Abstract
The potential of using centrifugation in the separation of suspended forms from dissolved and colloidal forms in the chemical analysis of river waters is demonstrated in the case of rivers in the south of the Russian Far East. The concentrations of colloidal and suspended particles in nonfiltered water, as well as in centrifugates and filtrates, were characterized with the use of the method of dynamic light scattering (DLS). The comparison of DLS intensity in centrifugates and 0.45-µm filtrates was used to calculate the native density of pelitic and coarse colloidal particles of river suspension, which made it possible to correlate the centrifugation regimes with the size of settling particles within the range from 0.45 to 3 µm. Chemical analysis of supernatants of river water, obtained at different centrifugation regimes was used to evaluate the distribution of chemical elements between dissolved/colloidal (<0.45 µm), coarse-colloidal (0.45–1 µm), and pelite (1–3 µm) fractions. A significant linear relationship was found between the DLS intensity and the concentrations in centrifugates of Fe, Al, Ti, Th, Sc, REE, i.e., chemical elements with a high fraction of coarse colloidal and suspended forms, which confirms the possibility to assess the concentration of colloidal particles in centrifugates by the intensity of DLS. A disadvantage of centrifugation is that it is difficult to use in the field.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
INTRODUCTION
The separation of suspended and dissolved/colloidal forms of chemical elements and compounds is a necessary procedure of chemical analysis of natural, especially river, waters in most countries, including the Russian Federation [3, 4, 25]. This is due to the considerable proportion of suspended forms in the total concentrations of many chemical elements in river water [1, 5]. At the same time, the suspended and colloidal/dissolved forms have different migration capacity and bioavailability. Water quality in terms of the concentration of many chemical compounds is standardized by the concentration of dissolved/colloidal forms [3].
The most common method used to separate suspended forms from dissolved colloidal ones is the filtration of a water sample through various membranes with a pore size of 0.45 or 0.2 µm. The application of 0.45-µm filters has a long history [16], but it lacks a rigorous scientific substantiation, being rather the result of a convention. The growing application in the recent time of 0.2 µm filters is due to the fact that these membranes retain practically all bacteria [13], the result being that, in addition to the separation of suspended and dissolved/colloidal forms, the filtrates are sterilized. At the same time, from the viewpoint of colloidal chemistry, the boundary between the suspended and colloidal particles is 1 µm [10]. Thus, some part of large-size colloids 0.45–1.0 µm (0.2–1.0 µm) is now classified as suspension at filtration, and the concentration of dissolved/colloidal forms, determined in the filtrate is underestimated. However, the main drawback of the use of membrane filters 0.45/0.20 µm is that the filtration process is accompanied by clogging of pores, and their effective diameter can decrease by an order of magnitude and more [17, 19, 23]. This will be accompanied by an uncontrolled decrease in the concentrations in the filtrate of Fe, Al, Ti, and other elements, for which a great role of colloidal form (<0.45 µm) in river water is typical [7, 19]. If the effect of colmatation is not taken into account, an obvious shift will take place in water quality estimates, calculations of sorption equilibria, and geochemical fluxes. Several methods were proposed to reduce the effect of colmatation: the use of capsule filters with a high filtering capacity [25], change of membrane filters in the course of filtration [19], an increase in the volume of filtered water [23].
An alternative method for separating suspended and dissolved/colloidal forms, at which no colmatation takes place, is centrifugal separation, when suspended particles settle onto the bottom of centrifuge tubes, while dissolved and colloidal forms remain in the centrifugate (supernatant). The conditions of centrifugation are determined in accordance with Stokes law:
where t is the time of centrifugation of particles (aggregates) with diameter D (cm) and density dp (g/cm3) in water with density of dw = 1.0 g/cm3 and dynamic viscosity η = 0.01 g/(cm s), angular rotation velocity ω (rad/s), and the distance from the axis of rotation to the deposition level R1 and to the suspension surface level R2 [15].
