Abstract
Cyclic thermal analysis is used to study the effect of overheating ΔT+ of a melt on the presolidification supercooling \(\Delta T_{{\text{L}}}^{ - }\) of gallium–indium alloys. The existence of deep supercooling \(\Delta T_{{\text{L}}}^{ - }\) with respect to the liquidus is found for hypoeutectic alloys, whereas, for hypereutectic alloys, the supercooling is found only with respect to solidus TE (eutectic transformation line). The activities and the coefficients of activity of constituents are calculated at the onset of quasi-equilibrium and nonequilibrium explosive solidification. When the composition approaches the eutectic one, the activity of both constituents is found to decrease.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
The alloys of low-melting metals, such as gallium and indium, are widely used in various fields of industry, in particular, as cold solders, dispersion pasts in performing repair works of cast glass and ceramic blanks, thermal lubricants in stomatology [1–4], and a heat-conducting layer between cooled and heat-removing surfaces (thermal interface) [5]. Gallium, indium, and their eutectic alloys are also used in nuclear industry as a liquid-metal coolant in breeder reactors [6].
The final physicomechanical properties of an alloy and the formed microstructure are known to depend on the thermal prehistory of the melt and conditions of their solidification [7–9]. The Ga–In phase diagram is classified among the eutectic diagrams with a terminal solubility [10]. The indium solubility in gallium is low, ~0.3 mol %, (gallium-based α solid solution), and the solubility of gallium in indium is ~2.2 mol % (indium-based β solid solution) at a eutectic temperature TE = 288.3 K. The solidus at the indium corner of the diagram exhibits the clear retrograde behavior, and at ~325 K, the gallium solubility in indium increases to 3.1 mol %.
The present study is aimed at the investigation of the effect of the melt overheating on the presolidification supercooling with respect to the liquidus and solidus temperatures of the Ga–In system, construction of nonequilibrium phase diagram with indicated metastable regions with respect to the liquidus and solidus, and calculation of the activities of gallium and indium at the onset of solidification.
EXPERIMENTAL
For the investigation, we use the following samples: Ga (I), Ga + 5.3 mol % In (II), Ga + 10.1 mol % In (III), Ga + 14.2 mol % In (IV, eutectic), Ga + 20.0 mol % In (V), Ga + 30.1 mol % In (VI), Ga + 40.1 mol % In (VII), Ga + 50.2 mol % In (VIII), Ga + 60.1 mol % In (IX), Ga + 70.3 mol % In (X), Ga + 80.0 mol % In (XI), Ga + 90.2 mol % In (XII), Ga + 98.1 mol % In (XIII), and In (XIV).
The alloys were prepared using special purity grade metals. The binary Ga–In alloys were prepared by melting of the constituents taken in certain proportions; they were heated in an alundum crucible to a temperature of 480 K (i.e., above the melting temperatures of gallium (305 K) and indium (429 K)) and mixed to form the homogeneous state. Subsequently, the cyclic thermal analysis (CTA) was performed. The essence of CTA consists in the fact that the lower temperature always remains unchanged (250 K), whereas the top temperature decreases or increases by 1–2 K with respect to the liquidus temperature of the preceding cycle. A gradient-free resistance furnace was located in a cooling chamber with a temperature of 239 K. The temperature was measured by chromel–alumel thermocouple using a UT325 digital thermometer; heating and cooling curves were recorded automatically using Microsoft Office Excel software. The measurement error was 0.2 K. The heating and cooling rates were within a range of 6–7 K/s. Two samples of each composition were studied; for the reliability of data, by 10 thermal cycles were recorded. The thermal curves were processed using a procedure available in [11, 12].
RESULTS AND DISCUSSION
The effect of the melt overheating on the solidification kinetics of pure gallium and indium and Ga–In eutectic alloy was studied earlier; the results are described in [13–15]. The reliability of the results is confirmed by near coincidence of the melting, liquidus, and solidus temperatures with the literature data [10].
