Abstract—The cathodic reduction of Dy(III) ions to the metal in molten cesium chloride in the temperature range from 963 to 1063 K at inert molybdenum and active gallium electrodes in an inert gas atmosphere was studied by cyclic and square-wave voltammetry and open-circuit potentiometry. All procedures on assembling an experimental cell were carried out in a dry box excluding oxygen and moisture impurities in the reagents used. The cyclic voltammograms of the CsCl–DyCl3 molten mixture recorded at the inert molybdenum electrode at different scan rates and at 987 K were characterized by one reduction current peak and the corresponding oxidation current peak. This fact indicates that the cathodic reduction of Dy(III) ions to the metal proceeds in one step possibly involving three electrons. The square-wave voltammogram exhibits an asymmetric cathodic curve of the Gaussian shape with one distinct current peak. The number of electrons involved in the electrochemical reduction and calculated from the width of the half-peak of the cathodic curve was close to three (n = 2.89 ± 0.05). The mechanism of the cathodic deposition of metallic dysprosium at the inert Mo electrode was determined. The electrode reaction is shown to proceed irreversibly in one step and is controlled by the charge transfer rate. The diffusion coefficients of complex ions [DyCl6]3– were calculated at different temperatures, and the activation energy of diffusion was determined. The temperature dependence of the diffusion coefficients of dysprosium(III) ions obeys the Arrhenius law and is described by the equation \(\log D = - 3.15 - {{2010} \mathord{\left/ {\vphantom {{2010} T}} \right. \kern-0em} T} \pm 0.02\). The diffusion activation energy is calculated to be –38.7 kJ/mol. An experimental temperature dependence of the apparent standard potential of the Dy(III)/Dy couple is found. It is described by the linear equation \(E_{{{{{\text{Dy}}\left( {{\text{III}}} \right)} \mathord{\left/ {\vphantom {{{\text{Dy}}\left( {{\text{III}}} \right)} {{\text{Dy}}}}} \right. \kern-0em} {{\text{Dy}}}}}}^{{\text{*}}} = \) \( - \left( {3.921 \pm 0.007} \right) + \left( {6.8 \pm 0.1} \right) \times {{10}^{{ - 4}}}T \pm 0.005\,\,{\text{V}}.\) The main thermodynamic characteristics of dysprosium trichloride were calculated. The electrochemical deposition of dysprosium at the active gallium electrode was found to be related to alloy formation and occurs with depolarization. The equilibrium potentials of the Dy–Ga alloy are measured. The temperature dependence of the apparent standard potential of the alloy was determined and described by the equation \(E_{{{\text{Dy}}\left( {{\text{Ga}}} \right)}}^{{{\text{**}}}} = \) \( - \left( {3.069 \pm 0.005} \right) + \left( {3.2 \pm 0.2} \right) \times {{10}^{{ - 4}}}T \pm 0.006\,\,{\text{V}}{\text{.}}\) A scheme was proposed for the formation of Dy–Ga intermetallic compounds. The activity coefficients and the partial excess Gibbs free energy change for dysprosium in liquid gallium are calculated.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
High-purity rare earth metals (REMs) are applied in diverse industrial areas: radioelectronics, instrument-making and machine-building, metallurgy, chemical and ceramic industries, and so on. In nature, REMs are divided into two subgroups: the cerium subgroup including light lanthanides from lanthanum to gadolinium and the yttrium subgroup consisting of yttrium and other lanthanides. Pure metals or their alloys cannot be obtained from aqueous solutions because of a high reactivity of lanthanide compounds and, therefore, they are prepared by the metallothermy and electrolysis of molten salt media. For the development and improvement of technological procedures of the electrolytic synthesis and refining of REMs, comprehensive data on the physicochemical characteristics of molten salts containing rare earth elements are needed; in particular, one has to know the electrochemical and thermodynamic properties [1–3].
Molten alkaline metal chlorides are good reaction media for the selective dissolution or deposition of REMs. They are ionic liquids with the long-range Coulomb interaction of particles. The dissolution of multicharge cations in the molten chloride media is accompanied by the formation of complex ions of various configuration, for example, tetrahedral or octahedral ions [4, 5].
The literature data on the electrochemical properties of the dysprosium compounds in the molten salt mixtures are contradictory. The electronic absorption spectra of such rare earth metal ions as Sm(III), Dy(III), Ho(III), and Er(III) were studied in the row from LiCl to CsCl [6]. It was shown that the complex \([{\text{LnCl}}]_{6}^{{3 - }}\) ions were formed in the melts. The electrochemical behavior of Dy(III) ions in the halide electrolytes at the inert and active electrodes was studied. The cathodic reactions at the active electrodes were found to occur with depolarization to form intermetallic compounds of diverse composition [7‒13]. The electroreduction of Dy(III) ions in the 3LiCl–2KCl melt at the inert tungsten and active aluminum electrodes was studied [14]. For instance, at the inert electrode the process proceeds in two consecutive steps: Dy(III) + ē → Dy(II) and Dy(II) + 2ē → Dy, whereas at the active electrode one step with depolarization occurs. The electrochemical behavior and efficiency of dysprosium extraction from 3LiCl–2KCl solutions containing DyCl3 and GdCl3 were studied by stationary and nonstationary electrochemical methods. It was found that at the W electrode Dy(III) ions were reduced to the metallic state in two consecutive steps [15]. The mechanisms of the reduction of Sm(III) and Dy(III) ions at the tungsten and binary Bi–Pb electrodes were studied [16]. The reduction of Dy(III) and Sm(III) at the inert electrode was found to proceed in one step for dysprosium and in two steps for samarium. As proved later [17–19], the cathodic reduction of Dy(III) ions to the metal at the inert electrodes proceeded irreversibly in one step involving three electrons.