Centrifugation is most often and successfully used in soil sciences to separate particles of different size [20, 24]. Since, genetically, river suspension is largely represented by products of soil cover denudation, the use of centrifugation to separate suspended and colloidal forms of chemical elements in river water looks quite reasonable. The main problem here is the lack of direct data on the density of river suspension particles, which are required to calculate the time and rate of centrifugation. The material of river suspension, collected on membranes or with the use of flow centrifugation from a large water volume and dried at 80–105°C, has a density of 2.0–2.5 g/cm3 in accordance with its mostly aluminosilicate nature. The bulk chemical composition of the suspension [1, 5] confirms its aluminosilicate base with a possible admixture of Fe hydroxides and organic matter. However, the data on the density of dried material do not suggest that the river suspension in its native state will have the same density. For example, aggregates that form by erosion and roiling of river silt have a density of 1.65 g/cm3 [12]. Electronic microscopy of a supernatant after five-hour centrifugation of river water at 4000 rpm reveals particles ≥0.4 µm in size, which the authors attributed to the presence of organomineral aggregates with low density in river suspension [14]. Clearly, independent methods are required to control the degree of separation of suspended and colloidal particles by centrifugation. The most powerful and universal are the varieties of flow field flow fractionation (FFFF) [15], which are increasingly used to study the distribution of chemical elements over the entire dimensional spectrum of colloidal and suspended particles in river water [11]. However, these methods are laborious and require expensive equipment. A simpler method used to determine the amount of colloidal and suspended particles remaining in supernatant fluid is the dynamic light scattering (DLS) [18]. This method evaluates the fluctuations of dispersion of a laser beam due to the Brownian motion of particles with sizes 0.001–10.0 µm, which depend, in particular, on particle sizes, thus making it possible, under some conditions, to calculate the distribution of particle size. M. Filella et al. [14] showed that, in polydisperse natural water, this calculation can be approximate at best. However, the data on the general intensity of DLS provide some information about the concentration of colloidal and suspended particles in water [26], in particular, in filtrates and centrifugates [8]. We suppose that the intensity of DLS is proportional to the total concentration of particles in water, and capsule filters with a pore size of 0.45 µm, which are little susceptible to clogging, ensure effective separation of particles with the appropriate size. In such case, equating the intensity of DLS in the filtrate after the capsule filter 0.45 µm to the DLS intensity in the supernatant, we can calculate the density of the settling particles >0.45 µm and then correlate the centrifugation regimes used in the experiment with the river suspension particles of appropriate size. Such an approach is expected to characterize the potential of centrifugation at different regimes for separating particles in the ranges 0.45–1 and 1–3 µm, with DLS method used to control the concentrations of suspended and colloidal particles in centrifugates and filtrates. This is the first task of this work. Its second task is to assess the distribution of chemical elements between the fine pelitic suspension (1–3 µm), large colloids (0.45–1 µm), and dissolved/colloidal forms (<0.45 µm) by changes in the concentrations of elements in supernatants, obtained under different centrifugation conditions.
MATERIALS AND METHODS
Study Objects
Centrifugation at different regimes was applied to water samples taken in the lower reaches of the Razdol’naya River—a river in the boreal zone in the southern Primorskii krai, RF, with a drainage area of 16.8 thous. km2, an average water discharge of 75 m3/s, and an average suspension concentration of 55 mg/L. The upper half of the basin lies in the territory of PRC. The population density varies from 30 pers/km2 in the territory of PRC to 39 pers/km2 in the Primorskii Krai. Almost the entire catchment of the Razdol’naya River is actively used for agriculture. In addition, residential areas are located on the banks of the river, including Ussuriisk City with a population of 180 000 (ranking third in size in the Primorskii krai, RF) and developed industry. Accordingly, the chemistry of the Razdol’naya River water features higher concentrations of some chemical elements and compounds [2, 6]. Water samples for centrifugation were taken in the lower reaches of the Razdol’naya River (Terekhovka Settl.) under different regimes from September to December 2022 at a gradual decrease in the water flow from monsoon flood to late-autumn low-water period and at an appropriate decrease in suspension concentration from 125.7 to 14.2 mg/L. The main characteristics of river water samples used for centrifugation at different regimes are given in Table 1.
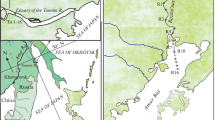
In addition, to characterize the dependence of DLS intensity on the amount of suspension in river water and to compare the filtrates through the capsule filter 0.45 µm and supernatants at the most intense regime of centrifugation (30′ at 4500 rot/min), 20 more samples were taken from other rivers in the southern regions of FE RF (the Tumannaya, Narva, Amba, Razdol’naya, Ussuri, and Amur) with suspension concentration from 4.6 to 450 mg/L, taken since October 2022 to April 2023. The study area with sampling points is shown in Fig. 1a.
Sampling and Sample Separation
River water samples were taken from the bank into a 2-L polyethylene vessel attached to a plastic pole. The vessels were prewashed with detergent, soaked for several days with double-distilled water, rinsed with deionized water (18 MΩ), and dried; before sampling, they were rinsed several times with the sampled water. The conductivity, temperature, and pH were measured in situ. The samples were packed in plastic bags and sealed plastic boxes and delivered to the laboratory within several hours.