An analysis of the thermograms of both pure metals and Ga–In alloys allowed us to find that the melt overheating affects the presolidification supercooling \(\Delta T_{{\text{L}}}^{ - }\) with respect to the melting temperature (\(\Delta T_{{\text{L}}}^{ - }\) = TL – Tmin, where TL is the melting temperature of pure substance or the liquidus temperature of alloy and Tmin is the minimum temperature in the supercooling range) and the supercooling with respect to the liquidus \(\Delta T_{{\text{L}}}^{ - }\) and solidus (eutectic temperature TE) \(\Delta T_{{\text{E}}}^{ - }\) = TE – Tmin temperatures.
Let us consider the solidification of samples of different Ga–In alloys. For gallium (sample I), the existence of two kinds of solidification is found; these are the quasi-equilibrium and nonequilibrium explosive solidification (QES and NES, respectively) processes. The dependence of presolidification supercooling \(\Delta T_{{\text{L}}}^{ - }\) on the melt overheating ΔT+ (ΔT+ = T+ – TL, where T+ is the temperature, to which the melt is heated) exhibits the monotonous ascending behavior [13]. The critical overheating was determined to be \(\Delta T_{{\text{c}}}^{ + }\) ≈ 20 K, at which gallium solidifies with the maximum supercooling \(\Delta T_{{\text{L}}}^{ - }\) ≈ 34 K, and this value remains unchanged during subsequent overheating to 100 K. During cooling of gallium from temperatures below \(\Delta T_{{\text{c}}}^{ + }\), the supercooling \(\Delta T_{{\text{L}}}^{ - }\) varies in accordance with ΔT+ [13], whereas the solidification of gallium is equilibrium only at very low overheating (~0.5 K, Fig. 1, curve 3).
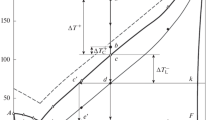
Dependences of the supercoolings ΔT+ and \(\Delta T_{{\text{E}}}^{ - }\) on the overheating \(\Delta T_{{\text{L}}}^{ - }\) of indium (curve 1, \(\Delta T_{{\text{L}}}^{ - }\) ≈ 2 K), Ga–14.2 mol % In alloy (eutectic, curve 2, \(\Delta T_{{\text{E}}}^{ - }\) ≈ 26 K), gallium (curve 3, \(\Delta T_{{\text{L}}}^{ - }\) ≈ 34 K), Ga–10.1 mol % In alloy (curve 4, \(\Delta T_{{\text{L}}}^{ - }\) ≤ 35 K), and Ga–10.1 mol % In alloy, (curve 5, \(\Delta T_{{\text{E}}}^{ - }\) ≈ 38 K).
In contrast to gallium, indium (sample XIV) solidifies with a low supercooling \(\Delta T_{{\text{L}}}^{ - }\) ≈ 1.5–2 K at any overheating to 100 K, i.e., the QES mechanism is realized [14].
In the case of eutectic Ga–In alloy, like in the case of gallium, \(\Delta T_{{\text{E}}}^{ - }\) increases as the overheating ΔT+ increases; the dependence \(\Delta T_{{\text{E}}}^{ - }\) = f(ΔT+) exhibits the monotonous ascending behavior [15] to the critical overheating \(\Delta T_{{\text{c}}}^{ + }\) ≈ 25 K (Fig. 2, curves 1–4). During further overheating to 100 K, the supercooling remains unchanged (\(\Delta T_{{\text{E}}}^{ - }\) ≈ 26 K; Fig. 2, curves 4–6).
The performed studies arouse the interest to the appearance of the dependence of supercooling on the melt overheating with respect to the liquidus (\(\Delta T_{{\text{L}}}^{ - }\)) and solidus (\(\Delta T_{{\text{E}}}^{ - }\)) for the hypo- and hypereutectic Ga–In alloys.
The analysis of thermograms (experimentally measured curves plotted on coordinates temperature T–time τ) allowed us to determine a number of regularities found upon solidification of Ga–In alloys, which are described below.
