathologies. Active development of gerontology is indicative of the demand for a special approach to assessing the changes in the functional state of the physiological systems during aging. Aging is usually described as a gradual loss of the physiological integrity, leading to disturbances of its functions and increased risk of death over time. So, age is a risk factor for many diseases, including cardiovascular diseases, dementia, osteoporosis, osteoarthritis, cancer, type 2 diabetes, idiopathic pulmonary fibrosis, glaucoma, etc. [1]. However, our understanding of the aging process per se remains insufficient, and its biological causes are largely unknown.
The aging phenomenon includes complex interrelated mechanisms at different biological levels. At the organismal level, aging leads to a decrease in the ability to maintain homeostasis due to disturbances in organ functioning. At the tissue level, chronic inflammation is observed, which functions as a driver of many age-related diseases (primarily the cardiovascular and neurodegenerative ones). The basis of these pathological changes is modifications at the cellular level, which manifest themselves in changing both qualitative and quantitative cell composition of tissues and disruption of intercellular communication [2, 3]. The ratio between differentiated and progenitor cells also changes, and the proportion of the so-called senescent cells with significantly modified morphology and functions increases.
Studies performed in recent decades have revealed cellular and molecular signs associated with aging, which can be tentatively divided into three categories: primary, antagonistic, and integrative [4]. The primary signs (genomic instability, telomere shortening, epigenetic changes, and impaired proteostasis) are unequivocally negative and function as triggers initiating pathological changes. The antagonistic signs (impaired nutrient recognition, mitochondrial dysfunction, and cell senescence) represent the response of the organism to the primary symptoms. Depending on the extent and context of manifestation, they may have either positive or negative effect. However, chronic activation and imperfection of these mechanisms give rise to the integrative signs (depletion of the stem cell pool and changes in intercellular interactions). For example, DNA damage (primary sign) may lead to carcinogenesis. Senescent state activation (antagonistic sign) in this case has a positive effect, preventing the proliferation of cells with damaged genome. On the other hand, this mechanism leads to a gradual depletion of the pool of dividing stem cells (integrative trait) [4–6].
The above signs were distinguished on the basis of three criteria: (1) the sign must be observed in normal aging; (2) its experimental enhancement must lead to accelerated aging; and (3) its experimental attenuation must decelerate the normal aging progression, thereby increasing the healthy life expectancy [4]. It should be noted once again that such classifications are quite voluntary, because the key signs during the aging are manifested simultaneously and are interconnected closely. Identification of the causality between them is one of the main challenges of gerontology.
CELL SENESCENCE
The proportion of senescent cells is increased with age [7–10]. In particular, it was shown that more than a half of cardiomyocyte progenitors in elderly patients with cardiovascular diseases show the signs of senescence [11]. However, the nature of these changes and the way of their contribution to degeneration and development of diseases in the elderly remain uncovered.
More than 50 years ago, it was found that human fibroblasts can undergo only a certain, limited number of divisions in culture [12]. The phenomenon was named after the author—the Hayflick limit. The search for the causes of the restriction of cell proliferative activity in vitro has since become the main stream of research in gerontology [13]. It was subsequently shown that the Hayflick limit is characteristic of many types of cells: keratinocytes, endothelial cells, lymphocytes, adrenocortical cells, chondrocytes, etc. The maximum number of cell divisions in culture varies considerably depending on the cell type and the species. It is believed that the existence of the Hayflick limit is determined by the replicative cellular senescence.
Currently, replicative and stress-induced cell senescence are distinguished. The replicative senescence is the cell state when the proliferative activity irreversibly decreases to complete stop of divisions. It is believed that the telomere shortening is the main cause of the cell-cycle arrest, which can be regarded as a particular case of genomic instability. The stress-induced cell senescence also causes cessation of proliferation; however, in contrast to the replicative senescence, it may occur at any time in response to sublethal exposure or activation of oncogenes, regardless of the number of divisions [13].
Both in vivo and in vitro, the cell senescence is activated after considerable DNA damage (usually in the case of double-strand breaks) [14]. Cell senescence can be induced with various physical and chemical exposures. Ionizing radiation and topoisomerase inhibitors are a particularly potent inducers of the senescent state. The chemotherapeutic drugs exerted a pronounced cytotoxic and cytostatic effects [15]. DNA damage caused by oxidative stress can also lead to cell-cycle arrest [16]. Oxidative stress can damage DNA bases and/or cause single-strand breaks. However, during replication or base excision repair, this damage can be transformed to the double-strand breaks [17]. Interestingly, oxidative stress may also accelerate telomere shortening [18], probably because of the high content of guanine (G), a base that is most susceptible to reactive oxygen species (ROS) [19]. Slight DNA defects leads to a transient cessation of proliferation. After a successful repair, the cell can start replication again. More significant damage, not repairable for a long time, leads to chronic activation of the signaling cascade responding to genome damage. This response usually occurs after multiple DNA defects and lead to the cell cycle arrest—the main cause of the senescent state [13, 19].
Thus, the genome stability is constantly subjected to danger from the exogenous (physical, chemical, and biological agents) and endogenous (DNA replication errors, spontaneous hydrolysis reactions, and ROS) factors. Genetic disorders that arise due to damage and repair system imperfections may include point mutations, translocations, chromosome shortening or lengthening, telomere shortening, and gene function caused by insertions or deletions of DNA sequences, including the integration of viruses or transposons. All these types of mutations may affect gene expression, which leads to the appearance of cells with disturbed functions, which may endanger the tissue and organismal homeostasis [4].
