Abstract—
The acid-base properties of cobalt ferrite synthesized by the mechanochemical method with different ratios of the main components Fe2O3 : Co3O4 have been investigated using the temperature-programmed desorption of ammonia, IR spectroscopy using adsorbed pyridine with ammonia, and the Hammett indicator method. A comparison of the results for the total content of Lewis and Brønsted acid sites by three methods has shown that all three samples were quite similar, with a probability of 0.5%. The introduction of cobalt oxide into the composition of cobalt ferrite leads to an increase in the concentration of Brønsted and Lewis centers with a simultaneous increase in the number of strong basic centers determined by the temperature-programmed desorption of ammonia. The indicator method is the most informative method for a quantitative study of the acid-base properties of the cobalt ferrite surface, since it allows one to assess the concentration and strength of Lewis and Brønsted acid sites and basic sites.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
INTRODUCTION
The heterogeneous processes involving the catalyst are based on the donor–acceptor properties of its surface, characterizing its acid-base characteristics. Following modern concepts, the surface of solid catalysts is characterized by the presence of acidic and basic centers [1]. The acid-base properties of solid catalysts affect the activity and selectivity of many catalytic reactions not only in acid-base transformations, but also in reduction and oxidation reactions [2–4]. Numerous catalytic reactions are catalyzed by solid acid catalysts, which are widely used in petroleum refining and chemical industries. Zeolites, simple and complex oxides, ferrites, and phosphates exhibiting acid-base properties of various natures are widely used in important industrial processes, such as ammonia oxidation, CO conversion with water vapor, etc. [5]. Heterogeneous solid catalysts, which exhibit acid and basic properties, have many advantages over liquid acid and base catalysts, since they are not corrosive, safe for the environment, and their separation from gaseous or liquid products is not difficult [6].
The examination of the acid-base properties of the surface of the catalyst is an interesting and important problem not only from a theoretical, but also from a practical point of view, since these properties determine the quality of adsorbents, catalysts, and semiconductors. It is known that the surface properties of oxides and oxide compounds are determined by the properties of their surface OH–groups, which depend on the type of metal atom and its coordination with oxygen atoms in the crystal structure of compounds [7].
Based on the crystallography data, metal atoms coordinated with a different number of oxygen atoms can exist on different faces even for an ideal crystal. Accordingly, the acid-base properties of OH-groups bound to such metal atoms can differ significantly. The existence of several acid-base centers on the surface of various oxides has been proven by IR spectroscopy [11–14], EPR spectroscopy [15], and the indicator adsorption method [16].
In recent years, spinel-type ferrites have received a lot of attention due to their structural, acid-base, and catalytic properties. These materials are good catalysts for many processes, for example, N2O decomposition, the Fischer–Tropsch synthesis, oxidation of CO to CO2, and the reforming of CH4 into synthesis gas [17–19]. Ferrites have strong acid and basic centers. The physicochemical properties of ferrites are determined by the valence state of metal ions and their distribution in the crystal lattice, which is also characterized by certain types and levels of point defects. Ferrites are complex compounds with different coordination of metal atoms with oxygen atoms. The surface of the ferrites is similar to polybasic acids or polyacid bases; i.e., it has several dissociation constants. The coordination states of atoms (cations and anions for ferrites) and the reaction properties of the catalyst surface depend on the crystal plane on which the adsorption occurs [20].
It was found that the reactivity of catalysts to the reducing gases is primarily determined by the activity of redox interaction with the participation of chemisorbed oxygen species on the surface. In this regard, it is important to choose a method for determining the acid-base properties of the adsorption centers surface to develop methods for changing the concentration and ratio between adsorption centers of different natures. The aim of this work was to study the acid-base properties of cobalt ferrite and evaluate them by physicochemical methods.