The purpose of this work was to determine the mechanisms of the cathodic reduction of Dy(III) ions to the metal on inert Mo and active Ga electrodes and to calculate the thermodynamic characteristics of the dysprosium compounds in molten CsCl.
EXPERIMENTAL
Cesium chloride (reagent grade, 99.9%, Nevskii khimik, Russia), metallic gallium (99.9999%, Pikalevskii glinozemnyi zavod), and all other reagents were used as received. All procedures on assembling the cell and reagent loading were carried out in SPEKS GB02 glove box (oxygen content was <1 ppm, moisture content was <1 ppm).
Experiments were carried out in a three-electrode quartz cell placed in a glassy carbon crucible in a dry argon atmosphere in the temperature range from 963 to 1063 K. A glassy carbon rod with a diameter of 3 mm served as the counter electrode. A molybdenum wire with a diameter of 1 mm and a quartz microcrucible with liquid gallium were used as the working electrodes. The surface area of the solid electrode was calculated from the immersion depth, and that of the liquid electrode was calculated from the microcrucible diameter. The measurements were carried out versus standard chloride reference electrode.
The electrochemical behavior of Dy(III) ions in molten cesium chloride was studied by cyclic and square-wave voltammetries and open-circuit potentiometry. The measurements were carried out on an AUTOLAB PGSTAT302N potentiostat/galvanostat equipped with the NOVA 1.11 software.
Samples of dysprosium-containing solutions were analyzed by the ICP-MS method on an OPTIMA 4300 DV (Perkin Elmer) inductively coupled plasma optical emission spectrometer.
RESULTS AND DISCUSSION
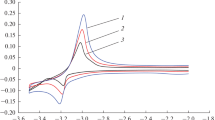
The first part of the work is associated with studying the mechanism of the cathodic reduction of Dy(III) ions to the metal. These studies are necessary to search for optimum conditions of the electrolytic preparation of high-purity metallic dysprosium in molten salts. The stable oxidation states of the dysprosium compounds were determined by different electrochemical methods. The cyclic voltammograms of the molten CsCl–DyCl3 mixture obtained at the inert molybdenum electrode at different scan rates at 987 K were shown in Fig. 1. They are characterized by one cathodic reduction peak and one anodic oxidation peak. This indicates that the cathodic reduction of Dy(III) ions to the metal proceeds in one step possibly involving three electrons. An increase of the scan rate resulted in the shift of the potential of the cathodic current peak to the negative side. At the same time, the cathodic current peaks were directly proportional to the square root of the scan rate.
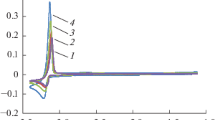
Square-wave voltammetry was used to determine the number of electrons of the electrode reaction. The asymmetric cathodic curve of the Gaussian shape was obtained in the CsCl–DyCl3 solution at 695 K (Fig. 2). Inset in Fig. 2 shows an interrelation between the cathodic current peak and square root of the frequency. This dependence is linear and passes through the origin, which makes it possible to use Eq. (1) to calculate the number of electrons in the electrode reaction in the frequency range from 10 to 18 Hz
where W1/2 is the half-peak width (V), R is the universal gas constant (J mol–1 K–1), T is the absolute temperature (K), n is the number of the exchange electrones, and F is Faraday’s constant (C/mol).
The calculated number of the exchange electrones of the cathodic reaction was close to three (n = 2.89 ± 0.05).
Based on an analysis of the obtained results and according to the theory of cyclic voltammetry, we can conclude that the cathodic reduction of Dy(III) ions to the metal at the inert electrode is irreversible, proceeds in one step, is controlled by the charge-transfer rate [20], and in dilute solutions is described by the equation
The diffusion coefficients of the [DyCl6]3– complex ions in the molten salt were determined using cyclic voltammetry by Eq. (3) [20], which is valid for the irreversible system at the scan rates used.
where Ip is the peak current (A), S is the surface area of the working electrode (cm2), C0 is the concentration of dysprosium ions (mol/cm3), D is the diffusion coefficient (cm2/s), and ν is the scan rate (V/s).