The general scheme of sample processing is given in Fig. 1b. In the laboratory, the samples were divided into several aliquots at intense mixing. The untreated source sample was analyzed to determine the DLS intensity. The second aliquot (0.3–0.4 L) was filtered through a Pall GWV capsule filter with a pore size of 0.45 µm, which is little susceptible to clogging because of the large filtering surface area (700 cm2). A parallel aliquot 0.5–1.0 L was filtered through pre-weighed Millipore Durapore membrane filters 47 mm in diameter with a pore size of 45 µm for determining suspended matter concentration gravimetrically [4].
Other 7 aliquots were centrifuged in 50-mL polypropylene test tubes on Elmi and Heraus Multifuge centrifuges at different regimes: 5′/1500, 12′/1500, 6′/3000, 10′/3000, 15′/3000, 20′/3500, and 30′/4500 rpm. The centrifuge tubes, filters, and filtration equipment were treated by 0.1 H НСl, and next many times rinsed by distilled water (18 MΩ) and the sample. All procedures of centrifugation and filtering were accompanied by blank samples, i.e., the operations were performed with deionized water, which next was subject to chemical analysis as a sample.
Samples of 5–10 mL of centrifugates (supernatants) and filtrates were taken for determining the intensity of DLS. The remaining part (40 mL) was acidified by 0.8 mL of double-distilled concentrated HNO3 to pH 1 for subsequent determination of the concentrations of chemical elements from Li to U by ICP-MS method. In addition, centrifugates 10′/3000 and 30′/4500 rpm and filtrates through a capsule filter were analyzed to determine the concentration of dissolved carbon (DOC, mg/L), sulfates, chlorides, and nitrates. ICP-MS analysis was carried out in the Center for Shared Use, FEGI FEB RAS, all other analyses were made in the Center for Shared Use, TSLEDGIS PIG FEB RAS.
Assessing DLS Intensity
The rate of DLS was determined on a PHOTOCOR Compact Z device. This device uses a thermally stabilized 638 nm semiconductor laser with adjustable power of 10–200 mW and the ability to reduce the intensity of scattered light (attenuator), has a temperature control system with an accuracy of 0.1°C and a built-in “Photocor FC” correlator with a minimum sampling time of 10 ns. The sample volume is 1.5–3 mL. The results were processed in the DynaLS program. The snaps were taken in cuvettes made of optical polymethyl methacrylate. The source homogenized samples, as well as filtrates and centrifugates were snapped in two or three replicates. In addition, the source samples were re-snapped with intervals from 1–3 days to 2–3 months to assess possible instrument drift and irreversible coagulation processes in samples when stored at 4°C. In parallel measurements and storage, the changes in DLS intensity were ≤5 from the initial value. The laser power was 15 mW with attenuator 1, the signal accumulation time was 3 min.
The DLS method, which is also called photon correlation spectroscopy (PCS), is based on the analysis of variations in laser light scattering due to the Brownian motion of suspended and colloidal particles. Analysis of the correlation function of these fluctuations in the intensity of scattering can be used to calculate the diffusion coefficient of dispersed particles and next to calculate the hydrodynamic size of the particles that cause scattering [22]. For monodisperse systems, mathematically accurate determination of particle size is possible based on fluctuation of the scattering rate. For polydisperse systems, the calculated distribution of particle size should be regarded as tentative [9, 14]. However, the total intensity of DLS scatter is found to be proportional to suspension concentration in the original river water with a high determination coefficient (Fig. 2), which allows the rate of scattering intensity DLS to be used as a characteristic of particle concentrations in both centrifugates and filtrates [8].
Determining the Concentrations of Chemical Elements and Compounds in Centrifugates and Filtrates
Centrifugates (supernatants) and filtrates, acidified with nitric acid to pH 1, were stored in a refrigerator for several weeks before analysis on an Agilent 7700 instrument (Primorsky Center for Local Elemental and Isotope Analysis, FEGI FEB RAS, Vladivostok). The accuracy of the results was verified by regular analyses of diluted standards of bottom sediments BCSS-1 and water CRM-TMDW-A. The difference with the passport data was ≤15%. The results of blank samples at filtration were <10 of concentrations determined in the samples for most microelements.