An additional factor contributing to the genome damage may be the impairment of the spatial architecture of chromatin, which makes DNA more susceptible. These disturbances include the well-known nuclear lamina defects causing progeroid syndromes—the Hutchinson–Gilford and Nestor–Guillermo syndromes. Interestingly, the aberrant isoforms of prelamin A (progerin) are detected not only in progerias but also in normal human aging [20, 21]. Telomere dysfunction increases progerin production in normal fibroblasts in vitro, which suggests the presence of additional relationships between the telomere length maintenance and progerin expression in normal aging [22]. In addition, the chromatin structure can be significantly affected by the epigenetic modifications of histones and DNA. Researchers pay particular attention to protein sirtuin, which exhibit the properties of histone deacetylase and mono(ADP-ribosyl)transferase. Sirtuins affect the chromatin packing density and, hence, regulate a wide range of cellular processes, including transcription, repair, and metabolism [1].
The factors that may significantly affect senescence processes include cellular metabolism and maintenance of proteostasis (qualitative and quantitative protein composition) [1, 4]. Caloric restriction leads to a significant reduction in the manifestation aging-associated signs [23]. At the molecular level, this effect is associated with the mTOR protein functioning and insulin/IGF signaling. mTORC1 integrates several signaling pathways, including the recognition of nutrients and the growth signals, as well as regulates the synthesis of proteins and lipids, the level of autophagy, and metabolism [24]. Autophagy processes increase with aging, which may be associated with an increased level of intracellular damage. Inhibition of autophagy may activate the senescent state due to metabolic disturbances and accumulation of damaged proteins [25–27].
Recently, researchers tend to believe that cell senescence is one of the main components of aging. Activation of the senescent state, in addition to an irreversible cell-cycle arrest, is accompanied by phenotypic variations of varying degrees of expression, including chromatin remodeling, modulation of metabolism, increased autophagic processes, and production of proinflammatory cytokines [28, 29]. The most well-known hallmarks of the cell senescence are the morphological changes—flattening and increase in size [30], increase in senescence-associated β-galactosidase (SA-β-gal) activity [31], and increase in the frequency of γH2AX heterochromatin foci [32]. The results of studies by various authors show that the kinetics of the formation of γH2AX foci largely correlates with the occurrence of double-strand breaks, due to which γH2AX is considered a reliable marker of cell senescence [33].
PARACRINE CHANGES (SASP)
In the last decade, the attention of researchers is increasingly focused on paracrine changes. Along with cell-cycle arrest, one of the most characteristic (and, probably, the most important from the viewpoint of aging of the whole organism) hallmarks of senescent cells is the senescence-associated secretory phenotype (SASP). The paracrine profile comprises hundreds of secreted factors, including the proinflammatory cytokines, chemokines, growth factors, and proteases [34–36]. The exact composition may vary depending on the cell type and the aging induction method. Despite the difficulties, many of the key factors and methods of their regulation were identified and described. It was found that the main regulator of SASP is the nuclear factor κB (NF-κB). However, the complex composition also involves other independent ways of regulation of individual secretome elements [1].
Senescence-associated secretory phenotype is a most important case of disturbance of intercellular communication, which leads to various consequences in the surrounding tissues [34]. It was shown that some factors can stimulate cell proliferation through activation of the growth-regulated oncogene (GRO) [35] and the growth factor amphiregulin. Some factors may be involved in neovascularization via VEGF activation [36]. Others can modulate Wnt-activation [37] and production of IL-6 and IL-8 [15], which, in turn, may either stimulate or inhibit the Wnt-signaling and cell proliferation depending on the physiological microenvironment. Studies on premalignant epithelial cells (SASP-affected fibroblasts after stress-induced aging) showed an increased frequency of epithelial–mesenchymal transition and the ability of cells to invade. These effects are largely associated with the influence of proinflammatory cytokines such as IL-6 and IL-8. In addition, SFRP1, GROα, and IL-6 may influence the proliferative and differentiation activity of stem cells as well as modulate their niche parameters [19]. In vitro studies performed on cultured fibroblasts showed that the direct cocultivation of “young” cells with “old” ones increases the frequency of formation of DNA damage foci, one of the signs of the presenescent state [38].
One of the most important effects caused by SASP elements (IL-6 and IL-8, various monocyte chemoattractant proteins (MCP), macrophage inflammatory proteins (MIP), granulocyte/macrophage colony stimulating factor (GM-CSF), etc.) is the induction and/or enhancement of the inflammatory process. Chronic inflammation, which is maintained by senescent cells, is considered as one of the most adverse factors affecting the development of diseases associated with aging, including osteoarthritis, pulmonary fibrosis, Alzheimer’s disease, tumorigenesis, etc. [32, 35, 39, 40]. Moreover, chronic inflammation leads to the dysfunction of epidermal stem cells [41], which further confirms the complex relationships between various key features enhancing the aging process [4].
It should also be noted that chronic inflammation functions according to the positive feedback principle. An increase in the level of proinflammatory cytokines activates white blood cells, which produce more cytokines. Thus, even weak stimuli, which are constantly produced by the senescent cells, may lead to serious systemic consequences over time [39].
MESENCHYMAL STROMAL CELLS
One of the causes of the change in the cellular composition and disturbance of intercellular communications with aging is the depletion of the stem cell pool in adult humans, as well as the changes in their physiology [42, 43]. Stem cell populations are essential for the maintenance of tissue homeostasis. Depletion of their pool and their modifications contribute to the development of progressive age-related changes. The populations of hematopoietic stem cells (HSCs) and mesenchymal stromal/stem cells (MSCs) are the best studied stem cells of adult humans. HSCs are precursors of all blood cells, including the myeloid (monocytes, macrophages, neutrophils, basophils, eosinophils, erythrocytes, megakaryocytes, platelets, and dendritic cells) and lymphoid (T, B, and natural killer cells) lineages. MSCs are differentiated mostly into the cells of the mesodermal origin.