EXPERIMENTAL
The IR spectra were obtained at room temperature using a TENSOR II IR spectrometer (Bruker AXS Gmbb). To determine the characteristics of the acid sites on the surface of the experimental samples, pyridine was used as a probe molecule. The concentration of acid sites was estimated from the integral intensity of the absorption bands corresponding to these centers in the spectra of adsorbed pyridine using the integral absorption coefficient [20] calculated as
The concentration of centers was based on the use of the Bouguer–Lambert–Beer law as applied to some bands in the spectra of adsorbed molecules. In the case of adsorbed probes, it is convenient to represent this law in the form
where А3 is the observed band integral absorption, cm–1; A0 is integral absorption of the band for the adsorbate concentration, μmol/g; C is the concentration of the adsorbed probe, μmol/g; and ρ is the amount of catalyst per 1 cm2 of the luminous flux section, mg [21].
The adsorption and desorption of ammonia were carried out using a thermal desorption unit assembled based on a Tsvet-500 gas chromatograph. The total surface acidity of the samples was estimated under the assumption of single-site adsorption of ammonia from the number of chemisorbed molecules, the desorption of which was almost complete when the temperature in the reactor with the catalyst was raised to 400–450°C.
The number of the acid sites Ni (mmol/g) distributed over the corresponding maxima of the thermal desorption peaks Тmax,I was calculated from the values of the area under the desorption curves using the formula
where 6 × 1023 is the Avogadro constant; S(Тmax,i) is the area under the corresponding maximum on the thermal desorption curve, mm2; Ssp is the specific surface area of the substrate, m2/g; G is the mass of the sample, g; and ΣS(Тmax, i) is the total area of peaks on the thermal desorption curve, mm2. The number of moles of desorbed ammonia was calculated as V/22400, where V is the desorbed volume of ammonia, mL.
The optical densities of the initial solutions of indicators (D0) were measured on a KFK-2MP photocolorimeter. Then, suspensions of catalysts in these solutions were prepared and optical densities (D1) were measured after establishing adsorption equilibrium and separating the solution from the precipitate by decantation or centrifugation. To evaluate the effect of the pH change on the optical density upon the contact of the sample with the solution, a suspension of catalysts was prepared in distilled water; then precipitate was separated after 30 min and the indicator solution was added, followed by the measurement of the optical density (D2). The content of active sites (qpKa, mol/g) with a given pKa value was calculated by the formula
where Cind and Vind are the concentration and volume of the indicator solution, respectively; m1 and m2 are the mass of the catalyst when measuring D1 and D2, respectively; the dash corresponds to the unidirectional change of D1 and D2 relative to D0 and the plus sign refers to the multidirectional change, respectively [3].
The distribution of adsorption centers was presented in the coordinates \({{q}_{{{\text{p}}{{{\text{K}}}_{{\text{a}}}}}}}\) = f(pKa).
RESULTS AND DISCUSSION
Infrared spectroscopy is one of the most widespread and effective methods for studying acid-base sites on the surface of catalysts [2, 4, 5]. The use of IR spectroscopy to study donor–acceptor surface properties is based on the spectral probe method. The properties of adsorption centers are evaluated from the absorption spectra of adsorbed molecules, as well as from the change in the position of absorption bands observed during adsorption. This method makes it possible to reliably identify the interaction of probe molecules with proton, aprotic, and basic surface centers and to quantitatively characterize the strength of individual centers, and it is suitable for the independent determination of the concentration of each type of surface center. The use of IR spectroscopy revealed some fundamental aspects of heterogeneous catalysis, namely, to detect the presence of Lewis and Brønsted centers on the surface, to establish their structure, and to reveal their role in catalytic transformations. Pyridine and ammonia are widely used as probe molecules [8]. In this work, pyridine was chosen as a probe to characterize the nature of the acidity of cobalt ferrite.
Pyridine, being a weaker base compared to ammonia, reacts only with strong acid sites on the surface [9]. The specific adsorption of pyridine on the surface of cobalt ferrite is molecular and occurs due to the formation of hydrogen bonds with surface hydroxyl groups [23].
