Abstract
Dibenzothiophene oxidation has been carried out in the presence of catalysts based on transition metal salts and acids. The effect of the nature of the catalyst, acid, and oxidant, as well as the time and temperature of oxidation exerted on the conversion level of dibenzothiophene, is studied. It has been shown that an optimal catalyst composition such as Na2MoO4 : HCOOH : S = 0.08 : 7.5 : 1 makes it possible to selectively obtain dibenzothiophene sulfone with a 70% yield. The effect of the extractant nature on the level of sulfone extraction from the reaction mixture and on the viability of anaerobic sludge cells that can be used for the subsequent bioconversion of sulfones into hydrogen sulfide has been studied. It is established that, when using oxidized sulfur compounds of ethyl or isopropyl alcohol as an extractant, it is possible to extract sulfone from the organic phase rather efficiently, as well as obtain a 100% bioconversion level of sulfone under anaerobic conditions.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
INTRODUCTION
In connection with the growing attention to the environment around the world, the problem of automobile emissions is of particular interest. Sulfur compounds present in fuels adversely affect catalysts in oil refining, as well. As a result, stringent environmental requirements are imposed all over the globe that state that the sulfur content in motor fuels should not exceed 10 ppm.
Sulfur compounds contained in the petroleum fractions represent sulfides, mercaptans, thiophenes, benzothiophenes, dibenzothiophenes, and alkyl derivatives thereof. The main process of oil purification from sulfur—hydrorefining—addresses the problem of removing sulfur to ultralow values, which leads to an increase in temperature and pressure of processing, as well as high hydrogen consumption. At present, hydrogen-free desulfurization methods are being widely developed as an alternative to methods of hydrorefining.
Among alternative technologies, the method of oxidative desulfurization has received the greatest development [1, 2]. As the oxidants, one uses NO2 [3], tert-butyl peroxide [4] and hydrogen peroxide, which is preferred owing to its availability and environmental safety. Organic acids [5, 6] and the salts of transition metals such as molybdenum, tungsten, and vanadium are most often used as oxidation catalysts [7]. The oxidation of sulfur compounds results in the formation of sulfones:
The isolation of sulfones from the reaction mixture can be quite easily performed by adsorption or extraction [8]. The optimal conditions for oxidative desulfurization are currently being sought after; in particular, catalysts, temperature and time modes, and methods for extracting sulfur-containing compounds from the organic phase are being chosen. At the same time, the problem of finding efficient ways to further use the resulting extracts containing organosulfur compounds such as sulfones remains urgent. The problem of purifying and disposing of solvents used to extract sulfones from hydrocarbon fractions is also of importance.
The sulfones that are contained in the extracts can be reduced to hydrogen sulfide using various methods, in particular, in the course of bioconversion under anaerobic conditions using activated sludge (AS) as a biocatalyst. This environmentally friendly and promising method of bioconversion under anaerobic conditions has been chosen in this work to purify the extractant from sulfur compounds, since waste inorganic sulfides and monomers obtained from renewable raw materials are considered nowadays in the scope of implementing environmentally safe chemistry principles as a raw-materials base for the synthesis of polymers and functional materials [9].

Since sulfur-containing organic compounds are toxic for cells, it is worth using stabilized (immobilized) forms of biocatalysts in their bioconversion processes. As for using the immobilized cells of sulfate-reducing bacteria in the purification of various organic effluents with the formation of hydrogen sulfide, there are known immobilization methods based on the sorption of these cells on a variety of carriers (activated carbon, polyethylene rings, silica gel, expanded clay, zeolite, and others) [10].
However, the sorption process, as is known, is not an efficient method for immobilization, since it has a number of disadvantages, The main disadvantage consists of the desorption of cells from the carrier, and as well as in the variability of the composition of the immobilized anaerobic biocatalyst obtained in this way. In this case, one could consider the immobilization via the incorporation of the cells into the structure of the carrier as the most appropriate method. In this work, the immobilization of biocatalysts has been performed via incorporating AS cells into polyvinyl alcohol cryogel.