MSCs attract particular interest either for both basic science or application in regenerative medicine. Researchers come to the conclusion that the functional state of organs and tissues strongly depends on MSCs, which occupy the perivascular niche and are involved in the regulation of angiogenesis, immunomodulation, maintaining hematopoiesis, etc. [44, 45]. These cells were found in almost all tissues of an adult organism, as well as in the tissues of newborns, including the placenta and umbilical cord. For example, MSCs were derived from the bone marrow, adipose tissue, amniotic fluid, amniotic membrane, tooth pulp, endometrium, limb anlages, menstrual blood, peripheral blood, salivary glands, skin, foreskin, synovial fluid, Wharton’s jelly, etc. [46]. Nevertheless, despite the compliance of the secreted cells with the minimum criteria [47], which allows them to be classified as MSCs, the derived cultures differ significantly, which determines the necessity of studying each individual tissue-specific population [46, 48–50].
Many researchers attribute the beneficial effects exerted by MSCs to their ability to secrete a number of biologically active factors, including the cytokines and the extracellular matrix components [51, 52]. MSCs are involved in the physiological tissue renewal and repair of traumatic injuries, performing the important function of tissue homeostasis maintenance. Numerous studies in vivo showed the efficiency of administration of this cell population for the reparative purposes in heart attack, stroke, ulcers, burns, injuries of internal organs, etc. [53–56]. Previously it was assumed that the observed effects are determined by the ability of MSCs to differentiate into the functionally active tissue elements or to fuse with them, thereby ensuring the delivery of healthy mitochondria and other cellular compartments. However, accumulated data strongly suggest that the secretion of various paracrine mediators by MSCs in the damaged area plays the main role. They produce a wide range of cytokines, thus exhibiting the antiapoptotic, immunomodulatory, chemoattractant, antifibrotic, and angiogenic effects [45, 57–59].
MORPHOLOGICAL AND FUNCTIONAL PROPERTIES OF SENESCENT MSCS
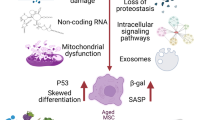
Due to their role in the tissue niche in maintaining homeostasis and auto-/paracrine regulation, MSCs are particularly interesting in terms of cell senescence. Similarly to other cells, MSCs change their morphological and functional characteristics as a result of activation of the senescent state. This leads to an irreversible cell-cycle arrest and changes in morphology and activity of organelles and in gene expression and to the appearance of heterochromatin foci (γH2AX) and a number of other hallmarks of cell senescence. Reduction of proliferation does not lead to cell growth arrest, which determines the increase in the size of senescent MSCs. It should be noted that the cells with a complete cell-cycle arrest are able to retain their viability and functional activity for a long time without triggering apoptotic cascades and continue to increase in size and accumulate a wide range of cytoplasmic inclusions. Thus, the increase in the average cell size and the decrease in the proliferative activity are closely interrelated processes. However, MSCs continue to interact with their environment, exerting local and systemic effects [42, 43, 60–62].
It is believed that one of the important features of senescent MSCs is the decrease in multipotency [42, 43], which may limit their reparative functions in tissues. At the same time, the balance between the osteogenic and adipogenic directions is shifted, although the direction of this shift is still a matter of controversy. Several studies showed that the osteogenic potential of MSCs gradually deteriorates with increasing duration of culturing or with aging [63, 64]. Authors of other studies showed no such changes or even demonstrated the opposite result and reported the enhancement of the osteogenic properties [65, 66]. Such ambiguous results obtained by different research groups are usually explained by the use of different experimental models and methodological approaches as well as by the lack of definitive tests for osteogenic differentiation [63, 67]. The optimal indicator of osteogenic differentiation in vitro is the mineralization of the matrix, which can be detected using the Alizarin red dye. However, the increased cell death may determine a greater degree of staining by this compound, leading to false positive results due to the release of large amounts of calcium from the dying cells and its binding to the matrix [42]. A greater consensus between researchers was reached regarding the changes in the adipogenic potential. Despite the wide variety of results, the majority of researchers agree that the adipogenic potential decreases after long-term passaging under the standard culturing conditions [43].
Particular attention should also be given to the complex regulation of the key transcription factors of differentiation—RUNX2 (positive regulator of osteogenesis) and PPARγ (positive regulator of adipogenesis). These factors are reciprocally regulated by several signaling pathways that may differentially change in different model systems of cellular senescence. It was shown that PPARγ downregulates RUNX2 at the transcriptional level by suppressing the activity of the Wnt signaling pathway [68]. In this case, another cause of the differences in the obtained data on the osteogenic potential of MSCs may be determined by the disturbance of the fine balance between these transcription factors. Among other things, it is worth noting that PPARγ activates a number of genes that are responsible for lipid metabolism and maintenance of glucose levels. This protein is responsible for the accumulation of the fat layer and the development of aging-related insulin resistance; it also inhibits the chronic inflammation, which accompanies aging [3]. In addition, in aging PPARγ is involved in the induction of autophagy, which is required for the maintenance of cellular homeostasis [69].
Recent studies of the immunomodulatory activity of senescent MSCs that were obtained using radiation exposure showed a reduction in their protective regulatory capacity in the sepsis model in mice [70]. On the one hand, the senescent MSCs retained the ability to regulate the inflammatory response of macrophages in vitro and partly continued to inhibit lymphocyte proliferation. On the other hand, their migratory activity in response to proinflammatory stimuli decreased, which was probably due to the inhibition of AP-1-signaling. It is worth noting that many of the SASP components that are secreted, in particular, by the senescent MSCs are associated with the immune processes. For example, IL-8 is a chemoattractant of neutrophils and other granulocytes [71] as well as a potent inducer of angiogenesis [72]. VCAM1 mediates adhesion of leukocytes to endothelium, and the elevated level of its circulating soluble form is associated with systemic inflammatory diseases such as systemic lupus erythematosus and ischemic heart disease [73]. Finally, MCP1 (CCL2) is a chemoattractant for monocytes and basophils and plays an important role in a number of inflammatory diseases such as multiple sclerosis [74] and inflammatory bowel disease [75]. Understanding the physiological and pathological factors influencing the immunomodulatory activity of MSCs is of great importance both for the autoimmune/inflammatory diseases and for degenerative pathologies.
