Abstract
The Mitidja-plain is very important in size and role, it contains two important aquiferous tanks exploited to serve Algiers and the surrounding agglomerations, these resources used for the population, industry and the hydro agricultural needs are nowadays threatened by contamination. The Eastern region of this area is the most affected by this pollution. Groundwater from the alluvial aquifer have been analyzed in order to investigate the mineralization processes, water origin and to evaluate the levels of pollution. In addition principal component analysis was used to identify the sources of pollution. Groundwater from this aquifer fall into Cl–SO4–Ca water type. Data inferred from 18O and 2H isotopes in groundwater samples indicated recharge with non-evaporated rainfall originating from Mediterranean air masses. Measurable tritium concentration allowed qualitative identification of modern recharge by recent precipitation. The groundwater quality is strongly influenced by the content of nitrates and heavy metals. The nitrates are certainly linked to pollution due to agricultural activities and the used of fertilizers. The concentrations of Fe, Mn, Pb and Cd were found higher than the prescribed limits defined by the World Health Organization. The application of principal component analysis for traces metals identified two sources of pollution—natural and anthropogenic sources.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
INTRODUCTION
The Eastern Mitidja plain constitutes a subterranean water reservoir essential for the agricultural, urban and industrial development of the capital Algiers. Recently, industrial activities, the spreading of exhaustive agricultural practices and the urbanization of the plain have lowered the quality of water. Different wads that recharge the alluvial reservoir flowing from south to north starting at Blida Mountain cross the Eastern Mitidja plain, these wads drain all kind of materials while crossing urban, agricultural and industrial zones, where wastes are thrown without treatment, in fact an increase of nitrate and heavy metals concentrations in the Eastern Mitidja groundwater was observed and a severe pollution problem is under consideration. In view of the importance of these resources, the present work was initiated. In this work hydro chemical and isotopic information from the alluvial aquifer of the Eastern Mitidja will be combined and used to determine the mechanism controlling the chemistry and to identify natural or anthropogenic processes that control the groundwater quality, the source and origin of recharge. The water pollution evaluation was carried out using hydro-chemical parameters, heavy metals to measure the levels of pollution, determine the sources of contaminants and evaluate the suitability of groundwater, specifically for human consumption by analyzing the collected samples to assess the quality with reference to nitrates, heavy metals contamination and their human health effects. For its multipurpose use, their toxicity and persistence in the environment, heavy metals are considered as one of the most serious pollutants in the aquatic environment. Almost, all types of water contain heavy metals, many of which result from the natural weathering of the earth’s surface. In addition, wastewater used for irrigation land, besides effluent from city sewage and industrial wastewater, could significantly affect water quality. Heavy metals from anthropogenic activities could migrate or infiltrate into aquifers and interact with groundwater. This study deals with the investigation of the environmental isotopes (18O, 2H) and the radioactive isotope 3H, the objects are to investigate the origin and timing of recharge. The principal component analysis (PCA) was employed to evaluate the sources of pollution.
STUDY AREA
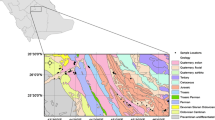
Mitidja plain is located in the north of Algeria with a total area of 1450 km2, is spread along a WSW–ENE direction on about 80 km length and 10 to 20 km width. Mitidja is divided into three parts: Eastern, Central and Western (Fig. 1). The Eastern part of the plain, covering an area of 576 km2, is limited by the Mediterranean Sea to the north, the Blidean Atlas to the south, El Harrach River to the west and the Reghaia River to the east. In the NE–SW direction, the altitude of the plain varies from 20 to 50 m [14].
Geographically, the Eastern Mitidja watershed includes three major units. From the north to the south, one can distinguish:
a) The Sahel A coastal (Sahelian) region bordering the Mitidja to the north. It is a quite wide ripple-like coastal portion of land with an altitude ranging from 200 to 250 m. Only the southern area of this somehow hilly area is part of the Mitidja basin. Rainfall taking place on its northern part runs-off directly towards the Mediterranean Sea and is lost.