The electrical conductivity as a measure of TDS was measured by instrument YSI Pro Plus. pH was measured at sampling by Hanna HI9126 with an accuracy of ±0.02 pH unit. The filtrates and centrifugates for the analysis of DOC and anions were stored in a refrigerator. DOC was determined by high-temperature catalytic oxidation on a Shimadzu TOC-V cpn instrument with an accuracy of ±5% relative. The blank samples at determining DOC were ≤0.1 mg/L. The anions \({\text{SO}}_{4}^{{2 - }}\), Cl–, and \({\text{NO}}_{3}^{ - }\) were determined by the method of ion chromatography on a Shimadzu LC-10 with an accuracy of ±4–5%. The concentration of suspension SS was evaluated by weighing filters before and after filtration of 500-mL water samples and drying at 80°C.
RESULTS AND DISCUSSION
Estimation of Particle Size in River Suspension Settling under Different Centrifugation Conditions
The intensity of DLS of supernatants steadily decreased at an increase in the time and speed of centrifugation (Fig. 3a). This is in agreement with the initial hypothesis regarding the proportionality of particle content and DLS intensity in river water and confirms the possibility of using centrifugation to separate colloidal and suspended particles. At the same time, at the majority of centrifugation regimes, calculated based on particle densities of 2.5 and 1.65 g/cm3, used in studies on separation of soil particles [12, 24], the intensity of DLS in supernatants was found to be much higher than in solutions obtained after filtration through a 0.45 µm capsule filter (Fig. 3a). Only at an increase in the centrifugation time to 30′ at 4500 rpm, the DLS intensity in the supernatant becomes comparable with that in filtrate 0.45 µm (Fig. 3b). This suggests the conclusion that the centrifugation regime of 30′/4500 rpm leads to settling of all particles larger than 0.45 µm or their majority.
(a) DLS intensity (I, cps) in water samples from the Razdol’naya River (susp), and at different regimes of centrifugation and filtering through a capsule filter (0.45_GWV); the vertical lines show the standard deviation of parallels and repetitions; (b) the comparison of DLS intensity (I, cps) in supernatants obtained by centrifugation 30′ at 4500 rpm (CF 0.45), and in filtrates through capsule filters (GWV 0.45) in all examined rivers in the southern FE of RF, sampled since September 2022 to May 2023.
Now, formula (1) can be used to calculate the density of river suspension particles at centrifugation, which gives 1.184 g/cm3. The volumetric density as low as that, indicates to the migration of aluminosilicate suspension in the Razdol’naya River flow in the form of low-density aggregates, likely, with an admixture of organic material. At least, this is true for the pelitic component of the suspension.
The calculated density of 1.184 g/cm3 was used to evaluate by formula (1) the minimal size of the particles that settle at the centrifugation regimes used: 5′/1500—2.96 µm, 12′/1500—1.9 µm, 6′/3000—1.35 µm, 10′/3000—1.0 µm, 15′/3000—0.85 µm, 20′/3500— 0.63 µm, 30′/4500—0.45 µm.
It should be mentioned that, although the DLS intensity of supernatants at centrifugation within 30′ at 4500 rpm is at the same level as the DLS intensity of 0.45 µm filtrates, the variations of the DLS intensity in the filtrates and centrifugates of the same sample can reach 30%. However, on the average for river waters in the southern RF FE, sampled from September 2022 to May 2023, the intensity of DLS in centrifugates 30′/4500 was only 13.5% less than in the filtrates through a 0.45 µm capsule filter (Fig. 3b).
More detail analysis of the dynamics of the decrease in DLS intensity for centrifugates with decreasing diameter of precipitating river suspension particles indicates to an appreciable change in the dynamics at a decrease in the minimal size of particles remaining in the supernatant (Fig. 4). At a decrease in the minimal size of precipitating particles from 3 to 1 µm, the intensity of DLS decreases by 20–30%; however, at a further decrease from 1 to 0.45 µm, the intensity of DLS drops by 3–4 times, i.e., the decrease follows a logarithmic law with a significant coefficient of determination. If we assume that the DLS intensity is proportional to the concentration of particles, this means that the mass of the fraction 1–3 µm in the examined water samples from the Razdol’naya River is appreciably less than that of the fraction 0.45–1.0 µm.
Now, there are no reliable and correct methods for determining the particle masses of individual river suspension fractions in samples with a volume of 10–50 mL, in which chemical analysis was carried out and the intensity of DLS was measured. However, the accelerated decrease in DLS intensity after centrifugation of the fraction <1 µm, may indicate to the considerable fraction of large colloids 0.45–1.0 µm in the composition of the pelite component of suspension in the Razdol’naya River smaller than 3 µm. In addition, an obvious steady decrease can be seen in the scale of decrease in the DLS rate from sample R223, taken during flood and containing 125.7 mg/L of suspension, to sample R226, taken in the low-water season before freeze-up and containing as little as 14.2 mg/L of suspended matter. In the sample R223, the intensity of DLS decreases by 5.5 times at the intensification of centrifugation, while in the sample R226, it decreases only by 3.7 times (Fig. 4).