On the other hand, it is known that MSCs may promote tumorigenesis. Additional studies showed the enhancement of their carcinogenic effect with aging via stimulation of proliferation and migration of tumor cells [76–79]. In addition, the senescent MSCs, secreting large amounts of IL-6 and IL-8, increase the resistance of breast cancer cells to cisplatin and contribute to the increase in the tumor volume in vivo [78]. The significance of IL-6 in tumorigenesis was also confirmed in other studies. In particular, the secretion of this cytokine stimulates proliferation and migration of breast cancer cells in vitro and in vivo [77]. The assessment of gene expression in the senescent MSCs showed an increased amount of transcripts and other secreted factors, many of which have the proinflammatory effect, including GRO1, MCP-2, RANTES, GM-CSF, metalloprotease MMP3, and the adhesion molecule ICAM-1 [42, 79]. Taken together, these data indicate that SASP modifies the paracrine communication between MSCs and their physiological/pathological microenvironment. It should be noted that, depending on the tissue source and the method of senescentce induction, the secretome composition may considerably vary. Moreover, MSCs obtained from different tissues may respond to stress inducers with different sensitivity [80].
One of the key elements of the MSC secretome is extracellular vesicles (EVs), comprising exosomes and ectosomes, which mediate intercellular communication and exhibit biological activity by delivering functional molecules, such as RNA and proteins, into the recipient cells [81, 82]. It was shown that the EVs produced and secreted by human MSCs can replace the intact MSCs for tissue restoration and regeneration. A recent study showed that the EVs obtained from the “young” MSCs can improve the state of senescent MSCs, reducing the intensity of manifestation of the aging-associated hallmarks. In particular, a decrease in the activity of the enzyme SA-β-gal, the expression of p21 and p53, and the production of cytokines IL-1a and IL-6 (i.e., the proinflammatory SASP components) was detected. The proteome analysis of EV showed that they are enriched in antioxidant enzymes, peroxyredoxins, which apparently partially explains their “rejuvenating” effect [83].
Another important function of MSCs is to maintain the activity of the hematopoietic stem cells (HSCs), which can self-renew and differentiate into all blood components, serving as a source of mature blood cells throughout life. However, the regenerative capacity of HSCs transplanted to recipients is disturbed with age [84].
The studies emphasized the key role of MSCs in the regulation of activity of HSCs and in stimulation of their engraftment [85]. Recent studies confirmed the contribution of external signals from the tissue niche to the HSC dysfunction with aging [84, 86]. Changes in the cellular composition of the hematopoietic niche in the course of aging promote suppression of osteogenesis, enhancement of adipogenesis and inflammation in the bone marrow, as well as changes in the mutual influence of HSCs and MSCs [87, 88].
There is evidence that inflammatory stimuli alter the functionality of HSCs, influencing the proliferation, differentiation potential, and the interaction between HSCs and the tissue niche. In particular, it was reported that chronic inflammation leads to depletion of HSCs during of aging [89, 90]. The senescence-associated secretory phenotype of MSCs may also enhance inflammation in the hematopoietic niche [9]. However, further studies are required to analyze the effect of individual factors of SASP on the physiology of hematopoiesis. Due to their unique immunomodulatory properties, MSCs began to be used in cell-based therapies, in particular, in the transplantation of HSCs, for treating acute graft versus host disease, improving engrafting, and stimulating tissue repair [91]. Numerous clinical trials based on the use of MSCs are already being performed. However, many of them are carried out on elderly patients with the use of autologous cells. Probably, a better approach would be to use MSCs derived from young patients or to use SASP inhibitors. Recent data also indicate that the pretreatment of senescent MSCs with SASP inhibitors (e.g., steroids or NF-kB inhibitor) eliminates the adverse effects on the functionality of HSCs [9].
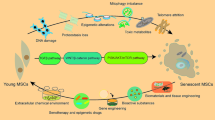
To use MSCs in medicine, it is necessary to continue developing methods that will allow obtaining large amounts of cells with retaining their original properties. Several potential approaches to maintaining or enhancing the therapeutic efficacy by controlling the specific factors that may affect the characteristics of MSCs, including their senescence, were developed. One of them is to introduce the telomerase gene hTERT. This method makes it possible to extend the lifespan of culture while retaining the normal karyotype and differentiation activity [92]. In addition, several low-molecular-weight compounds, such as aspirin, vitamin C, and cytokine FGF-2, were proposed for the endogenous telomerase activation [93]. However, this method is not recommended for use in clinical practice because of the risk of malignant transformation.
The second approach is to use antioxidants or inhibitors of certain signaling pathways. N-acetyl-L-cysteine (NAC), a precursor of glutathione, or other antioxidants can be used as therapeutic agents for scavenging ROS and reducing their hazardous effects on the cell [94]. Other antioxidants, such as ascorbic acid and inhibitors of p38/MAPK or mTOR, may also contribute to mitigating the impact of ROS [43]. Modification of culturing conditions (particularly, the change in the level of oxygen) may lead to similar effects [42, 95, 96].
The third approach is genetic engineering. The p16INK4a/CDKN2A knockdown or RB (retinoblastoma) silencing in MSCs suppress the manifestation of the senescent phenotype and increase the proliferation rate [97, 98]. However, this technique affects the differentiation potential and increases the risk of tumorigenesis.