(b) The plain of the Eastern Mitidja is a subsidence area, with Mio-Plio-Quaternary fillings. The mean altitude varies between 40 and 50 m and increases regularly to the Atlas Piedmont in the south as well as to the Sahel region in the north.
(c) The Blidian Atlas, representing the southern boundary of the considered watershed with a mean altitude that ranges between 1200 and 1600 m. It is an area of active erosion phenomena due to intense runoff favored by both topography and the low vegetation cover.
The Eastern Mitidja is characterized by a Mediterranean climate type with 2 seasons: one season is cold and wet, generally from September to May, the second season is hot and dry, from June to August with an average annual temperature of 18°C.
The mean annual rainfall is estimated to 600 mm, over a period of 29 years (from 1978 to 2007), a maximum annual precipitation of 913.8 mm was recorded in 1984 and a minimum of 282.6 mm in 2000, and the evapotranspiration rate is up to 465.8 mm/year. The hydrographic net consists of three main rivers (oueds) with irregular flow, being completely dry in the summer except near the outlet. The rivers in the plain are: Oued El-Harrach in the west, Oued El-Hamiz in the center of the plain and Oued Réghaia in the Est.
GEOLOGICAL AND HYDROGEOLOGICAL SETTINGS
The Eastern Mitidja basin represents a clearly distinct geographic and geologic unit limited by the massif of Blida to the south, the Mediterranean Sea to the north, Boudouaou wadi to the east and to the west El-Harrach wadi [6].
Geologically, this basin consists of a series of sedimentary and metamorphic terrains, as well as some igneous rocks at some locations. A large hydrogeological basin is found in the midst of the plain. It includes a complex Astian‑Quaternary aquifer system in the form of an asymmetric syncline structure overlying a marly Plaisancian substratum, occasionally of Miocene and Cretaceous ages in some places. Each horizon has its own hydraulic properties. The geological formations, which fill the basin, are very heterogeneous and were randomly deposited considering the large number of erosive episodes that are found intercalated between the tectonic phases. Geophysical studies conducted in 1967 revealed the existence of two superimposed aquifer under the plain of Mitidja: the aquifer of Eeocen (astian aquifer) (formed during the tertiary age) and the aquifer of the quaternary alluvia which are separated by the impermeable marl layer of El-Harrach on almost the whole territory with an exception in the east part [17].
Astian Aquifer (Upper Pliocene)
The Astian aquifer is a confined aquifer formed by sandstones and sandy limestones of the Pliocene. The Astian aquifer has as upper limit El-Harrach clays, except in the Rouiba-Réghaia zone where the aquifer is in direct connexion with the alluvial aquifer of Mitidja. Its substratum is composed by the blue marls of the pleostene age and its roof by semi-permeable yellow marls named marl’s of El-Harrach. Its average thickness varies from 100–150 m. this aquifer is very deep, generally located between 250 and 300 m below ground in the major section of the plain. The Astian aquifer is less important for the storage of water, but its hydraulic role is not negligible, particularly with regard to the exchange of water with the Mitidja aquifer. As mentioned above the aquifer of Eocene is not only a confined aquifer but also a very deep one, these natural characteristics make it less vulnerable to pollution compared to the phreatic aquifer of the quaternary.
Alluvial Aquifer (Mitidja Aquifer)
The alluvial aquifer of the quaternary is mainly composed of sands, gravels and rollers alternating with silts and clays. A part from the zone of Mazafran, this aquifer is entirely unconfined and based on the yellow marls of El-Harrach. Its thickness varies from 100–200 m. Its edge Eastern and Western limit is ensured by the rise of the blue marls of the pleostene. The depth of water table varies between 4 and 30 m. This aquifer is the main reservoir for groundwater storage in the region.