A method for desulfurizing hydrocarbon fractions by means of the sequential combination of the chemical catalytic oxidation of sulfur-containing compounds and the biotechnological reduction of the obtained oxidized forms of sulfur using immobilized biocatalysts under anaerobic conditions has been developed and tested. In the course of the development of methods for the biological destruction of organic sulfur-containing compounds that compose extracts taken from the organic phase, special attention has been paid to studying the extractant nature effect exerted not only on the efficiency of the extraction procedure, but also on anaerobic biocatalysts. The choice of catalysts based on transition metal salts and organic acids for the chemical oxidation of sulfur-containing compounds is caused by the need to assess the effect of the residual amount of the catalyst entering the organic extractant exerted on the possibility of sulfone bioconversion under the action of AS.
EXPERIMENTAL
The model mixture consisted of dibenzothiophene (DBT, 98%, Sigma-Aldrich) dissolved in dodecane (99%, Sigma-Aldrich). The initial concentration of total sulfur in the mixture amounted to 500 ppm. The mixtures of methylphenyl sulfide (MeSPh, 99%, Acros Organics), dibenzyl sulfide (Bn2S, 98%, Sigma-Aldrich), and benzothiophene (BT, 98%, Sigma-Aldrich) were prepared in a similar way.
The following transition metals complexes and acids were used as catalysts: sodium molybdate (Na2MoO4·2H2O, 99%, Sigma-Aldrich), sodium tungstate (Na2WO4·2H2O, 99%, Sigma-Aldrich), phosphomolybdenic acid (PMC, reagent grade, Lenreaktiv), phosphotungstic acid (PVA, reagent grade, Lenreaktiv), ammonium paramolybdate ((NH4)6Mo7O24·4H2O, pure, RusKhim), sulfuric acid (H2SO4, 95.6%, Sigma Tech), formic acid (HCOOH, 90%, Komponent-reaktiv), acetic acid (CH3COOH, 99.8%, Komponent-reaktiv), propionic acid (C2H5COOH, 99.5%, ChemicalLine), and stearic acid (C18H36O2, 99.3%, Komponent-reaktiv).
Hydrogen peroxide (H2O2, 50%, Prime Chemicals Group) and tert-butyl hydroperoxide (tert-BuOOH, 70%, ABCR) were used as oxidizing agents.
For the extraction of oxidized sulfur-containing compounds, we used dimethylformamide (DMFA, reagent grade, Komponent-reaktiv), acetonitrile (reagent grade, Komponent-reaktiv), N-methylpyrrolidone (reagent grade, Komponent-reaktiv), distilled water according to GOST (State Standard) 6709, isopropyl alcohol (C3H8O, 99.8%, Komponent-reaktiv), and absolute ethanol.
The composition of the reaction products and the purity of the starting reagents were determined by gas chromatography using a Kristall-2000M chromatograph equipped with a flame ionization detector, a Zebron column with L = 30 m, d = 0.32 mm, and ZB-1 liquid phase under temperature programming from 100 to 250°C. Chromatograms were registered and analyzed using a computer with a Chromatek Analytic 1.5 software package.
The conditions for analyzing the reaction mixture before and after oxidation were as follows:
(i) carrier gas: nitrogen (p = 200 kPa), volumetric flow rate 30 mL/min;
(ii) initial column temperature 100°C;
(iii) injector temperature 150°C;
(iv) detector temperature 250°C;
(v) column heating rate 20°С/min.
Preparation of Oxidative Catalytic Mixture
60 μmol of a transition metal salt was preliminarily dissolved in 0.01–0.1 mol of hydrogen peroxide under permanent stirring. After a complete dissolution of the salt, 0.005–0.1 mol of the chosen acid was added to the mixture. Stirring was continued for 5 min.