Alloys II and III
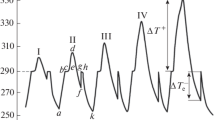
These alloys are hypoeutectic. According to the Ga–In phase diagram [10], the solidification of alloys II and III with respect to the liquidus temperature starts from the precipitation of almost pure gallium (with ~0.3 mol % In) and finishes at the solidus temperature by the formation of eutectic at TE = 288.3 K, i.e., the phase composition of solid alloys is α + E(α + β). The analysis of thermograms of alloys II and III allows us to find that, at any overheating ΔT+ of the melt, the solidification of all hypoeutectic alloys is nonequilibrium explosive. Figure 3 shows the heating–cooling curves of hypoeutectic alloy III (Ga + 10.1 mol % In, TL ≈ 295 K), which exhibit neither inflection points nor plateau; the explosive solidification starts below the solidus temperature. Taking into account the fact that, for gallium, \(\Delta T_{{\text{L}}}^{ - }\) = 34 K [13] and, for the eutectic, \(\Delta T_{{\text{E}}}^{ - }\) = 26 K [15], it is possible to assume that the solidification occurs with the deep presolidification supercooling \(\Delta T_{{\text{L}}}^{ - }\) with respect to the liquidus temperature. According to the heating–cooling curves given in Fig. 3, at overheating ΔT+ ≈ 2, 10, 15, and 25 K (curves 1, 2, 3, and 4, respectively), the supercooling was found to be \(\Delta T_{{\text{L}}}^{ - }\) ≈ 3, 15, 35, and 35 K, respectively. During thermal cycling of alloy II, the following values of supercooling were determined: \(\Delta T_{{\text{L}}}^{ - }\) ≈ 4, 17, 37, and 37 K at overheatings ΔT+ ≈ 2, 10, 15, and 25 K. Thus, at overheatings ΔT+ ≤ 15 K, the dependence of \(\Delta T_{{\text{L}}}^{ - }\) on ΔT+ for all hypoeutectic alloys exhibits the monotonic ascending behavior (like for Ga and eutectic alloy), and the maximum supercooling ~35 K is unchanged in accordance with subsequent overheating to 100 K.
Alloys V–XII
According to [10], the solidification of hypereutectic alloys V–XII with respect to the liquidus temperature starts from the precipitation of indium-based β solid solution and finishes by the formation of eutectic at the solidus temperature TE = 288.3 K; i.e., in the solid state, the phase composition of the alloys V–XII is β + E(α + β). During solidification of alloys V–XII, the presolidification supercoolings \(\Delta T_{{\text{L}}}^{ - }\) were found to be absent at any overheating of the melt, and the supercooling \(\Delta T_{{\text{E}}}^{ - }\) with respect to the eutectic temperature TE were found to exist. The value of \(\Delta T_{{\text{E}}}^{ - }\) for all alloys was ~38 K, and the solidification is nonequilibrium explosive.
Alloy XIII
The solidification of the alloy is quasi-equilibrium with respect to all temperatures of structural transformations.
The Ga–In phase diagram (Fig. 4) shows the drawn minimum temperatures Tmin of the onset of NES of the alloys over the whole composition range with respect to the solidus and liquidus for the hypoeutectic alloys and with respect to the solidus for the hypereutectic alloys. The boundaries of this temperatures region (shown by gray) correspond to the metastable state of all alloys of the system.
The metastable Ga–In phase diagram was constructed in [15] for slow cooling (the cooling rate is not given); the eutectic temperature and indium content were determined to be 244.7 K and 6.5 at %, respectively. Under our experimental conditions, \({\text{the}}\) solidification temperature of supercooled eutectic is 266.3 K.
The analysis of solidification processes of the Ga–In alloys and construction of the nonequilibrium phase diagram of the system arouse the interest to the determination of the activity and activity coefficients of components participating in the QES and NES processes. For the eutectic alloy, such calculations were performed earlier and described in [16].
Alloys II and III
Using the Shreder equation [17] for hypoeutectic alloys II and III, the activities of gallium (\(a_{\alpha }^{{{\text{Ga}}}}\)) and indium (\(a_{\alpha }^{{{\text{In}}}}\)), which comprise the Ga-based α solid solution and participate in the solidification at a moment of QES, namely, at the liquidus temperature TL are calculated by the equations:
where \(\Delta H_{{\text{L}}}^{{{\text{Ga}}}}\) and \(\Delta H_{{\text{L}}}^{{{\text{In}}}}\) are the enthalpies of melting of gallium (5.59 kJ/mol) and indium (3.27 kJ/mol), respectively [18]; \(T_{{\text{L}}}^{{{\text{Ga}}}}\) and \(T_{{\text{L}}}^{{{\text{In}}}}\) are the melting temperatures of gallium (305 K) and indium (429 K), respectively; and TL is the liquidus temperature (for alloy II, TL ≈ 300 K and, for alloy III, TL ≈ 295 K).