The fourth approach is the selective use of growth factors to maintain the proliferative and differentiation potential of MSCs. It is known that the use of exogenous FGF-2, PDGF, and EGF increases the ability of MSCs to proliferate and decelerates the aging of cells without affecting osteogenesis and adipogenesis [99].
CONCLUSIONS
Thus, the ongoing studies bring us closer to understanding the cell senescence physiology, which opens up prospects for the development of approaches to extend the productive life of older persons. Replicative senescence during long-term culture allows to analyze the modulation of the progenitor cell properties as well as to develop approaches to retaining the proliferative and functional activity of MSCs, which maintains their reparative functions. The modification of cell culture approaches, a profound understanding of the basic mechanisms of cell senescence, and the knowledge on the tissue-specific MSC properties will ensure significant advance in adult stem cell application for regenerative medicine of both young and elderly patients.
REFERENCES
McHugh, D. and Gil, J., Senescence and aging: causes, consequences, and therapeutic avenues, J. Cell Biol., 2018, vol. 217, no. 1, p. 65.
Zhang, R., Chen, H.Z., and Liu, D.P., The four layers of aging, Cell Syst., 2015, vol. 1, no. 3, p. 180.
Moskalev, A.A., Proshkina, E.N., Belyi, A.A., and Solovyev, I.A., Genetics of aging and longevity, Russ. J. Genet.: Appl. Res., 2017, vol. 7, no. 4, p. 369.
López-Otín, C., Blasco, M.A., Partridge, L., et al., The hallmarks of aging, Cell, 2013, vol. 153, no. 6, p. 1194.
Muñoz-Espín, D., Cañamero, M., Maraver, A., et al., Programmed cell senescence during mammalian embryonic development, Cell, 2013, vol. 155, no. 5, p. 1104.
Muñoz-Espín, D. and Serrano, M., Cellular senescence: from physiology to pathology, Nat. Rev. Mol. Cell Biol., 2014, vol. 15, no. 7, p. 482.
van Deursen, J.M., The role of senescent cells in ageing, Nature, 2014, vol. 509, no. 7501, p. 439.
Farr, J.N., Xu, M., Weivoda, M.M., et al., Targeting cellular senescence prevents age-related bone loss in mice, Nat. Med., 2017, vol. 23, no. 9, p. 1072.
Gnani, D., Crippa, S., Della Volpe, L., et al., An early senescence state in aged mesenchymal stromal cells contributes to hematopoietic stem and progenitor cell clonogenic impairment through the activation of a proinflammatory program, Aging Cell, 2019, vol. 18, p. e12933.
Patil, P., Dong, Q., Wang, D., et al., Systemic clearance of p16INK4α-positive senescent cells mitigates age-associated intervertebral disc degeneration, Aging Cell, 2019, vol. 18, p. e12927.
Lewis McDougall, F.C., Ruchaya, P.J., Domenjo Vila, E., et al., Aged-senescent cells contribute to impaired heart regeneration, Aging Cell, 2019, vol. 18, p. e12931.
Hayflick, L. and Moorhead, P.S., The serial cultivation of human diploid cell strains, Exp. Cell Res., 1961, vol. 25, p. 585.
de Magalhães, J.P. and Passos, J.F., Stress, cell senescence and organismal ageing, Mech. Ageing Dev., 2018, vol. 170, p. 2.
Nakamura, A.J., Chiang, Y.J., Hathcock, K.S., et al., Both telomeric and non-telomeric DNA damage are determinants of mammalian cellular senescence, Epigenet. Chromatin, 2008, vol. 1, no. 1, p. 6.
Coppé, J.P., Patil, C.K., Rodier, F., et al., Senescence-associated secretory phenotypes reveal cell non-autonomous functions of oncogenic RAS and the p53 tumor suppressor, PLoS Biol., 2008, vol. 6, p. 2853.
Pole, A., Dimri, M., and Dimri, G.P., Oxidative stress, cellular senescence and ageing, AIMS Mol. Sci., 2016, vol. 3, no. 3, p. 300.
Sedelnikova, O.A., Redon, C.E., Dickey, J.S., et al., Role of oxidatively induced DNA lesions in human pathogenesis, Mutat. Res., 2010, vol. 704, p. 152.
von Zglinicki, T., Oxidative stress shortens telomeres, Trends Biochem. Sci., 2002, vol. 27, p. 339.
Campisi, J., Aging, cellular senescence, and cancer, Ann. Rev. Physiol., 2013, vol. 75, p. 685.
Ragnauth, C.D., Warren, D.T., Liu, Y., et al., Prelamin A acts to accelerate smooth muscle cell senescence and is a novel biomarker of human vascular aging, Circulation, 2010, vol. 121, p. 2200.
Scaffidi, P. and Misteli, T., Lamin A-dependent nuclear defects in human aging, Science, 2006, vol. 312, p. 1059.
Cao, K., Blair, C.D., Faddah, D.A., et al., Progerin and telomere dysfunction collaborate to trigger cellular senescence in normal human fibroblasts, J. Clin. Invest., 2011, vol. 121, no. 7, p. 2833.
Mitchell, S.J., Madrigal-Matute, J., Scheibye-Knudsen, M., et al., Effects of sex, strain, and energy intake on hallmarks of aging in mice, Cell Metab., 2016, vol. 23, no. 6, p. 1093.
Saxton, R.A. and Sabatini, D.M., mTOR signaling in growth, metabolism, and disease, Cell, 2017, vol. 168, no. 6, p. 960.
Herranz, N., Gallage, S., Mellone, M., et al., mTOR regulates MAPKAPK2 translation to control the senescence-associated secretory phenotype, Nat. Cell Biol., 2015, vol. 17, no. 9, p. 1205.