Oxidation of Model Mixtures
A total of 0.01–0.1 mL of an oxidative catalytic mixture was added to 5 mL of a model mixture of an organosulfur compound placed in a thermostatic reactor. The oxidation was carried out for 0.5–6 h at a temperature of 20–80°C. After completing the reaction, the aqueous phase was separated; the mixture was washed with water to remove the catalyst residues; and sulfones were twice extracted by the extractant, 5 mL each.
Obtaining an Immobilized Anaerobic Biocatalyst
For testing the effect of extractants on the anaerobic biocatalyst in the course of the decomposition of sulfur-containing organic compounds involved in the extracts, we used the anaerobic sludge taken from the digester, wherein the alcohol stillage is processed (Moscow oblast, Russia). This AS has been successfully proven earlier from the standpoint of the possibility of the anaerobic bioconversion of sulfones to produce sulfides [11].
The AS was used in the experiment either in the suspension form or in the immobilized form. The AS was immobilized by incorporating the cryogel of polyvinyl alcohol (PVA) according to a technique described earlier [12].
Determining Intracellular Adenosine Triphosphate Concentration
In order to assess the effect of extractants on the biocatalyst, the suspension and immobilized AS cells at a concentration amounting to15 g of dry matter per liter were placed in 0.1 M potassium phosphate buffer (pH 7.2). After that, the extractant was introduced into the medium with AS at a ratio of 35 mL of extractant to 965 mL of buffer. The mixture was stirred. The AS was held in the resulting medium for 24 h, after which the concentration of intracellular adenosine triphosphate (ATP) was determined in the selected AS samples.
To obtain an extract of intracellular ATP, the AS biomass or granules containing such biomass were weighed (0.15 ± 0.05 g), transferred into dimethyl sulfoxide (1 mL), and held at 25°C for 1 h. The concentration of intracellular ATP in the suspension and immobilized cells was determined by means of a bioluminescence technique using a luciferin–luciferase reagent (Lumtek LLC, Russia) and a New Horizons Diagnostics 3560 microluminometer (United States) [13].
Anaerobic Fermentation
A total of 45 mL of 0.1 M K-phosphate buffer (pH 7.2) containing glucose (1 g/L), immobilized AS at a concentration amounting to15 g of dry matter (humidity 85%) per liter, and an extractant containing sulfone, was put into hermetically sealed vials (anaerobic reactors, 120 mL). The volume of extractant was calculated so that the final concentration of sulfone in the working reactor amounted to 0.15 mM. The anaerobic incubation was carried out at a temperature of 35°C.
Determination of Sulfone and Sulfide Concentration
The concentration of sulfide ions in the liquid phase was determined by spectrophotometry at a wavelength of 660 nm using a Shimadzu UV-1202 spectrophotometer (Japan) [14].
RESULTS AND DISCUSSION
The activity of the catalysts was analyzed using a model mixture of dibenzothiophene (DBT). Such a choice of the substrate is caused by the relative inertness of DBT with respect to oxidation. Most common systems based on the compounds of transition metals in combination with acids have been taken as the catalysts for the oxidation of sulfur compounds. The catalyst composition and DBT oxidation conditions have been optimized for further studying the effect of the residual catalyst amount entering the organic extractant exerted on the possibility of sulfone bioconversion in the presence of active sludge.
Effect of Acid Nature on the Conversion Level of DBT
Dibenzothiophene has been oxidized using hydrogen peroxide at a fourfold excess thereof with respect to the total sulfur content in the model mixture. It is known that, in an acidic environment, the oxidation of sulfur compounds proceeds more efficiently [2]; therefore, at the first stage, we studied the effect of the acid nature on the conversion of DBT. The oxidation was performed at 60°C for 1 h. The results are shown in Table 1.
As is shown in Table 1, formic acid is the most efficient acid additive, which in the presence of hydrogen peroxide forms a peroxy acid that acts as an additional oxidant of DBT.