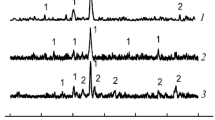
Figure 1 shows the IR spectra of cobalt ferrite nanoparticles with different ratios of the main components. When cobalt ferrite is saturated with an excess of cobalt(III) oxide, the band at 417–440 cm–1 shifts to a higher frequency region and the band at 520–630 cm–1 has a shoulder, which corresponds to the partial use of pyridine groups in coordination with Со3+ ions. The IR spectra (Fig. 1) of cobalt ferrite with different ratios of the main components exhibit absorption bands in the range of 1400–1700 cm–1, which indicates the presence of acidic Lewis and Brønsted centers on the surface and the presence of only a small amount of proton centers capable of the formation of pyridinium ions at the adsorption of pyridine ≥Ñ → М+. In ferrites, IR bands are created by vibrations of oxygen ions with cations present in octahedral and tetrahedral positions in the unit cell.
A sign of the formation of a pyridinium complex with Lewis centers is a shift of the pyridinium band at 1447 cm–1 by 2–4 cm–1, depending on the ratio of the main components of iron oxide and cobalt. Other closely spaced bands at 1488 and 1489 cm–1 are less intense and practically do not change their positions. In the presence of proton centers, Lewis centers can be identified only by the pyridine band at 1450–1460 cm–1.
The protonation of pyridine is the criterion for the presence of proton centers [8]. When pyridine is protonated, absorption bands of pyridinium ions appear in the spectrum, the characteristic of which is the band at 1608 cm–1 in Fig. 1. The pyridinium ions chemisorbed on the surface have an absorption band at 3340 cm–1, corresponding to NH+ stretching vibrations. These spectra have an absorption band at 2358 cm–1, which characterizes the antisymmetric linear vibrations of physically adsorbed CO2 molecules.
The main difficulty of the spectral method from the experimental side in determining the main centers of the surface of cobalt ferrite arises from masking the spectral manifestations of the molecular interactions with the surface as a result of absorption by the volume of the catalyst. Even the weak physical adsorption of a molecule on a surface can lead to the loss of some of the rotational and translational movements.
To overcome the difficulties in determining the acid-base centers, a method for studying the acidity of catalysts based on temperature-programmed desorption (TPD) of adsorbed ammonia from the surface of the samples has been proposed. Due to its usability, rapidity, and visualization of results, this method has gained the greatest popularity. By varying the corresponding experimental parameters using this method, it is possible to measure the total acidity of the surface and establish the distribution of acid sites by their strength [9]. The temperature range of saturation of the catalyst samples with ammonia in the thermal desorption method is close to the operating temperature of catalysts. This makes it possible to correctly identify the correlations of their activity with surface properties. To study the acid sites of cobalt ferrite, ammonia was used as a probe. The choice of ammonia is due to its small size, ease of dosage, and high basicity, making it possible to determine not only strongly acid centers, but also weak centers [22].
It was found that the TPD spectra of ammonia adsorbed on the surface of the modified cobalt ferrite (Fig. 2) exhibit three desorption maxima which change their position on the temperature coordinate Тmax1 (108–210)°С; Тmax2 (211–350)°С; Тmax3 (350–500)°С, depending on the experimental conditions. The observed maxima correspond to the desorption of ammonia from the surface acid sites, conventionally ranked as weakly acidic, moderately acidic, and strongly acidic. It can be seen that a gradual rise in temperature leads to a clearer manifestation of thermal desorption maxima while maintaining the qualitative distribution of acid sites in terms of their strength. This may be due to the specificity of the radiation effect on the desorption of ammonia molecules from the corresponding surface areas (local centers) of the active mass of cobalt ferrite, which absorbs radiation energy and transforms it into heat more efficiently than the carrier.
It was also revealed by the TPD that the number of acid sites decreases with an increase in the content of cobalt oxide in ferrite. Ammonia is desorbed mainly on acid sites with electron-withdrawing properties and their number decreases as the amount of cobalt oxide in ferrite increases.
Figure 3 shows the molecular adsorption of ammonia, which occurs during the specific interaction of ammonia molecules with the surface of cobalt ferrite, resulting in the formation of acid-base centers on the surface. This leads to a decrease in the intensity of the absorption bands at 3120 cm–1, which is attributed to ammonium ions formed during the reaction of ammonia molecules with water molecules remaining on the surface, as well as with a predominance of Lewis acid sites. These bands were interpreted as asymmetric stretching and bending vibrations of the ammonium ion. The absorption bands of molecular adsorbed ammonia were observed at 3401–3417 cm–1. The molecular adsorption of ammonia occurs during the specific interaction of ammonia molecules with surface hydroxyl groups with the formation of the hydrogen bond. The absorption band of ammonia molecules at 3335 cm–1 characterizes their coordination bond with the Lewis center. The absorption band at 3280 cm–1 can be attributed to the stretching vibrations of NH+ in ammonia molecules, which formed coordination bonds with the surface of cobalt ferrite.