The activity coefficients of gallium (\(g_{\alpha }^{{{\text{Ga}}}}\)) and indium (\(g_{\alpha }^{{{\text{In}}}}\)) were determined as the ratio of the activities to the gallium (\(X_{\alpha }^{{{\text{Ga}}}}\)) and indium (\(X_{\alpha }^{{{\text{In}}}}\)) concentrations in the hypoeutectic alloy:
The metastable region of the Ga–In phase diagram is of particular interest for the analysis of the activities of components and activity coefficients. Taking into account the fact that the eutectic alloys solidify upon deep presolidification supercooling \(\Delta T_{{\text{L}}}^{ - }\) with respect to the liquidus, the activities were calculated at temperatures of the onset of NES, i.e., at temperatures Tmin (Tmin = TL – \(\Delta T_{{\text{L}}}^{ - }\)). Since the Tmin temperature of hypoeutectic alloys and gallium depends on the melt overheating, the maximum supercoolings for the alloys were taken for the calculations; these are 37 K for alloy II, 35 K for alloy III, 34 K for gallium, and 2 K for indium. Denote the activities upon NES by \(b_{\alpha }^{{{\text{Ga}}}}\) and \(b_{\alpha }^{{{\text{In}}}}\) and activity coefficients by \(\gamma _{\alpha }^{{{\text{Ga}}}}\) and \(\gamma _{\alpha }^{{{\text{In}}}}\). For calculation, we adapt Eqs. (1)–(3) to NES,
where \(T_{{\min }}^{{{\text{Ga}}}}\), \(T_{{\min }}^{{{\text{In}}}}\), and \(T_{{\min }}^{{\text{L}}}\) are the minimum temperatures in the supercooling region for gallium, indium, and respect to the liquidus temperature TL of corresponding alloy, respectively.
Alloys V–XIII
The experiments showed that the solidification of β solid solution in all hypereutectic alloys V–XIII with respect to the temperature TL is quasi-equilibrium. We denote the activities by \(a_{\beta }^{{{\text{Ga}}}}\) and \(a_{\beta }^{{{\text{In}}}}\) and activity coefficients \(g_{\beta }^{{{\text{Ga}}}}\) and \(g_{\beta }^{{{\text{In}}}}\) and determine them by the expressions:
where \(X_{\beta }^{{{\text{Ga}}}}\) and \(X_{\beta }^{{{\text{In}}}}\) are the gallium and indium concentrations in the hypereutectic alloy, respectively.
Table 1 and 2 give all values of the activity and activity coefficients determined by Eqs. (4)–(9) (method is available in [19]).
As follows from data given \({\text{in}}\) Table 1, the gallium activity is higher than that of indium in the case of both QES and NES during solidification of the α solid solution in hypoeutectic alloys with respect to the liquidus temperature. As the concentration of the second component (indium) increases, the gallium and indium activities decrease in the case of both QES and NES. For the hypereutectic alloys, the solidification of which is only quasi-equilibrium, the gallium activity also is higher than that of indium; as the indium concentration increases, the activities of both components increase, and the activity coefficient of gallium in all alloys increases as the content of the second component increases, whereas, in this case, the activity coefficient of indium decreases.
CONCLUSIONS
(1) Thermographic studies of the effect of overheating of the melt on the presolidification supercooling of Ga–In alloys were performed. Using the supercooling of the alloy, a nonequilibrium Ga–In phase diagram was constructed.
(2) Using the procedure developed by V. D. Aleksandrov with colleagues, we determined the activities and activity coefficients of the components of the Ga–In system during quasi-equilibrium and nonequilibrium explosive (only for hypoeutectic alloys) solidification. The activity of gallium in Ga–In alloys was found to be higher than that of indium.
(3) Solid gallium has an orthorhombic crystal lattice, the sites of which are occupied with Ga2 molecules with a covalent bond, whereas indium has a tetragonal single-atom lattice.