MATERIALS AND METHODS
Within the sampling campaign carried out in July 2014, 12 groundwater samples (3 wells and 9 boreholes) from the alluvial aquifer of the Eastern Mitidja groundwaters were collected (Fig. 1). The measurements of pH, temperature, electrical conductivity (EC) and dissolved oxygen were performed in the field. Tight-capped high quality polyethylene bottles were used for sample storage. Samples for laboratory analysis were filtered by means of 0.45 µm membranes. Filtered and acidified (1% v/v HNO3) samples were collected for heavy metals and major cations samples were immediately transported to the laboratory in iceboxes and stored at 4°C up to analysis. Major cations (Na+, Ca2+, Mg2+, K+, Li+) and anions (\({\text{HCO}}_{3}^{ - },\)\({\text{SO}}_{4}^{{2 - }},\) NO3–, Cl–, F–, Br–) were analyzed through high performance ion liquid chromatography (HPILC, Dionex DX-120). The dissolved silica in water was determined by a colorimetric method. The total alkalinity (as \({\text{HCO}}_{3}^{ - }\)) was estimated in the laboratory by potentiometric titration method using 0.1 N HCl. The chemical results were accepted when the charge balance error was within ±5%. The concentrations of heavy metals (Cu, Zn, Mn, Fe, Cr, Cd, Pb) were analyzed using a flame atomic absorption spectrometer (Model Perkin Elmer A. Analyst 400). Stable isotope values of water samples (δ18O, δ2H) were analyzed using the laser absorption spectrometer Picarro L2110-i in the isotope hydrology laboratory of Algiers Nuclear Research Centre (ANRC). The results are given as relative deviations δ (in per mil) from the Vienna standard mean ocean water (VSMOW). The analytical precision was 0.56‰ for δ2H and 0.06‰ for δ18O.
The tritium content was measured at the ANRC Laboratory by means of electrolytic enrichment and liquid scintillation spectrometry [24]. 3H is expressed in Tritium Units (TU) (one TU is defined as the isotope ratio 3H/1H = 10–18) [8] with the analytical precision of ±1.4 TU. The piper diagram was established to determine the different water types. In the aim to identify the most important factors controlling the groundwater geochemistry, PCA was carried out using the program XL STAT 2013.
FIELD PARAMETERS
The physicochemical parameters measured in situ are presented in Table 1. Samples in this work were collected from groundwater, given the fact that they were protected, their temperature was stable and not affected by climatic conditions, it varies from 20.2 to 24.8°C with an average value of 22.04°C.
The pH of groundwater samples vary into a relatively narrow range between 6.02 and 7.3 with a mean of 6.95, neutral and slightly acid; these waters are within the limits of the guidelines values established by the World Health Organization (WHO) which gives as a guide level a variation of 6.5 to 8.5, except well ME5 which have a value of 6.02. The oxygen content in the water is a function of water origin. The shallows waters may contain relatively large amounts, near the saturation; however, deep waters contain just a few milligrams per liter. Dissolved oxygen concentrations ranges from 0.7 to 7.6 mg/L; this is the case of deep waters that contain, only a few milligrams per liter of dissolved oxygen.
Values of conductivity covers the range between 714 and 2560 µS/cm, with an average of 1649 µS/cm, 42% of the samples gave value within the permissible limit (1500 µS/cm), prescribed by WHO, and 58% were found above their recommended limits for drinking uses. The high value of salinity is attributed to over exploitation, and the seawater intrusion inside groundwater because of intense pumping from the coastal aquifer. The high concentration of Na+ and Cl– in the groundwater samples (ME1, ME4, ME5, ME7, ME9, ME10, ME11) and the strong correlation between these two elements (0.814) provide insight into phenomena of seawater intrusion. A strong correlation is also shown between Na+ and Cl– with EC (0.753 and 0.82 respectively), indicating that this two elements contribute to the mineralization of groundwater (Table 2).