Effect of Catalyst Nature on DBT Conversion Level
In previous papers it has been shown that the most active catalysts intended for oxidative desulfurization contain molybdenum or tungsten atoms [7] forming peroxo complexes in the presence of hydrogen peroxide. Table 2 shows data on the oxidation of dibenzothiophene in the presence of catalysts based on molybdenum and tungsten salts and formic acid.
As can be seen from Table 2, sodium molybdate is the most efficient catalyst. It has been used in the further work as a catalyst.
Effect of Acid on DBT Conversion Level
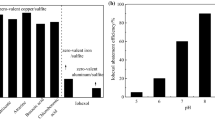
At the next stage, we investigated the effect of the amount of formic acid on the conversion level of dibenzothiophene (Fig. 1). As one can see from Fig. 1, a 7.5-fold excess of formic acid gives the best results, while an increase in the amount of acid does not lead to an increase in the conversion level. The use of the excess acid is caused by a slow formation of peroxy acid that serves as a source of active oxygen for the oxidation of dibenzothiophene, as well as by an insufficiently efficient area of interaction between DBT and the oxidizing agent, since they dwell in different phases. These restrictions can be compensated by the addition of an excess of formic acid.
Effect of Sodium Molybdate on DBT Conversion Level
In order to increase the conversion level of DBT, we have studied the effect of sodium molybdate on the level of DBT oxidation. The amount of sodium molybdate has been varied from 1 : 50 to 1 : 6 (mol) with respect to the total sulfur. As can be seen from Fig. 2, the ratio of 1 : 12 chosen earlier is optimal, since both an increase and a decrease in the amount of catalyst in the system negatively affects the DBT conversion level.
Effect of Temperature on DBT Conversion Level
The effect of the oxidation temperature on the DBT conversion level has been studied in the presence of two different oxidizing agents: hydrogen peroxide and tert-butyl hydroperoxide. The temperature ranged from 20 to 80°C (Table 3).
Data presented in Table 3 show that hydrogen peroxide is a more efficient oxidant in this system. The optimal oxidation temperature amounts to 60°C, and a further increase in temperature leads to the decomposition of hydrogen peroxide, which negatively affects the conversion level of DBT.
Effect of Oxidant on the DBT Conversion Level
For the formation of sulfone that, according to chromatographic data, represents the only reaction product, a twofold excess of an oxidizing agent is required. However, in the course of oxidation, hydrogen peroxide is permanently consumed with the formation of water, which leads to its dilution and, as a consequence, to a decrease in the activity of hydrogen peroxide. In this regard, for the oxidation of sulfur compounds, a larger excess of the oxidizing agent should be added. The oxidation of DBT has been performed under a two- to tenfold molar excess of hydrogen peroxide with respect to the sulfur content. The data are presented in Table 4.
As one can see from Table 4, the highest conversion level is achieved in the case of a sixfold excess of hydrogen peroxide, whereas the further increase in the amount of hydrogen peroxide does not lead to any significant increase in the DBT conversion level.
Effect of Oxidation Time on DBT Conversion Level
Kinetic studies have been carried out at a fourfold and sixfold excess of the oxidizing agent. The data are shown in Fig. 3. As can be seen from Fig. 3, the oxidation proceeds almost completely in 1 h, and the further increase in the oxidation time does not lead to any increase in the DBT conversion level.
Oxidation of Sulfur Compounds Belonging to Different Classes
Since real petroleum fractions contain a large number of classes of sulfur compounds, we have performed the oxidation of methyl phenyl sulfide (MeSPh), dibenzyl sulfide (Bn2S), benzothiophene (BT), dibenzothiophene and methyldibenzothiophene (MeDBT). The results are presented in Fig. 4.