Summarizing, the IR spectroscopy of adsorbed probe molecules establishes the total surface acidity without dividing into Lewis and Brønsted centers, while the chemisorption of ammonia allows one to identify surface centers by the type and their strength.
The method of adsorption of acid-base indicators should be mentioned among the methods of analysis used to determine the features of the functional-chemical composition of the surface. This method is the oldest and experimentally simplest way to measure the acid-base properties of a solid surface [4]. It is based on the feature of the indicator to change its color upon adsorption on the surface, which is a measure of the acidity (basicity) of the surface containing Lewis and Brønsted active centers. A complete description of the acid-base properties of the solid surface implies the determination of the concentration and strength of active centers, i.e., obtaining their distribution with differentiation into acids and bases.
Figure 4 shows the distribution spectra of acid-base sites on the surface of cobalt ferrite with different ratios of the main components. It can be seen that the distribution of acid-base sites on the surface of cobalt ferrite is nonuniform, resulting in the discreteness and a rather clear deformation of sorption bands with maxima of different intensities corresponding to a certain pKa value.
The surface of cobalt ferrite is characterized by a complex distribution of centers, which is due to the presence of oxygen impurities in their structure. Intense bands with рKа = 6.4, 7.3, 9.45, and 12.8 are assigned to Mе–(OH) and Mе–(OH)– proton-donor adsorption centers, formed by coordinated water molecules or OH-groups with alkaline (MeO)- or acidic Me2+ Lewis centers. Less intense bands in the region of рKа = 2.5–5 and рKа = 1.3 are associated with the presence of proton acceptor centers on the surface.
Lewis acid regions with pKa > 14 are unsaturated surface sites, which, in the case of ferrite, are metal cations (Со3+). These centers can interact with molecules containing an unshared electron pair or carrying a positive charge according to the donor–acceptor mechanism.
For cobalt ferrite containing cobalt and iron oxides, intense bands of three types of Brønsted centers were recorded: acidic Mе–(OH)δ + (рKа = 2.5; 3.46; 4.1), neutral Mе–(OH)δ0 (рKа = 6.4), and basic Mе–(OH)δ – (рKа = 8.8; 9.45; 12). It was noted that the bands related to the main centers are more intense and their number increases with an increase in the content of cobalt oxide in the catalyst (Fig. 4).
The results of determining the acid-base properties of the surface of the iron-cobalt catalyst by three methods are given in Table 2, which also reports the total content of centers for cobalt ferrite with different ratios of cobalt and iron oxides.
Based on the TPD of ammonia and the Hammett indicator method, it can be concluded that the state of adsorbed ammonia on the surface of the modified cobalt ferrite is different. This is reflected in the presence of various types of centers, which differ in nature. In addition, each of these types is energetically heterogeneous.
CONCLUSIONS
The acid-base properties of cobalt ferrite are significantly influenced by the interaction between the components of the mixed phases. A change in the content of cobalt oxide in the composition of the mixed phase makes it possible to additionally vary the number of acid-base centers. The experimental data indicated a change in the acid-base state of the surface of the examined cobalt ferrite samples during their modification and contributed to the development of theories about the mechanism of interaction of the main catalyst during the conversion of nitrous oxide. A comparison of the results for the total content of Lewis and Brønsted acid sites obtained by the IR spectroscopy of adsorbed ammonia, TPD of NH3, and the Hammett indicator method showed fairly close values for all three samples with a probability of 0.5%. The number of centers capable of chemisorbing ammonia is comparable to the total concentration of Brønsted (B2) and Lewis (L2) centers of medium strength, which was calculated using all methods in Table 4. Weak Lewis centers (L1) and partially proton-donating groups with low acidity can probably form weak and strong physical bonds with the probe molecule under the temperature-programmed desorption of ammonia. The chemisorption of ammonia was used to determine the total surface acidity of the proton donor (Brønsted) and electron acceptor (Lewis), while the IR spectroscopy of adsorbed probe molecules was used to identify surface centers by type and their strength. In this case, the temperature range of the sample saturation with a probe molecule in the TPD of ammonia is close to the operating temperatures of catalysts in comparison with the IR spectroscopy of adsorbed pyridine, which makes it possible to more correctly compare the surface properties of cobalt ferrite with its catalytic characteristics.