The aquifer of the Eastern Mitidja is threatened mainly by the intensive use of agricultural chemicals (fertilizers and pesticides), untreated wastewater discharge into surface waters that recharge the alluvial reservoir, and the accelerated industrialization often carried out in an anarchistic way, which might contribute to these elevated salinity levels [1]. The increase in the electrical conductivity value can indicate also leaching of bicarbonate formations of the Blidian Atlas as well as the bicarbonate formations of the Pliocene (Astian) which borders the alluvial aquifer of the southern Mitidja considered the high values of bicarbonates. The presence of high value of EC in groundwater samples may cause gastrointestinal irritation to the consumers. The concentration of dissolved silica in water’s samples varies between 11 and 18.6 mg/L with a mean value of 14.49 mg/L.
HYDROCHEMISTRY
The piper’s diagram indicates that 66.67% of groundwater samples fall into the Cl–SO4–Ca type, whereas 16.66% are classified as HCO3-Ca facies, and remaining Ca–Cl type (Fig. 2). Therefore, facies classification indicates that maximium groundwater samples belong to Cl–SO4–Ca type. Table 1 shows that the dominant trend of major cations was Ca2+ > Na+ > Mg2+ > K+ > Li+, while the order of anions is Cl– > \({\text{HCO}}_{3}^{ - }\) > \({\text{SO}}_{4}^{{2 - }}\) > \({\text{NO}}_{3}^{ - }\) > Br– > F–. Table 2 presents the matrix of correlation coefficients for the hydrochemical data in the Eastern Mitidja alluvial reservoir. The positive correlation between the major elements (Cl–, Ca2+, Na+, Mg2+, \({\text{HCO}}_{3}^{ - },\)\({\text{SO}}_{4}^{{2 - }},\)\({\text{NO}}_{3}^{ - }\)) and the conductivity reveals that the process of the mineralization is controlled by the presence of this elements [18].
To determine the origin and processes that contribute to groundwater mineralization, some plots of relations between major elements are introduced. In the sodium-chloride diagram, values are plotted along a linear trend (Fig. 3a), indicating a strong relationship between this two elements, indicating halite dissolution as the principal control of Na+ and Cl– contents. This process is verified by the linear evolution of halite saturations indices, calculated with the help of the Water F program and the sum of sodium and chloride ions (Fig. 3b). Ca2+ is linearly correlated with \({\text{SO}}_{4}^{{2 - }}\) indicating the same origin of both ions (anhydrite and/or gypsum) minerals [4], the effect of this dissolution is linked to the proportional evolution between the negative saturation indexes of gypsum and anhydrite evaporites and the sum of ions (Ca2+ + \({\text{SO}}_{4}^{{2 - }}\)) (Fig. 3c‒3d). The values of saturation index for halite/gypsum/anhydrite show an under saturation state (Table 3).
NITRATE
Nitrate concentrations in the groundwater samples vary from 2.2 to 116.2 mg/L, and 50% of samples are greater than 50 mg/L, exceeding the current drinking water quality limits advocated by WHO. These high nitrate values are the result of industrial activities, the urbanization of the plain and especially the spreading of intensive agricultural practices. As the investigated region is one of the most important agricultural areas of the Mitidja plain, intensive use of nitrogen fertilizers has increased the agricultural production, with a negative impact on water quality [12]. The high correlation between \({\text{NO}}_{3}^{ - }\)/Ca2+ (0.737), Mg2+/\({\text{SO}}_{4}^{{2 - }}\) (0.746) and \({{{\text{NO}}_{3}^{ - }} \mathord{\left/ {\vphantom {{{\text{NO}}_{3}^{ - }} {{\text{SO}}_{4}^{{2 - }}}}} \right. \kern-0em} {{\text{SO}}_{4}^{{2 - }}}}\) (0.701) (Table 2) indicates a common source of this elements, i.e., from anthropogenic activities (use of fertilizers) and provide insight into the excessive use of Ca(NO3)2, MgSO4 and ammonium sulphate fertilizers respectively [23].