Thus, the change in DLS intensity of supernatants at an increase in the centrifugation speed and time corresponds to the general trend in the dependence of DLS intensity on the concentration of particles in water and can be used to characterize centrifugation efficiency at the separation of large colloids and suspended particles. The comparison of DLS intensity in centrifugates and filtrates shows that the coarse colloid and pelite suspension occurs in river water in the form of aggregates with the volumetric density of 1.184 g/cm3. The centrifugation regime of 30′ at 4500 rpm leads to sedimentation of suspended and colloidal particles larger than 0.45 µm, while that for 10′ at 3000 rpm, larger than 1 µm. Judging by the dynamics of the decrease in DLS intensity with an increase in centrifugation speed, the concentration of large colloids in the Razdol’naya R. water (0.45–1.0 µm) is greater than that of pelite material (1–3 µm).
Therefore, DLS data show that river water centrifugation gives a correct separation of fractions <0.45, 0.45–1.0, and 1–3 µm. The advantage of centrifugation compared with filtration is the absence of clogging, which is inevitable at the use of standard membrane filters [7]. A drawback of the use of centrifugation to separate colloidal and suspended particles is that the intensity of DLS, which is used to assess centrifugation efficiency, can be directly correlated with the mass concentration of suspended particles only for the original samples of river water (Fig. 2). The direct determination of the mass concentration of coarse colloids and fine suspension in individual fractions separated by centrifugation is impossible, and the intensity of DLS is a relative integral estimate of particle concentration in water, depending on their number and shape, as well as their size distribution [14]. In addition, the use of centrifugation for sample separation in the field at the sampling site is less convenient than filtration and sometimes even impossible.
Changes in Supernatant Chemistry under Different Centrifugation Regimes
Clearly, the changes in chemical element concentrations in supernatants, obtained at different centrifugation speeds will depend on the proportions of suspended and large colloidal forms capable of settling in the total concentration of the element in water. If the dominating fraction is dissolved ionic forms and complexes or fine colloids that do not settle at the centrifugation regimes used in the experiment, then the concentration of chemical elements in supernatants will remain constant. However, the forms associated with suspension (>1.0 µm) or large colloids (0.45–1.0 μm) will settle, and the concentration in the supernatant should decrease with an increase in centrifugation rate, i.e., with a decrease in the size of settling particles. Indeed, the concentration in centrifugates of elements with an abrupt predominance of dissolved ionic forms in river water (Na, K, Ca, Mg, B, Sr) does not depend on centrifugation intensity, as has been seen in all examined water regimes of the Razdol’naya River (Figs. 5a, 5b, example for Na and Sr). The concentration of dissolved organic carbon (DOC) also shows no appreciable decrease at the intensification of centrifugation, nor a decrease in the estimated size of precipitated particles from 3 to 0.45 µm (Fig. 5c). This indicates the presence of organic carbon in river water in dissolved and fine colloidal forms, which is confirmed by detail studies of river water with the use of ultrafiltration [21]. For Mo, constant concentration in centrifugates is recorded in most cases, except for flood (R223), when Mo concentration decreased 1.5 times with intensification of centrifugation (Fig. 5d). At the same time, for a large number of elements with the predominance of dissolved forms in river water (Li, Si, Mn, Co, Ni, Cu, As, Se, Rb, Cs, Ba, U), a relatively constant concentration in centrifugates is observed only in the sample R226, taken in the late-autumn low-water period in December at low concentration of suspension (14.2 mg/L). At an increase in suspension concentration to 29–32 mg/L (R224, 225), especially, during flood (R223), at the amount of suspension of 126 mg/L, the concentration was found to decrease appreciably (by a factor of 1.6–2.2) at an increase in centrifugation speed and a decrease in the size of precipitating particles (Figs. 5e, 5f, example for Ni and As). This indicates the migration of an appreciable part of these elements during high flood in the form of a fine pelitic suspension (1–3 µm) and large colloids (0.45–1.0 µm).
The decrease in the concentration in supernatants at the intensification of centrifugation was most pronounced for Fe, Al, Ti, Th, Sc, and REE (Fig. 6), i.e., for the chemical elements for which maximal proportion of suspended and coarse-colloidal forms is typical during the migration in river water [1, 21]. At a decrease in the calculated size of precipitating particles from 3 to 0.45 µm, the concentration of those metals decreases by 2–9 times for Fe, Al, and Ti and by 1.8–6 times for REE and other hydrolyzed elements.