Laberge, R.-M., Sun, Y., Orjalo, A.V., et al., MTOR regulates the pro-tumorigenic senescence-associated secretory phenotype by promoting IL1A translation, Nat. Cell Biol., 2015, vol. 17, no. 8, p. 1049.
García-Prat, L., Martínez-Vicente, M., Perdiguero, E., et al., Autophagy maintains stemness by preventing senescence, Nature, 2016, vol. 529, no. 7584, p. 37.
Campisi, J. and d’Adda di Fagagna, F., Cellular senescence: when bad things happen to good cells, Nat. Rev. Mol. Cell Biol., 2007, vol. 8, no. 9, p. 729.
Salama, R., Sadaie, M., Hoare, M., and Narita, M., Cellular senescence and its effector programs, Genes Dev., 2014, vol. 28, no. 2, p. 99.
Imai, Y., Takahashi, A., Hanyu, A., et al., Crosstalk between the Rb pathway and AKT signaling forms a quiescence-senescence switch, Cell Rep., 2014, vol. 7, no. 1, p. 194.
Dimri, G.P., Lee, X., Basile, G., et al., A biomarker that identifies senescent human cells in culture and in aging skin in vivo, Proc. Natl. Acad. Sci. U.S.A., 1995, vol. 92, no. 20, p. 9363.
Watanabe, S., Kawamoto, S., Ohtani, N., and Hara, E., Impact of senescence-associated secretory phenotype and its potential as a therapeutic target for senescence-associated diseases, Cancer Sci., 2017, vol. 108, no. 4, p. 563.
Firsanov, D.V., Solovjeva, L.V., and Svetlova, M.P., H2AX phosphorylation at the sites of DNA double-strand breaks in cultivated mammalian cells and tissues, Clin. Epigenet., 2011, vol. 2, no. 2, p. 283.
Kuilman, T. and Peeper, D.S., Senescence-messaging secretome: SMS-ing cellular stress, Nat. Rev. Cancer, 2009, vol. 9, no. 2, p. 81.
Coppé, J.P., Desprez, P.Y., Krtolica, A., and Campisi, J., The senescence-associated secretory phenotype: the dark side of tumor suppression, Annu. Rev. Pathol., 2010, vol. 5, p. 99.
Coppé, J.P., Kauser, K., Campisi, J., and Beauséjour, C.M., Secretion of vascular endothelial growth factor by primary human fibroblasts at senescence, J. Biol. Chem., 2006, vol. 281, no. 40, p. 29568.
Elzi, D.J., Song, M., Hakala, K., et al., Wnt antagonist SFRP1 functions as a secreted mediator of senescence, Mol. Cell Biol., 2012, vol. 32, no. 21, p. 4388.
Nelson, G., Wordsworth, J., Wang, C., et al., A senescent cell bystander effect: senescence-induced senescence, Aging Cell, 2012, vol. 11, no. 2, p. 345.
Freund, A., Orjalo, A., Desprez, P.Y., and Campisi, J., Inflammatory networks during cellular senescence: causes and consequences, Trends Mol. Med., 2010, vol. 16, p. 238.
Campisi, J. and Robert, L., Cell senescence: role in aging and age-related diseases, Interdiscip. Top Gerontol., 2014, vol. 39, p. 45.
Doles, J., Storer, M., Cozzuto, L., et al., Age-associated inflammation inhibits epidermal stem cell function, Genes Dev., 2012, vol. 26, no. 19, p. 2144.
Turinetto, V., Vitale, E., and Giachino, C., Senescence in human mesenchymal stem cells: functional changes and implications in stem cell-based therapy, Int. J. Mol. Sci., 2016, vol. 7, no. 7, p. E1164.
Li, Y., Wu, Q., Wang, Y., et al., Senescence of mesenchymal stem cells, Int. J. Mol. Med., 2017, vol. 39, no. 4, p. 775.
Payushina, O.V., Localization and functions of mesenchymal stromal cells in vivo, Biol. Bull. Rev., 2016, vol. 6, no. 1, p. 1.
Lunyak, V.V., Amaro-Ortiz, A., and Gaur, M., Mesenchymal stem cells secretory responses: senescence messaging secretome and immunomodulation perspective, Front. Genet., 2017, vol. 8, p. 220.
Ullah, I., Subbarao, R.B., and Rho, G.J., Human mesenchymal stem cells—current trends and future prospective, Biosci. Rep., 2015, vol. 35, no. 2, p. e00191.
Dominici, M., Le Blanc, K., Mueller, I., et al., Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement, Cytotherapy, 2006, vol. 8, no. 4, p. 315.
Hoogduijn, M.J., Betjes, M.G., and Baan, C.C., Mesenchymal stromal cells for organ transplantation: different sources and unique characteristics? Curr. Opin. Organ Transplant., 2014, vol. 19, no. 1, p. 41.
Mattar, P. and Bieback, K., Comparing the immunomodulatory properties of bone marrow, adipose tissue, and birth-associated tissue mesenchymal stromal cells, Front. Immunol., 2015, vol. 6, p. 560.
McLeod, C.M. and Mauck, R.L., On the origin and impact of mesenchymal stem cell heterogeneity: new insights and emerging tools for single cell analysis, Eur. Cell Mater., 2017, vol. 34, p. 217.
Andreeva, E.R. and Buravkova, L.B., Paracrine activity of multipotent mesenchymal stromal cells and its modulation in hypoxia, Hum. Physiol., 2013, vol. 39, no. 3, p. 315.
Richardson, S.M., Kalamegam, G., Pushparaj, P.N., et al., Mesenchymal stem cells in regenerative medicine: focus on articular cartilage and intervertebral disc regeneration, Methods, 2016, vol. 99, p. 69.