From Fig. 4, one can see that the oxidizing ability of sulfur compounds decreases in the order of MeSPh > Bn2S > DBT > MedBT > BT, which is in good agreement with the literature data concerning the oxidation of sulfides [16]. A lower reactivity of benzothiophene in comparison with dibenzothiophene is associated with a lower electron density inherent in the former. One can also see from the data that the presence of more branched substituents at the sulfur atom leads to a decrease in the conversion level, which could be associated with steric hindrances in the oxidation reaction.
Sulfone Extraction
The removal of oxidized sulfur compounds from fuels is an important stage in oxidative desulfurization. The most efficient way to remove sulfur compounds consists of extraction.
In this work, we studied the effect of the extractant nature on the extraction level of dibenzothiophene sulfone. The data are shown in Table 5. The extraction was performed twice at room temperature.
Data presented in Table 5 show that DMFA and N-methylpyrrolidone make it possible to remove dibenzothiophene oxidation products to the most complete extent. The obtained solutions of sulfones in the corresponding extractants have been bioconverted in order to decompose sulfones to study the effect of the extractant nature on the efficiency of bioconversion level in the presence of AS.
Effect of Extractant Nature on Anaerobic Sludge Cells
In the course of the development of methods for the biological destruction of organic sulfur-containing compounds that compose the extracts from the organic phase, it is important to assess the effect of extractants on the anaerobic biocatalysts. It is known that the process of methanogenesis in the presence of sulfones lasts for at least 8 days [12]. Therefore, to assess the effect on the cells and choose the most preferable extractants, a more rapidly implemented method based on monitoring changes in the level of intracellular ATP has been used [17, 18]. The concentration of intracellular ATP is a universal indicator of the viability of a living cell. The ATP assay makes it possible to assess the inhibitory effect of the system components on the target activity of biocatalysts.
A number of extractants readily miscible with water, characterized by different classes of toxicity, have been tested as potential candidates for the extraction of organic sulfur-containing compounds from a hydrocarbon medium. In order to determine the effect of these potential extractants on the viability of the suspension and immobilized forms of AS, the AS was exposed to the substances under investigation for 24 h (Table 6).
For comparison with other extractants in the presence of ethyl and isopropyl alcohols, an insignificant positive change in the ATP level has been observed (see Table 6). This is explained by the fact that AS cells can use these substances as a substrate, which is known from the literature [19]. In the presence of water, changes in the concentration of intracellular ATP are observed within the measurement error. The concentration of intracellular ATP decreases most significantly when acetonitrile, DMF, and N-methylpyrrolidone are added to the AS. In the case of immobilized AS cells, the observed changes in the concentration of intracellular ATP are less pronounced than in the case of suspension cells.
The higher residual level of intracellular ATP in immobilized cells when compared with suspension cells indicates that the metabolic activity of the immobilized cells is higher than that of the suspension cells. It is known that the cells immobilized in PVA cryogel are tolerant with respect to the presence of toxicants; they can be used for the biotransformation of wastes into various commercial products [20, 21].
Thus, it has been found that, in the course of choosing extractants from the standpoint of minimum inhibitory effect exerted on the activity of biocatalysts for the extraction of sulfones, it is most expedient to use water and ethyl and isopropyl alcohols (see Table 6). For 10 days of DBTO2 bioconversion in the anaerobic conditions under the action of immobilized AS using the mentioned extractants, the decomposition level of sulfone and the yield of sulfide amounted to 100%.
In general, the changes in the concentration of intracellular ATP in AS cells observed for 24 h in the presence of water, ethanol, and isopropanol indicate that, within the entire range of potential applicants, these extractants are most expedient to use for biotechnological processes involving anaerobic biocatalysts in the form of sludge. The biocatalysts themselves are most expedient for use in the immobilized form on the carrier based on PVA cryogel. The results obtained in this work could be useful for researchers engaged in solving complicated problems concerning the transition of existing production cycles to environmentally safe chemical technologies.