Noteworthy is the obvious similarity in the rates of decrease in DLS intensity (Fig. 4) and the decrease in the concentrations of these elements-hydrolysates (Fig. 6) in centrifugates at a decrease in the minimal size of precipitating particles. Accordingly, a significant positive linear relationship can be seen between the concentrations of Fe, Al, Sc, Ce, Dy, Th in centrifugates and the rate of DLS in them. A relationship with high determination coefficients between metal concentrations and the rate of DLS in centrifuges can also be seen both for individual samples and for the series of all examined samples, whatever the water regime (Fig. 7). This confirms the assumption that the concentrations of Fe, Al, Th, Sc, and REE in supernatants is controlled by the concentration of large colloids (0.45–1.0 µm) and pelitic suspension (1–3 µm), remaining there at centrifugation, which, in turn, is reflected in the DLS intensity. In addition, this proves that the DLS intensity in centrifugates is proportional to the concentration of coarse colloids and fine suspension, because the data of ICP-MS analysis are mass concentrations.
For the chemical elements that migrate in river water in ionic forms (Na, Sr, Mo), the concentrations in centrifugates are not related with DLS intensity, while for elements with a high seasonal variability of concentrations (Mn, Cu, As), such a relationship is observed only in individual cases.
A significant relationship between the concentrations of some metals and DLS intensity in centrifugates (Fig. 7) suggests that the concentration of these chemical elements is proportional to the concentration of colloid particles. In addition, the stable trend toward a decrease in the concentration at an increase in centrifugation intensity (Figs. 5, 6) makes it possible to calculate the distribution of Fe, Al, Ti, REE, and other elements-hydrolysates between fine pelite (1–3 µm), coarse colloids (0.45–1.0 µm) and forms <0.45 µm. To do this, it is proposed to use the concentration of chemical elements in centrifugates after 30′ at 4500 rpm (<0.45 µm), 10′ at 3000 rpm (1 µm), and 5′ at 1500 rpm (3 µm). The results of calculations (Figs. 8a–8e) confirm that, at least for the Razdol’naya River, the proportion of Fe, Al, Ti, and REE, that occur in water as components of large colloids 0.45–1.0 µm, often exceeds the concentration of dissolved forms <0.45 µm. At the same time, there is no direct relationship between the concentration and the proportion of coarse-colloidal forms of those metals and the suspension concentration. The concentrations of the same metals in the fine-pelite fractions are commonly less than in coarse colloidal (0.45–1.0 µm) and dissolved (<0.45 µm) forms, and, unlike them, it is controlled primarily by suspension concentration in river water (Fig. 8a, the example for Fe).
Variations of the concentration (µg/L) of dissolved/colloidal (0.45 µm), coarse colloidal (0.45–1 µm), and fine pelite (1–3 µm) forms of some chemical elements in the Razdol’naya R. water from the autumn flood (R223) to prewinter low water period (R226) at (a) a decrease in suspension concenration (SS) from 126 to 14 mg/L.
A similar calculation made for chemical elements with the predominance of dissolved/colloidal forms <0.45 µm (Figs. 8f–8h) showed that the proportion of large colloidal forms during low-water season is ≤20% of the total concentration of forms <3 µm. However, during floods (R223), the proportion of large colloidal forms of Ni, Mo (Figs. 8f, 8h), as well as Cu, As, Rb (not shown) increases to 40–50% of the total concentration. The effect of water regime is most clearly seen in Mn distribution: during floods, the proportion of large colloidal (0.45–1.0 µm) and fine pelite (1–3 µm) forms increases to 80%, while during the low-water season, it is ≤5% (Fig. 8g). The chemical elements with a high predominance of dissolved forms <0.45 µm (Na, К, Ca, Mg, B, Sr), which are characterized by similar concentrations in supernatants, whatever the centrifugation intensity, demonstrate an insignificant proportion of coarse colloidal and fine pelitic forms (Fig. 8i, example of Sr).