Rubina, K.A., Kalinina, N.I., Efimenko, A.Yu., et al., Mechanism of stimulation of angiogenesis in ischemic myocardium with the help of adipose tissue stromal cells, Kardiologiya, 2010, no. 50, p. 51.
Kalinina, N.I., Sysoeva, V.Yu., Rubina, K.A., et al., Mesenchymal stem cells in tissue growth and repair, Acta Nat., 2011, vol. 3, no. 4, p. 32.
Zuk, P.A., The adipose-derived stem cell: looking back and looking ahead, Mol. Biol. Cell, 2010, vol. 21, p. 1783.
Natesan, S., Zhang, G., Baer, D.G., et al., A bilayer construct controls adipose-derived stem cell differentiation into endothelial cells and pericytes without growth factor stimulation, Tissue Eng., Part A, 2011, vol. 17, nos. 7–8, p. 941.
Gnecchi, M., Danieli, P., Malpasso, G., and Ciuffreda, M.C., Paracrine mechanisms of mesenchymal stem cells in tissue repair, Methods Mol. Biol., 2016, vol. 1416, p. 123.
Hodgkinson, C.P., Bareja, A., Gomez, J.A., and Dzau, V.J., Emerging concepts in paracrine mechanisms in regenerative cardiovascular medicine and biology, Circ. Res., 2016, vol. 118, no. 1, p. 95.
Gornostaeva, A., Andreeva, E., and Buravkova, L., Factors governing the immunosuppressive effects of multipotent mesenchymal stromal cells in vitro, Cytotechnology, 2016, vol. 68, no. 4, p. 565.
Gu, Y., Li, T., Ding, Y., et al., Changes in mesenchymal stem cells following long-term culture in vitro, Mol. Med. Rep., 2016, vol. 13, no. 6, p. 5207.
Legzdina, D., Romanauska, A., Nikulshin, S., et al., Characterization of senescence of culture-expanded human adipose-derived mesenchymal stem cells, Int. J. Stem Cells, 2016, vol. 9, no. 1, p. 124.
Ratushnyy, A., Lobanova, M., and Buravkova, L.B., Expansion of adipose tissue-derived stromal cells at “physiologic” hypoxia attenuates replicative senescence, Cell Biochem. Funct., 2017, vol. 35, no. 4, p. 232.
Kim, M., Kim, C., Choi, Y.S., et al., Age-related alterations in mesenchymal stem cells related to shift in differentiation from osteogenic to adipogenic potential: implication to age-associated bone diseases and defects, Mech. Ageing Dev., 2012, vol. 133, no. 5, p. 215.
Despars, G., Carbonneau, C.L., Bardeau, P., et al., Loss of the osteogenic differentiation potential during senescence is limited to bone progenitor cells and is dependent on p53, PLoS One, 2013, vol. 8, no. 8, p. e73206.
Wagner, W., Horn, P., Castoldi, M., et al., Replicative senescence of mesenchymal stem cells: a continuous and organized process, PLoS One, 2008, vol. 3, no. 5, p. e2213.
Digirolamo, C.M., Stokes, D., Colter, D., et al., Propagation and senescence of human marrow stromal cells in culture: a simple colony-forming assay identifies samples with the greatest potential to propagate and differentiate, Br. J. Haematol., 1999, vol. 107, no. 2, p. 275.
Cheng, H., Qiu, L., Ma, J., et al., Replicative senescence of human bone marrow and umbilical cord derived mesenchymal stem cells and their differentiation to adipocytes and osteoblasts, Mol. Biol. Rep., 2011, vol. 38, no. 8, p. 5161.
Stechschulte, L.A. and Lecka-Czernik, B., Reciprocal regulation of PPARγ and RUNX2 activities in marrow mesenchymal stem cells: fine balance between p38 MAPK and protein phosphatase 5, Curr. Mol. Biol. Rep., 2017, vol. 3, no. 2, p. 107.
Lee, Y.H., Lee, H.Y., Kim, T.G., et al., PPARγ maintains homeostasis through autophagy regulation in dental pulp, J. Dent. Res., 2015, vol. 94, no. 5, p. 729.
Sepúlveda, J.C., Tomé, M., Fernández, M.E., et al., Cell senescence abrogates the therapeutic potential of human mesenchymal stem cells in the lethal endotoxemia model, Stem Cells, 2014, vol. 32, no. 7, p. 1865.
Baggiolini, M. and Clark-Lewis, I., Interleukin-8, a chemotactic and inflammatory cytokine, FEBS Lett., 1992, vol. 307, p. 97.
Li, A., Dubey, S., Varney, M.L., et al., IL-8 directly enhanced endothelial cell survival, proliferation, and matrix metalloproteinases production and regulated angiogenesis, J. Immunol., 2003, vol. 170, p. 3369.
Blankenberg, S., Rupprecht, H.J., Bickel, C., et al., Circulating cell adhesion molecules and death in patients with coronary artery disease, Circulation, 2001, vol. 104, p. 1336.
Tanuma, N., Sakuma, H., Sasaki, A., and Matsumoto, Y., Chemokine expression by astrocytes plays a role in microglia/macrophage activation and subsequent neurodegeneration in secondary progressive multiple sclerosis, Acta Neuropathol., 2006, vol. 112, p. 195.
Spoettl, T., Hausmann, M., Herlyn, M., et al., Monocyte chemoattractant protein-1 (MCP-1) inhibits the intestinal-like differentiation of monocytes, Clin. Exp. Immunol., 2006, vol. 145, p. 190.
Li, Y., Xu, X., Wang, L., et al., Senescent mesenchymal stem cells promote colorectal cancer cells growth via galectin-3 expression, Cell Biosci., 2015, vol. 5, p. 21.
Di, G.H., Liu, Y., Lu, Y., et al., IL-6 secreted from senescent mesenchymal stem cells promotes proliferation and migration of breast cancer cells, PLoS One, 2014, vol. 9, no. 11, p. e113572.