HEAVY METALS
We evaluated the extent of heavy metal contamination in groundwater by comparing concentration of metals detected in the study area with the limits as prescribed by WHO [27] for the drinking purposes. Table 4 shows total concentrations of seven heavy metals (Cu, Cr, Cd, Fe, Mn, Zn and Pb) of groundwater at different locations of the study area. The mean concentration of studied metals in groundwater followed a decreasing order of Fe > Mn > Zn > Pb > Cu > Cd > Cr. The mean concentrations of Fe, Mn, Cd, and Pb in the groundwater exceeded drinking water standards established by WHO, the average concentrations of Mn, Pb, Fe and Cd was 110.5; 51.6; 611.9 and 4.5 µg/L respectively, but Zn, Cr and Cu were found below their recommended limits for drinking purposes (67.5; 0.236 and 25.1 µg/L respectively). Fe concentrations were in the range (59‒1927 µg/L), the highest concentration (1927 µg/L) were found in sample (ME5) collected from Reghaia. Ingestion of Fe in large quantities results in a condition known as “haemochromatosis,” which results in tissue damage causing due to high iron concentration [25]. The level of Mn fell in the range 27‒398 µg/L, the maximum concentration of Mn (398 µg/L) was also found in sample (ME5) (Reghaia).
The overexposure to the manganese can lead to a permanent neurological disorder known as manganese similar to idiopathic Parkinson’s disease with symptoms that include facial muscle spasms, difficulty walking, and tremors [15]. Pb values varied from 0 to 250 µg/L, the highest value (250 µg/L) was measured in sample (ME1) situated in the industrial area of Rouïba city. Pb is a highly toxic heavy metal and carcinogenic. The high level of Pb exposure can damage the kidneys and brain and ultimately cause death [3]. Total Cd content ranged from 0 to 10 µg/L, hight level of Cd (10 µg/L) was observed in sample (ME9) from El-Hamiz city. Cd is a very toxic and carcinogenic heavy metal for humans. Cd and its compounds irritate the stomach, cause diarrhea and leading to vomiting. Long-term exposure to higher levels leads to its accumulation in the kidneys and possible kidney disease, fragile bones, and lung damage.
Source Identification of Trace Metals in Groundwater
PCA was used to explore the extent of metal pollution and for source identification, using standard procedures. Varimax was used to maximize the sum of the variance of the factor coefficients, which better elucidate the possible groups/sources that influenced water systems [5]. Using varimax normalized rotation, three principal components (PC) with eigenvalues >1 were extracted for trace metals in groundwaters, accounting for 82.88% of variance in the dataset (Fig. 4). The first PC represents 39.98% of total variance and has major loadings of Fe, Mn and Zn. The second PC with 29.45% variance revealed greater loadings for Cd. The third PC explained 13.45% of the total variance and was associated with distributions of Cu. A negative correlation was recorded for Pb and Cr with PC1 and PC2.
The correlation coefficients between the concentrations of Cu, Cr, Cd, Fe, Mn, Zn and Pb in groundwater were calculated and the results are introduced in Table 5. A strong positive correlation was found with Fe and Mn (r = 0.864), a significant correlation of Fe and Zn (r = 0.685) was observed, a significant correlation was also recorded for Mn with Zn (r = 0.691). Pb was correlated positively with Cr (r = 0.654). A negative correlation was recorded for Pb with other metals except Cr (r = 0.654). A weak correlation was found for Cu with Pb, Mn, Cr, Zn, Cd and Fe. From the above results it is shown that only some heavy metals were significantly correlated with one another that mean their sources were the same. Fe is abundant in the earth [19]; in the region there are several naturally occurring sources of Fe, which are expanded constituents of the alluvial sediments. The presence of Mn is linked to the natural lithological composition of the alluvial aquifers, the weathering of parent material and subsequent pedogenesis [22]. Zn can have a lithogenic source since it forms a number of soluble or insoluble salts.