Thus, chemical analysis of supernatants, obtained by separation of river water by centrifugation with different intensity, can be used to assess the distribution of chemical elements between fine pelitic (1–3 µm), coarse colloidal (0.45–1.0 µm), and dissolved/colloidal (<0.45 µm) fractions. In this case, three groups of elements are identified. The first group is elements-hydrolysates (Fe, Al, Ti, Th, Sc, REE), which tend to associate with coarse colloidal and fine-pelitic fractions even at low concentration of suspension. The second group contains a large group of elements (Li, Si, Mn, Co, Ni, Cu, As, Se, Rb, Cs, Ba, U), which differ in their chemical properties, but are similar in the predominance of dissolved forms in the Razdol’naya River water at most types of water regime, except for floods. The fraction of coarse colloids, especially, fine-pelitic suspension, in the migration of these elements depends on water regime and suspension concentration, reaching its maximum during floods. The third group includes elements with the predominance of dissolved forms at all water regimes (Na, K, Ca, Mg, B, Sr), for which coarse colloidal and fine pelitic forms are not typical, according to centrifugation data. According to these data, the specific features of the distribution of chemical elements between fractions of different size do not contradict to the results obtained by cascade filtration methods [13, 21].
CONCLUSIONS
Analysis of DNS intensity and the chemical composition of supernatants and filtrates showed that the centrifugation of river water within 30′ at 4500 rpm separates suspensions from dissolved and colloidal forms <0.45 µm with an efficiency close to the filtration through a 0.45 µm capsule filter. Assuming that the DLS intensity in centrifugates and filtrates is equal to 0.45 µm, the native density of pelite and coarse colloidal particles of river suspension was calculated, allowing the authors to correlate the centrifugation regimes with the sizes of precipitating particles within the range from 0.45 to 3 µm. The chemical analysis of supernatants of river water obtained at different centrifugation regimes was used to evaluate the distribution of chemical elements between dissolved/colloidal (<0.45 µm), coarse colloidal (0.45–1.0 µm), and fine pellitic (1–3 µm) fractions. It was shown that, in the examined waters of the Razdol’naya River, the concentration of coarse colloidal (0.45–1.0 µm) forms of elements-hydrolyzates (Fe, Al, Ti, Th, Sc, REE), is as a rule higher than the concentration of both dissolved/colloidal forms <0.45 µm and fractions 1–3 µm. A significant linear relationship was found between the DLS intensity and the concentrations in centrifugates of Fe, Al, Ti, Th, Sc, REE, i.e., chemical elements with a high proportion of coarse-colloidal and suspended forms, which confirms the possibility of assessing the concentration of colloidal particles in centrifugates by DLS intensity. A disadvantage of the centrifugation is that it is difficult to use in the field.
REFERENCES
Gordeev, V.V. and Lisitsyn, A.P., Geochemical interaction between freshwater and marine hydrospheres, Geol. Geofiz., 2014, vol. 55, no. 5–6, pp. 721–744.
Mikhailik, T.A., Tishchenko, P.Ya., Koltunov, A.M., Tishchenko, P.P., and Shvetsova, M.G., The effect of Razdol’naya River on the environmental state of Amur Bay (the Sea of Japan), Water Resour., 2011, no. 4, pp. 512–521.
RD 52.24.353-2012. Otbor prob poverkhnostnykh vod sushi i ochishchennykh stochnykh vod (Sampling Terrestrial Surface Water and Treated Wastewater), Moscow: Rosgidromet, GKhI, 2012.
RD 52.24.468-2019. Massovaya kontsentratsiya vzveshennykh veshchestv i sukhogo ostatka v vodakh (Mass Concentration of Suspended Matter and Dry Residue in Water), Moscow: Rosgidromet, GKhI, 2019.
Savenko, V.S., Khimicheskii sostav vzveshennykh nanosov rek mira (Chemical Composition of Suspended Sediments in World Rivers), Moscow: GEOS, 2006. 175 s.
Shul’kin, V.M., Bogdanova, N.N., and Perepelyatnikov, L.V., Space-time variations of river water chemistry in RF southern Far East, Water Resour., 2009, no. 4, pp. 406–417.
Shul’kin, V.M., Bogdanova, N.N., and Elovskii, E.V., Effect of filter clogging on the determination of concentrations of chemical elements migrating in river water as components of true solutions or in colloidal forms, Water Resour., 2022, vol. 49, no. 1, pp. 122–133.
Shul’kin, V.M., The use of the method of dynamic light scatter for assessing the efficiency of separation of suspended and colloidal particles in river water by filtration and centrifugation, Izv. Tomsk. Politekh. Univ. Inzhin. Geores., 2023, vol. 334, no. 10, pp. 88–97.
Bhattacharjee, S., DLS and zeta potential—What they are and what they are not, J. Controlled Release, 2016, vol. 235, pp. 337–351.
Buffle, J., Wilkinson, K.J., Stoll, S., Filella, M., and Zhang, J., A generalized description of aquatic colloidal interactions: the three-colloidal component approach, Environ. Sci. Technol., 1998, pp. 2887–2899.