CONCLUSIONS
As a result of these studies, the optimal conditions for the oxidation of dibenzothiophene in the presence of a catalyst based on a transition metal and an acid have been chosen: sodium molybdate as the catalyst, formic acid as the acid additive, and hydrogen peroxide as the oxidizer. The oxidation conditions are as follows H2O2 : S : HCOOH : Mo = 6 : 1 : 7.5 : 0.08 (mol), 60°C, 60 min. The effect of the extractant nature exerted on the extraction level of DBT sulfone and on the activity of AS has been studied. It is shown that for extraction it is most expedient to use ethyl or isopropyl alcohols as the extractants, which make it possible to achieve a high level of sulfone extraction and a complete bioconversion of sulfones isolated with the use of AS.
REFERENCES
Babich, I.V. and Moulijn, J.A., Science and technology of novel processes for deep desulfurization of oil refinery streams: A review, Fuel, 2003, vol. 82, no. 6, pp. 607–631. https://doi.org/10.1016/S0016-2361(02)00324-1
Akopyan, A.V., Fedorov, R.A., Andreev, B.V., Tarakanova, A.V., Anisimov, A.V., and Karakhanov, E.A., Oxidative desulfurization of hydrocarbon feedstock, Russ. J. Appl. Chem., 2018, vol. 91, no. 4, pp. 529–542.
Abro, R., Abdeltawab, A.A., Al-Deyab, S.S., Yu, G., Qazi, A.B., Gao, S., and Chen, X., A review of extractive desulfurization of fuel oils using ionic liquids, RSC Adv., 2014, vol. 4, no. 67, pp. 35302–35317.
Wang, D.H., Qian, E.W.H., Amano, H., Okata, K., Ishihara, A., and Kabe, T., Oxidative desulfurization of fuel oil—Part I. Oxidation of dibenzothiophenes using tert-butyl hydroperoxide, Appl. Catal., A, 2003, vol. 253, no. 1, pp. 91–99.
Dehkordi, A.M., Sobati, M.A., and Nazem, M.A., Oxidative desulfurization of non-hydrotreated kerosene using hydrogen peroxide and acetic acid, Chin. J. Chem. Eng., 2009, vol. 17, no. 5, pp. 869–874.
Yu, G.X., Lu, S.X., Chen, H., and Zhu, Z.N., Oxidative desulfurization of diesel fuels with hydrogen peroxide in the presence of activated carbon and formic acid, Energy Fuels, 2005, vol. 19, no. 2, pp. 447–452.
Polikarpova, P., Akopyan, A., Shigapova, A., Glotov, A., Anisimov, A., and Karakhanov, E., Oxidative desulfurization of fuels using heterogeneous catalysts based on MCM-41, Energy Fuels, 2018, vol. 32, no. 10, pp. 10898–10903.
Qiu, L., Cheng, Y., Yang, C.P., Zeng, G.M., Long, Z.Y., Wei, S.N., Zhao, K., and Luo, L., Oxidative desulfurization of dibenzothiophene using a catalyst of molybdenum supported on modified medicinal stone, RSC Adv., 2016, vol. 6, no. 21, pp. 17036–17045.
Or-Rashid, M.M., Onodera, R., and Wadud, S., Biosynthesis of methionine from homocysteine, cystathionine and homoserine plus cysteine by mixed numen microorganisms in vitro, Appl. Microbiol. Biotechnol., 2001, vol. 55, no. 6, pp. 758–764.
Khlebnikova, T.D., Khamidullina, I.V., Khusainov, M.A., Nasyrova, L.A., and Il’ina, S.F., Study of the effect of various immobilization materials on the growth of sulfate-reducing bacteria in the biochemical purification of wastewater, Mezhdunar. Zh. Eksp. Obraz., 2015, vol. 12, pp. 248–249.