Skolekova, S., Matuskova, M., Bohac, M., et al., Cisplatin-induced mesenchymal stromal cells-mediated mechanism contributing to decreased antitumor effect in breast cancer cells, Cell Commun. Signaling, 2016, vol. 14, p. 4.
Minieri, V., Saviozzi, S., Gambarotta, G., et al., A new paradigm in cardiac regeneration: The mesenchymal stem cell secretome, Stem Cells Int., 2015, vol. 2015, p. 765846.
Özcan, S., Alessio, N., Acar, M.B., et al., Unbiased analysis of senescence associated secretory phenotype (SASP) to identify common components following different genotoxic stresses, Aging (N.Y.), 2016, vol. 8, no. 7, p. 1316.
van Niel, G., D’Angelo, G., and Raposo, G., Shedding light on the cell biology of extracellular vesicles, Nat. Rev. Mol. Cell Biol., 2018, vol. 19, no. 4, p. 213.
Tkach, M. and Théry, C., Communication by extracellular vesicles: where we are and where we need to go, Cell, 2016, vol. 164, no. 6, p. 1226.
Liu, S., Mahairaki, V., Bai, H., et al., Highly purified human extracellular vesicles produced by stem cells alleviate aging cellular phenotypes of senescent human cells, Stem Cells, 2019, vol. 37, no. 6, p. 779.
Geiger, H., de Haan, G., and Florian, M.C., The ageing hematopoietic stem cell compartment, Nat. Rev. Immunol., 2013, vol. 13, no. 5, p. 376.
Kfoury, Y. and Scadden, D.T., Mesenchymal cell contributions to the stem cell niche, Cell Stem Cell, 2015, vol. 16, no. 3, p. 239.
Adams, G.B., Martin, R.P., Alley, I.R., et al., Therapeutic targeting of a stem cell niche, Nat. Biotechnol., 2007, vol. 25, no. 2, p. 238.
Mendez-Ferrer, S., Michurina, T.V., Ferraro, F., et al., Mesenchymal and hematopoietic stem cells form a unique bone marrow niche, Nature, 2010, vol. 466, no. 7308, p. 829.
Mendelson, A. and Frenette, P.S., Hematopoietic stem cell niche maintenance during homeostasis and regeneration, Nat. Med., 2014, vol. 20, no. 8, p. 833.
Haas, S., Hansson, J., Klimmeck, D., et al., Inflammation-induced emergency megakaryopoiesis driven by hematopoietic stem cell-like megakaryocyte progenitors, Cell Stem Cell, 2015, vol. 17, no. 4, p. 422.
Pietras, E.M., Mirantes-Barbeito, C., Fong, S., et al., Chronic interleukin-1 exposure drives hematopoietic stem cells towards precocious myeloid differentiation at the expense of self-renewal, Nat. Cell Biol., 2016, vol. 18, no. 6, p. 607.
Bernardo, M.E. and Locatelli, F., Mesenchymal stromal cells in hematopoietic stem cell transplantation, Methods Mol. Biol., 2016, vol. 1416, p. 3.
Takeuchi, M., Takeuchi, K., Kohara, A., et al., Chromosomal instability in human mesenchymal stem cells immortalized with human papilloma virus E6, E7 and hTERT genes, In Vitro Cell Dev. Biol. Anim., 2007, vol. 43, nos. 3–4, p. 129.
Wei, F., Qu, C., Song, T., et al., Vitamin C treatment promotes mesenchymal stem cell sheet formation and tissue regeneration by elevating telomerase activity, J. Cell Physiol., 2012, vol. 227, no. 9, p. 3216.
Lin, T.M., Tsai, J.L., Lin, S.D., et al., Accelerated growth and prolonged lifespan of adipose tissue-derived human mesenchymal stem cells in a medium using reduced calcium and antioxidants, Stem Cells Dev., 2005, vol. 14, no. 1, p. 92.
Choi, J.R., Pingguan-Murphy, B., Wan Abas, W.A., et al., In situ normoxia enhances survival and proliferation rate of human adipose tissue-derived stromal cells without increasing the risk of tumourigenesis, PLoS One, 2015, vol. 10, no. 1, p. e0115034.
Buravkova, L.B., Andreeva, E.R., Gogvadze, V., and Zhivotovsky, B., Mesenchymal stem cells and hypoxia: where are we? Mitochondrion, 2014, vol. 19, part A, p. 105.
Gharibi, B., Farzadi, S., Ghuman, M., and Hughes, F.J., Inhibition of Akt/mTOR attenuates age-related changes in mesenchymal stem cells, Stem Cells, 2014, vol. 32, no. 8, p. 2256.
Okada, M., Kim, H.W., Matsuura, K., et al., Abrogation of age-induced microRNA-195 rejuvenates the senescent mesenchymal stem cells by reactivating telomerase, Stem Cells, 2016, vol. 34, no. 1, p. 148.
Gharibi, B. and Hughes, F.J., Effects of medium supplements on proliferation, differentiation potential and in vitro expansion of mesenchymal stem cells, Stem Cells Transl. Med., 2012, vol. 1, no. 11, p. 771.
Funding
The study was supported by the Russian Foundation for Basic Research (project no. 19-015-00150) and the scholarship of the President of the Russian Federation (no. SP-960.2019.4).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
The authors declare that they have no conflict of interest. This article does not contain any studies involving animals or human participants performed by any of the authors.
Additional information
Translated by M. Batrukova
Rights and permissions
About this article
Cite this article
Ratushnyy, A.Y., Buravkova, L.B. Cell Senescence and Mesenchymal Stromal Cells. Hum Physiol 46, 85–93 (2020). https://doi.org/10.1134/S0362119720010132
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0362119720010132