The presence of Pb and Cd could be linked to the contribution of anthropogenic sources. The urban waste drained by the public sewage network is not fully cleaned and is discharged into the rivers traversing the Mitidja plain [21]. Aquifers in the Mitidja being alluvial, part of the polluted water feeds into the underlying groundwaters. The eastern region of Mitidja is currently experiencing heavy industrial activities, where the effluents are discharged without any treatment. Sources of Pb are chemical manufacturing products like batteries and accumulator, metal products like paints, solder and pipes, non-metallic minerals industry, metal processing industry, energy industry and petrochemistry. Concentrations of Cd are influenced by industrial activities such as electroplating and metal surface treatment processes, inorganic pigment manufacturing, wood processing industry. The principal component analysis identified two sources of pollution. Therefore, the first PC is indicative of a natural/geogenic sources, while the second principal component analysis can be identified as anthropogenic (industrial/urban) processes.
ISOTOPE DATA
Tritium and isotopes stables (18O, 2H) are components of the water molecule; they are perfect tracers for describing phenomena of water cycle and have a great significance in isotope hydrology. The isotope geochemistry techniques predicted useful information concerning the groundwater recharge processes, water origin and residences times which participate to sustainable use of groundwater.
Isotope Composition of Groundwaters
The nearest relevant IAEA/WMO GNIP station is Algiers (located on the Mediterranean coast (3°11 E; 36°43 N; Z = 18 m) and installed inside the USTHB University in Bab-Ezzouar), for which there is a permanent temporal record of monthly means of δ18O and δ2H for rainfall during the period 2000–2004. The weighted mean value of Algiers rainwater is ‒5‰ vs. VSMOW for δ18O and ‒28‰ vs. VSMOW for δ2H [13]. The δ18O and δ2H compositions of groundwater, for the present samples vary from ‒4.64 to ‒6.66‰ and from ‒29.2 to ‒36.3‰, respectively, with a mean value of ‒6.06‰ for δ18O and ‒33.95‰ for δ2H. Groundwater samples were presented in the conventional diagram of δ18O/δ2H, with respect to the Global Meteoric Water Line (GMWL: δ2H = 8δ18O + 10) [9]; the Local Meteoric Water Line for the Western Mediterranean, which was determined by Cell (LMWL: δ2H = 8δ18O + 14) [7] and the Regional Meteoric Water Line of Algiers (RMWL), given by δ2H = 8δ18O + 11.2 (Fig. 5) [13].
The majority of the samples values are situated above the GMWL and the RMWL and are scattered around the LMWL except well (ME4) situated bellow the GMWL. The scatter of the points above the LMWL implies that groundwaters were recharged by recent precipitation due to direct condensation of Mediterranean atmospheric moisture [11]; the relatively depleted isotopic values of samples demonstrate the modern origin of these groundwaters, which are not affected by evaporated open water or soil water [4, 10]. Elevated values of deuterium excess between 11.56 and 19.66 confirm the origin of these groundwaters [20]. On another side, these samples are more depleted in heavy isotopes than the mean weighted values of δ18O and δ2H observed at the Algiers station, this depletion is probably related to an altitude effect and/or temperature effect [26]. Furthermore the scatter of the majority of these points below the mean local precipitation (‒5‰ for δ18O and ‒28‰ for δ2H) can be attributed to seasonal recharge of groundwaters, however in Mediterranean countries as Algeria, the seasonal variation of isotopic composition of ambient moisture is relatively high [16].
The most enriched δ18O and δ2H values represent well ME4 (‒4.64 and ‒29.2‰ for δ18O and δ2H, respectively, and a value of deuterium excess around 7‰), highlighting evaporation process of meteoric water before or after groundwater recharge; it can also suggest the water-rock interaction into the alluvial aquifer of the study area [2].
Tritium
Tritium is a radioactive isotope that emits low-energy particles, with a maximum energy of 18.6 keV with the recently re-evaluated half-life of 12.32 years. Environmental tritium is extensively used in hydrology as an age indictor for young groundwaters. Tritium concentrations in analyzed groundwaters fluctuate between 0.05 and 0.90 TU. The presence of measurable concentrations of tritium denotes the presence of waters of modern infiltration in the Eastern Mitidja Basin [8].