Senko, O., Maslova, O., Gladchenko, M., Gaydamaka, S., Akopyan, A., Lysenko, S., Karakhanov, E., and Efremenko, E., Perspective approach to anaerobic bioconversion of benzo- and dibenzothiophene sulfones to sulfide, Molecules, 2019, vol. 24, pp. 1736–1748.
Senko, O., Gladchenko, M., Maslova, O., and Efremenko, E., Long-term storage and use of artificially immobilized anaerobic sludge as a powerful biocatalyst for conversion of various wastes including those containing xenobiotics to biogas, Catalysts, 2019, vol. 9, pp. 326–345.
Stepanov, N. and Efremenko, E., Immobilised cells of pachysolen tannophilus yeast for ethanol production from crude glycerol, New Biotechnol., 2017, vol. 34, pp. 54–58.
Trukhina, A.I., Gladchenko, M.A., and Kalyuzhnyi, S.V., Optimizations of sulphide and organic modifications of the DEAMOX process, Appl. Biochem. Microbiol., 2011, vol. 47, no. 9, pp. 841–845.
Zhao, H. and Baker, G.A., Oxidative desulfurization of fuels using ionic liquids: A review, Front. Chem. Sci. Eng., 2015, vol. 9, no. 3, pp. 262–279.
Houda, S., Lancelot, C., Blanchard, P., Poinel, L., and Lamonier, C., Oxidative desulfurization of heavy oils with high sulfur content: A review, Catalysts, 2018, vol. 8, no. 9, p. 344. https://doi.org/10.3390/catal8090344
Efremenko, E.N. and Tatarinova, N.Y., The effect of long-term preservation of bacterial cells immobilized in poly(vinyl alcohol) cryogel on their viability and biosynthesis of target metabolites, Microbiology, 2007, vol. 76, no. 3, pp. 336–341.
Efremenko, E.N., Azizov, R.E., Raeva, A.A., Abbasov, V.M., and Varfolomeyev, S.D., An approach to the rapid control of oil spill bioremediation by bioluminescent method of intracellular ATP determination, Int. Biodeterior. Biodegrad., 2005, vol. 56, no. 2, pp. 94–100.
Efremenko, E.N., Andryushina, V.A., and Balabanova, T.V., Immobilizovannye kletki: biokatalizatory i protsessy (Immobilized Cells: Biocatalysts and Processes), Moscow: RIOR, 2018.
Maslova, O.V., Sen’ko, O.V., and Stepanov, N.A., The use of the hydrolyzates of various agricultural wastes as substrates for the cultivation of microorganisms to produce various target products, Materialy Mezhdunarodnoi nauchno-prakticheskoi konferentsii “Novye podkhody k razrabotke tekhnologii proizvodstva i pererabotki sel’skokhozyaistvennoi produktsii” (Proc. International Research and Practice Conference “New Approaches to the Development of Technologies for the Production and Processing of Agricultural Products”) (Volgograd, 2018), Gorlov, I.F., Ed., Volgograd: Volgogr. Inst. Upr., 2018, pp. 417–421.
Stepanov, N.A., Sen’ko, O.V., Maslova, O.V., and Efremenko, E.N., Biocatalytic conversion of wastes from various production processes and renewable biomass into commercially important products, Materialy Mezhdunarodnoi nauchno-prakticheskoi konferentsii “Ekologicheskie chteniya-2018” (Proc. International Research and Practice Conference “Environmental Readings-2018”) (Omsk, 2018), Omsk: LITERA, 2018, pp. 297–299.
Funding
This work was financially supported by the Russian Foundation for Basic Research, project 18-29-05064.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated by O. Polyakov
Rights and permissions
About this article
Cite this article
Akopyan, A.V., Polikarpova, P.D., Anisimov, A.V. et al. Oxidation of Dibenzothiophene with the Subsequent Bioconversion of Sulfone. Theor Found Chem Eng 55, 778–785 (2021). https://doi.org/10.1134/S0040579521040035
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0040579521040035