(4) The high gallium activity can be explained by the fact that its solidification is two-stage and includes the formation of Ga2 molecules and, after that, crystal lattice formation. The solidification of indium is a single-stage process (joining of indium atoms to form a crystal lattice).
REFERENCES
L. I. Glushkova and S. G. Konnikov, “Interaction of components in a gallium-based solder paste,” in Transactions of Leningrad Polytechnic Institute. Metal Forming and Welding (Mashinostroenie, Leningrad, 1969), No. 308, pp. 205–208.
V. S. Kazakov, “Development of gallium paste solders for low-temperature soldering of copper and titanium alloys with ceramics,” Extended Abstract Cand. Sci. Dissertation, Krasnoyarsk, 2007.
A. I. Kablov and V. I. Krayukhin, “Composition of materials for the preparation of strengthening pastes,” RF Patent 2345865, 2009.
S. P. Yatsenko, in Gallium. Interaction with Metals (Nauka, Moscow, 1974), pp. 26–28.
S. Yu. Mel’chakov, L. F. Yamshchikov, V. A. Volkovich, A. G. Osipenko, V. A. Ivanov, and S. P. Raspopin, “Thermodynamic characteristics of praseodymium alloys with eutectic gallium–indium melts,” Izv. Vyssh. Uchebn. Zaved., Tsvetn. Metall., No. 6, pp. 24–28 (2014).
E. V. Sulim, N. G. Bogdanovich, O. V. Starkov, et al., Vopr. Atomn. Nauki Tekhn., Ser. Fiz. Yadern. Reakt., No. 4, 54–63 (2008).
V. I. Danilov, Structure and Solidification of Liquids (Izd. AN Ukr. SSR, Kiev, 1956).
B. I. Kudyarov, Kinetics of Formation of Crystals from Liquid (Nauka, Novosibirsk, 1970).
V. D. Aleksandrov and S. A. Frolova, “Effect of time–temperature treatment of liquid on the solidification of Sn–Bi alloys,” Rasplavy, No. 3, 14–21 (2003).
Phase Diagrams of Binary Metallic Systems: A Handbook, Ed. by N. P. Lyakishev (Mashinostroenie, Moscow, 1997), Vol. 2, pp. 601–603.
Ya. Shestak, Theory of Thermal Analysis (Mir, Moscow, 1987).
GOST P53293–2009. Fire Safety of Substances and Materials. Identification of Substances and Materials by Thermal Analysis Methods (TA, DTA, DSC, TGA) (Standartinform, Moscow, 2019).
V. D. Aleksandrov and S. A. Frolova, “Effect of the overheating of the gallium melt on its supercooling during solidification,” Russ. Metall. (Metally), No. 1, 14–19 (2014).
V. D. Aleksandrov and S. A. Frolova, “Study of supercoolings during solidification of In–Bi alloys,” in Starodub Readings-2003: Transactions of International Conference (Dnepropetrovsk, 2003), Vol. 1, pp. 164–169.
T. J. Anderson and I. Ansara, “The Ga–In (gallium–indium) system,” J. Phase Equil. 12 (1), 64–72 (1991).
V. D. Aleksandrov and S. A. Frolova, “Solidification of the eutectic alloy in the gallium–indium system,” Russ. Metall. (Metally), No. 3, 308–312 (2021).
V. M. Glazov, Foundations of Physical Chemistry (Vyssh. Shkola, Moscow, 1981).
Properties of Elements: A Handbook, Ed. by M. E. Drits (Metallurgiya, Moscow, 1985).
V. D. Aleksandrov, A. P. Zozulya, and S. A. Frolova, “Construction of nonequilibrium gallium–tin phase diagram and its analysis,” Izv. Vyshs. Uchebn. Zaved., Tsvetn. Metall., No. 1, 39–47 (2020).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The author declares that she has no conflicts of interest.
Additional information
Translated by N. Kolchugina
Rights and permissions
About this article
Cite this article
Frolova, S.A. Solidification of Gallium–Indium Alloys. Russ. Metall. 2022, 1059–1064 (2022). https://doi.org/10.1134/S0036029522090075
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0036029522090075