NOTATION
A 0 | integral absorption of adsorbate band for concentration, μmol/g |
А 3 | integral band absorption, cm–1 |
С | concentration of adsorbed probe, μmol/g |
C ind | concentration of indicator solution, mol/mL |
G | mass, g |
m1, m2 | mass of catalyst, g |
N i | content of active sites, mol/g |
S(Тmax, i) | area under corresponding maximum on thermal desorption curve, mm2 |
ΣS(Тmax, i) | total peak area on thermal desorption curve, mm2 |
S sp | specific surface area of carrier, m2/g |
V | desorbed volume of ammonia, mL |
V ind | volume of indicator solution, mL |
λ | absorption spectrum, cm–1 |
ρ | amount of catalyst per 1 cm2 of the luminous flux section, mg |
REFERENCES
Ermolovich, E.A., Effect of grinding on the donor-acceptor properties of surfaces of backfill mix components, J. Min. Sci., 2013, vol. 49, no. 5, pp. 839–846. https://doi.org/10.1134/S1062739149050195
Busca, G., Onida, B., Tichit, D., and Vaccari, A., Catalysis by acids and bases: New materials and surface studies: ABC-6, 6th World Congress on Catalysis by Acids and Bases, Genova (Italy), May 10–14, 2009, Catal. Today, 2010, vol. 152, nos. 1–4, p. 1. https://doi.org/10.1016/S0920-5861(10)00343-3
Vredrine, J.C., The role of redox, acid–base and collective properties and the crystalline state of heterogeneous catalysts in the selective oxidation reactions, Top. Catal., 2002, vol. 21, p. 97.
Fechete, I., Wang, Y., and Vredrine, J.C., The past, present and future of heterogeneous catalysis, Catal. Today, 2012, vol. 189, p. 2.
Isupova, L.A., Tsybulya, S.V., Kryukova, G.N., Budneva, A.A., Paukshtis, E.A., Litvak, G.S., Ivanov, V.P., Kolomiichuk, V.N., Pavlyukhin, Yu.T., and Sadykov, V.A., Mechanochemical synthesis and catalytic properties of calcium ferrite—Ca2Fe2O5, Kinet. Catal., 2002, vol. 43, no. 1, p. 122.
Romanova, R.G., Lamberov, A.A., Gil’manov, Kh.Kh., and Gil’mullin, R.R., Acid–base properties of the surface of bimetallic dehydrogenation catalysts, Vestn. Kazan. Tekhnol. Univ., 2010, no. 5, pp. 74–82.
Sitnikov, P.A., Kuchin A.V., Ryazanov, M.A., Belykh, A.G., Vaseneva, I.N., Fedoseev, M.S., and Tereshatov, V.V., Influence of acid-base properties of oxides surface on their reactivity towards epoxy compounds, Russ. J. Gen. Chem., 2014, vol. 84, pp. 810–815. https://doi.org/10.1134/S107036321405003X
Lauren-Pernot, H., Evaluation of surface acid-basic properties of inorganic-based solids by model catalytic alcohol reaction networks, Catalyst, 2006, vol. 48, p. 315.
Tanabe, K., Solid Acids and Bases: Their Catalytic Properties, New York: Academic, 1970.
Nikitenkov, N.N., Osnovy analiza poverkhnosti tverdykh tel metodami atomnoi fiziki (Fundamentals of an Analysis of the Surface of Solids by the Methods of Atomic Physics), Tomsk: Tomsk. Politekh. Univ., 2013.
Smith, A.L., Applied Infrared Spectroscopy: Fundamentals, Techniques, and Analytical Problem-Solving, Chemical Analysis: A Series of Monographs on Analytical Chemistry and Its Applications, New York: Wiley, 1979.