Cuss, C.W., Donner, M.W., Grant-Weaver, I., Noernberg, T., Pelletier, R., Sinnatamby, R.N., and Shotyk, W., Measuring the distribution of trace elements amongst dissolved colloidal species as a fingerprint for the contribution of tributaries to large boreal rivers, Sci. Total Environ., 2018, vol. 642, pp. 1242–1251.
Droppo, I.G., Nackaerts, K., Walling, D.E., and Williamset, N., Can flocs and water stable soil aggregates be differentiated within fluvial systems?, Catena, 2005, vol. 60, pp. 1–18.
Drozdova, O.Yu., Aleshina, A.R., Tikhonov, V.V., Lapitskiy, S.A., and Pokrovsky, O.S., Coagulation of organo-mineral colloids and formation of low molecular weight organic and metal complexes in boreal humic river water under UV-irradiation, Chemosphere, 2020, 250, p. 126216. https://doi.org/10.1016/j.chemosphere.2020.126216
Filella, M., Zhang, J., Newman, M.E., and Buffle, J., Analytical applications of photon correlation spectroscopy for size distribution measurements of natural colloidal suspensions: capabilities and limitations, Colloids Surfaces A. Physisochem. Eng. Aspects, 1997, vol. 120, pp. 27–46.
Gimbert, L.J., Haygarth, P.M., Beckett, R., and Worsfold, P.J., Comparison of centrifugation and filtration techniques for the size fractionation of colloidal material in soil suspensions using sedimentation field-flow fractionation, Environ. Sci. Technol., 2005, vol. 39, no. 6, pp. 1731–1735.
Goldberg, E.D., Baker, M., and Fox, D.L., Microfiltration in oceanographic research, J. Mar. Res., 1952, vol. 11, pp. 194–203.
Horowitz, A.J., Lum, K.R., Garbarino, J.R., Hall, G.E.M., Lemieux, C., and Demas, C.R., Problems associated with using filtration to define dissolved trace element concentrations in natural water samples, Environ. Sci. Technol., 1996, vol. 30, p. 954.
Langevina, D., Raspauda, E., and Mariota, S., Towards reproducible measurement of nanoparticle size using dynamic light scattering: important controls and considerations, NanoImpact, 2018, vol. 10, pp. 161–167.
Morrison, M. and Benoit, G., Filtration artifacts caused by overloading membrane filters, Environ. Sci. Technol., 2001, vol. 35, p. 3774.
Nguen, D.N., Grybos, M., Rabiet, M., and Deluchat, V., How do colloid separation and sediment storage methods affect water mobilizable colloids and phosphorus? An insight into dam reservoir sediment, Colloids Surf. A: Physicochem. Engin. Aspects, 2020, vol. 606, p. 125505.
Pokrovsky, O.S. and Schott, J., Iron colloids/organic matter associated transport of major and trace elements in small boreal rivers and their estuaries (NW Russia), Chem. Geol., 2002, vol. 190, pp. 141–179.
Schmitz, K.S., An Introduction to Dynamic Light Scattering by Macromolecules, Boston: Acad. Press, 1990.
Shiller, A.M., Syringe filtration methods for examining dissolved and colloidal trace element distributions in remote field locations, Environ. Sci. Technol., 2003, vol. 37, pp. 3953–3957.
Tang, Z., Wu, L., Luo, Y., and Christie, P., Size fractionation and characterization of nanocolloidal particles in soils, Environ. Geochem. Health, 2009, vol. 31, pp. 1–10. https://doi.org/10.1007/s10653-008-9131-7
Wilde, F.D., Radtke, D.B., Gibs, Jacob., and Iwatsubo, R.T., Processing of water samples (ver. 2.2). U.S. Geological Survey Techniques of Water-Resources Investigations, 2004 with updates through 2009, Book 9, Chap. A5, April 2004. 2015. http://pubs.water.usgs.gov/twri9A5.
Xu., R., Light scattering: A review of particle characterization applications, Particuol., 2015, vol. 18, pp. 11–21.
Funding
The study was supported by the Russian Science Foundation, project no. 23-27-00029.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The author of this work declares that he has no conflicts of interest.
Additional information
Publisher’s Note.
Pleiades Publishing remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Shulkin, V.M. The Use of Centrifugation for the Separation of Suspended and Colloidal Forms of Chemical Elements in the Analysis of River Waters: Possibilities and Limitations. Water Resour 51, 550–561 (2024). https://doi.org/10.1134/S0097807824700945
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0097807824700945