CONCLUSIONS
Based on the results of the present investigation, it can be concluded that:
Groundwaters from the quaternary alluvial aquifer of the Eastern Mitidja are dominated by the Cl–SO4–Ca facies, derived mainly from the dissolution of evaporates minerals (Halite, Anhydrite /Gypsum).
Environmental isotopes (18O, 2H) results revealed the presence of waters not affected by significant evaporation, which suggests rapid infiltration before evaporation of meteoric waters.
In the present investigation concentration of Cd, Pb, Mn and Fe were found higher than the safe recommended values for drinking purposes established by WHO for heavy metals and might create an adverse effect on this aquatic reservoir. In fact, the contamination of the water systems by heavy metals poses serious threat to human health and ecological habitat in study area, which therefore require attention.
The quality of waters is characterized also by the presence of nitrate that can be reasonably attributed to the uses of fertilizers in the agricultural area.
Tritium results confirm the modern water recharge within the alluvial aquifer of the Eastern Mitidja.
REFERENCES
Samey, A. and Gang, C., A GIS based drastic for the assessment of groundwater vulnerability to pollution in west Mitidja: Blida city, Algeria, Res. J. Appl. Sci., 2008, vol. 3, no. 7, pp. 500–507.
Banner, J.L., Wasseerburg, G.J., Dobson, P.F., Carpenter, A.B., and Moore, C.H., Isotopic and trace-element constraints on the origin and evolution of saline groundwaters from central Missouri, Geochim. Cosmochim. Acta, 1989, vol. 53, pp. 383–398.
Barakat, M.A., New trends in removing heavy metals from industrial wastewater, Arabian J. Chem., 2011, vol. 4, pp. 361–377.
Ben Moussa, A., Mzali, H., Zouari, K., and Hezzi, H., Hydrochemical and isotopic assessment of groundwater quality in the Quaternary shallow aquifer, Tazoghrane region, north-eastern Tunisia, J. Quatern. Int., 2014, vol. 338, pp. 51–58.
Bhuiyan, M.A.H., Islam, M.A., Dampare, S.B., Parvez, L., and Suzuki, S., Evaluation of hazardous metal pollution in irrigation and drinking water systems in the vicinity of a coal mine area of northwestern Bangladesh, J. Hazard. Mater., 2010, vol. 179, pp. 1065–1077.
Binnie, A., Schéma d’aménagement des ressources en eau dans la région d’Alger-Sebaou, Missions B et F, 1983.
Celle-Jeanton, H., Travi, Y., and Blavoux, B., Isotopic typology of the precipitation in the Western Mediterranean region at the three different time scales, Geophys. Res. Lett., 2001, vol. 28, pp. 1215–1218.
Clark, I.D. and Fritz, P., Environmental Isotopes in Hydrology, Boca Raton, USA: Lewis Publishers, 1997.
Craig, H., Isotopic variation in meteoric water, Science, 1961, vol. 133, pp. 1702–1703.
Edmunds, W.M., Guendouz, A., Mamou, A., Moulla, A., Shand, P., and Zouari, K., Groundwater evolution in the Continental Intercalaire aquifer of southern Algeria and Tunisia: trace element isotopic indicators, J. Appl. Geochem., 2003, vol. 18, pp. 805–822.
Farid, I., Trabelsi, R., Zouari, K., and Beji, R., Geochemical and isotopic study of surface and groundwaters in Ain Bou Mourra basin, central Tunisia, J. Quatern Int., 2013, vol. 303, pp. 210–227.
Garcia-Galan, M.J., Garrido, T., Fraile, J., Ginebreda, A., Diaz-Cruz, M.S., and Barcelo, D., Simultaneous occurrence of nitrates and sulfonamide antibiotics in two groundwater bodies of Catalonia (Spain), J. Hydrol., 2010, vol. 383, pp. 93–101.