Nechiporenko, A.P., Burenina, T.A., and Kol’tsov, S.I., Indicator method for studying the surface acidity of solids, Zh. Obshch. Khim., 1985, vol. 55, no. 9, pp. 1907–1912.
Zakharova, N.V. and Sychev, M.M., Evolution of donor–acceptor centers of the surface of ferroelectrics upon dispersion, Kondens. Sredy Mezhfaznye Granitsy, 2010, no. 1, pp. 56–62.
Romanova, R.G. and Petrova, E.V., Acid-base surface properties of binary systems based on aluminum and zirconium oxides, Kinet. Catal., 2006, vol. 47, no. 1, p. 138.
Glazneva, T.S., Kotsarenko, N.S., and Paukshtis, E.A., Acid-base surface properties of oxide catalysts: From the study of aqueous suspensions to in-situ studies, Kinet. Catal., 2008, vol. 49, no. 6, p. 859.
Tkhyui, D.M. and Mikhalenko, I.I., Pyridine adsorption for probing electron-acceptor sites on the surface of titanium oxide with supported silver, copper, and gold ions, Prot. Met. Phys. Chem. Surf., 2015, vol. 51, no. 6, p. 934.
Il’in, A.P., Smirnov, N.N., and Il’in, A.A., Development of a catalyst for medium-temperature conversion of carbon monoxide in ammonia production, Ross. Khim. Zh., 2006, vol. 60, no. 3, p. 84.
Manova, E., Paneva, D., Kunev, B., Estournès, C., Rivière, E., Tenchev, K., Léaustic, A., and Mitov, I., Mechanochemical synthesis and characterization of nanodimensional iron–cobalt spinel oxides, J. Alloys Compd., 2009, vol. 485, nos. 1–2, p. 356.
Guido, E.S. and Giaime, M.D., Mechanochemical route for the synthesis of cobalt ferrite–silica and iron–cobalt alloy–silica nanocomposites, J. Nanopart. Res., 2004, no. 6, p. 99.
Vasyutin, O.A., Study of the effect of synthesis conditions on the adsorption properties of spinel-type ferrite and the surface characteristics of garnet-type ferrite of yttrium by potentiometry and wetting methods, Extended Abstract of Cand. Sci. (Chem.) Dissertation, St. Petersburg, 2012.
Paukshtis, E.A. and Yurchenko, E.N., Study of the acid–base properties of heterogeneous catalysts by infrared spectroscopy, Russ. Chem. Rev., 1983, vol. 52, no. 3, p. 242.
Muradova, P.A., Zul’fugarova, S.M., Shakunova, N.V., Litvishkov, Yu.N., Tret’yakov, V.F., and Talyshinskii, R.M., The acidic properties of the surface of aluminum-reinforced alumina supports produced in a microwave field, Neftegazokhimiya, 2012, vol. 6, no. 2, p. 52.
Ivanovskaya, M.I., Tolstik, A.I., Kotsikau, D.A., and Pankov, V.V., The structural characteristics of Zn–Mn ferrite synthesized by spray pyrolysis, Russ. J. Phys. Chem. A, 2009, vol. 83, no. 12, p. 2081.
ACKNOWLEDGMENTS
We thank the Centre for Collective Use at the Ivanovo State University of Chemistry and Technology for providing access to equipment facilities.
Funding
This work was performed within the work plan of the laboratory for the synthesis, investigation, and testing of catalytic and adsorption systems for processing hydrocarbon raw materials (founded with financial support from the Ministry of Education and Science of the Russian Federation for 2012–2022, project no. FZZW-2020-0010). The theoretical part of the work was financially supported by the Scholarship of the President of the Russian Federation (grant no. SP-3800.2019.1).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated by D. Kharitonov
Rights and permissions
About this article
Cite this article
Denisova, K.O., Il’in, A.A., Rumyantsev, R.N. et al. Acid-Base Properties of Cobalt Ferrite Surface Examined by Different Physicoсhemical Methods. Theor Found Chem Eng 54, 1306–1313 (2020). https://doi.org/10.1134/S0040579520050309
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0040579520050309