IAEA, Isotopic Composition of Precipitation in the Mediterranean Basin in Relation to Air Circulation Patterns and Climate. Final Report of a Coordinated Research Project (2000–2004), IAEA-TECDOC-1453, IAEA, Vienna, 2005.
Imerzoukene, S. and Walraevens, K., Simulation of groundwater flow and evolution of the fresh/salt water interface in the coastal aquifer of the Mitidja plain (Algeria), Proc. SWIM, Ghent, 1999, pp. 249–262.
Iregren, A., Manganese neurotoxicity in industrial exposures: proof of effects, critical exposure level, and sensitive tests, Neurotoxicology, 1999, vol. 20, nos. 2−3, pp. 315–323.
Kebede, S., Travi, Y., and Rozanski, K., The d18O and d2H enrichment of Ethiopian lakes, J. Hydrol., 2009, vol. 365, pp. 173–182.
Khouli, M.R. and Djarbi, L., Impact of use of agricultural inputs on the quality of groundwater case of Mitidja plain (Algeria), Geographia Tech., 2011, vol. 2, pp. 35–44.
Kuitcha, D., Takounjou, A.L.F., and Ndjama, J., Apport de l’hydrochimie et de l’isotope de l’environnement a la connaissance des resssources en eaux souterraines de Yaoundé, Cameroun, J. App. Biosci., 2013, vol. 67, pp. 5194–5208.
Li, J., Li, F., Liu, Q., and Zhang, Y., Trace metal in surface water and groundwater and its transfer in a Yellow River alluvial fan: Evidence from isotopes and hydrochemistry, J. Sci. Total Environ., 2014, vol. 472, pp. 979–988.
Merlivat, L. and Jouzel, J., Global climatic interpretation of the deuterium-oxygen 18 relationship in precipitation, J. Geophys. Res., 1979, vol. 84–C8, pp. 5029–5033.
Mimouni, O., Cheikh Lounis, G., Kabouche, S., and Menceur, H., Vulnerability to pollution of mitidja palin alluvial aquifer (Algiers-Algeria), Int. J. Enhanced Res. Sci. Technol. Engineering, 2015, ISSN: 2319-7463, pp. 137–143.
Nriagu, J.O., A global assessment of natural sources of atmospheric trace metals, Nature, 1989, vol. 338, pp. 47–59.
Stigter, T.Y., Carvalho, A.M., Ribeiro, L., and Reis, E., An impact of the shift from groundwater to surface water irrigation on aquifer dynamics and hydrochemistry in a semi arid region in the south of Portugal, Agr. Water Manage., 2006, vol. 85, pp. 121–132.
Taylor, C.B., Tritium enrichment of environmental waters by electrolysis: development of cathodes exhibiting high isotopic separation and precise measurements and applications. High Tatras, Czechoslovakia, Bratislava, 1977.
Tiwari, M.K., Bajpai, S., Dewangan, U.K., and Tamrakar, R.K., Assessment of heavy metal concentrations in surface water sources in an industrial region of central India, Kerbala, Int. J. Modern Sci., 2015, vol. 1, pp. 9–14
Trabelsi, R., Abid, K., and Zouari, K., Geochemistry processes of the Djeffara palaeogroundwater (Southern Tunisia), J. Quatern Int., 2012, vol. 257, pp. 43–55.
World Health Organization (WHO), Guidelines for Drinking-Water Quality, 4th ed., Genewa, Switzerland, 2011.
Author information
Authors and Affiliations
Corresponding authors
Additional information
The article is published in the original.
Rights and permissions
About this article
Cite this article
Dalale Khous, Ait-amar, H., Belaid, M. et al. Geochemical and Isotopic Assessment of Groundwater Quality in the Alluvial Aquifer of the Eastern Mitidja Plain. Water Resour 46, 443–453 (2019). https://doi.org/10.1134/S0097807819030060
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0097807819030060