The temperature dependence of the diffusion coefficient obeys the Arrhenius law and was described by the following equation:
The determined diffusion coefficients were used to calculate the activation energy of the diffusion.
where ΔEA is the activation energy of the diffusion (kJ/mol), D0 is the preexponential term (cm2/s), and Δ is the experimental error.
The diffusion coefficients and activation energy of dysprosium(III) ions in molten cesium chloride at different temperatures are given in Table 1.
The equilibrium electrode potential of the Dy(III)/Dy couple was determined at different temperatures by open-circuit potentiometry. For this purpose, the inert molybdenum electrode was polarized with a current of 25–75 mA for 20–35 s and then the potential–time dependence was recorded. The typical chronopotentiogram of the CsCl–DyCl3 melt is shown in Fig. 3. The Nernst equation was applied to calculate the apparent standard potential
where
The experimental dependence obtained using the Origin Pro software (version 7.5) in the temperature range from 963 to 1063 K was described by the linear equation
The partial excess Gibbs free energy change upon the formation of dysprosium trichloride from the elements was calculated by Eq. (9). The resulting dependence is described by Eq. (10).
The results are summarized in Table 2.
The chronopotentiograms of the CsCl–DyCl3 melt recorded at the molybdenum and gallium electrodes at 965 K are shown in Fig. 4. The potential–time dependence after the short polarization of the Ga electrode is shown by line 1 in Fig. 4. The plateau at a potential of –2.58 V corresponds to the equilibrium potential of the cathodic product, i.e., Dy–Ga alloy. In the case of the cathodic polarization of the inert Mo electrode, the chronopotentiogram exhibits the distinct plateau corresponding to the equilibrium potential of the Dy(III)/Dy couple (Fig. 4, line 2). The studies showed that the depolarization during the discharge of dysprosium ions at the gallium electrode ranged from 0.50 to 0.55 V. This is related to the alloy formation when the active cathodes are used in the electrolysis of the melt.
The equilibrium standard potential of the alloy was determined by open-circuit potentiometry (Fig. 4, line 1). The Nernst equation was applied to calculate the apparent standard potential
where EMe(alloy) is the equilibrium potential of the alloy (V), \(E_{{{\text{Me}}\left( {{\text{alloy}}} \right)}}^{{{\text{*}}{\kern 1pt} {\text{*}}}}\) is the apparent standard potential of the alloy (V), n is the number of exchange electrones, CMe(III) is the concentration of metal ions in the solvent (molar fractions), and xMe(alloy) is the concentration of metal atoms in the alloy (molar fractions).
The change in the apparent standard potentials of the alloys at different temperatures was determined using the Origin Pro 7.5 software. The obtained dependence was approximated by the linear equation
The activity coefficients of dysprosium in the liquid gallium alloy were calculated by Eq. (13) [21]. The temperature dependence of the activity coefficient was described by the following equation [14]:
The low activity coefficients indicate a strong interaction between dysprosium and liquid gallium. An increase of the temperature resulted in system ordering [22].
The excess partial Gibbs energy for dysprosium in liquid gallium was calculated by Eq. (16) [21]
A scheme for the formation of the Dy–Ga intermetallic compounds can be represented as follows [23]:
The thermodynamic characteristics of the liquid Dy–Ga alloy are given in Table 3.
CONCLUSIONS
The cathodic reduction of Dy(III) ions to the metal in molten CsCl in the temperature range from 963 to 1063 K on molybdenum and gallium electrodes was studied by cyclic and square-wave voltammetry and open-circuit potentiometry. The mechanism of the cathodic deposition of metallic dysprosium at the inert Mo electrode was determined. The electrode reaction was shown to proceed irreversibly in one step and be controlled by the charge-transfer rate. The temperature dependence of the diffusion coefficients of dysprosium(III) ions was determined to obey the Arrhenius law. The activation energy of the diffusion process was calculated. The temperature dependence of the apparent standard potential for the Dy(III)/Dy couple was determined. The main thermodynamic characteristics of dysprosium trichloride were calculated.
The electrochemical deposition of dysprosium at the active Ga electrode was found to be related to the alloy formation and to occur with depolarization. The equilibrium potentials of the Dy–Ga alloy were measured, and the temperature dependence of the apparent standard potential of the alloy was determined. A scheme was proposed for the formation of Dy–Ga intermetallic compounds. The activity coefficients and the partial excess Gibbs free energy change for dysprosium in liquid gallium were calculated.
REFERENCES
B. Yezhovska-Trshebiatowska, S. Kopacz, and T. Mikulski, Rare Elements (Polish Academy of Sciences, Warsaw, 1976).
A. I. Mikhailichenko, Rare Metals (Metallurgiya, Moscow, 1987).
A. N. Zelikman and B. G. Korshunov, Metallurgy of Rare Metals (Metallurgiya, Moscow, 1991).
A. N. Baraboshkin, Electrocrystallization of Metals from Molten Salts (Nauka, Moscow, 1976).
M. V. Smirnov, Electrode Potentials in Molten Chlorides (Nauka, Moscow, 1973).
T. Fujii, T. Nagai, A. Uehara, and H. Yamana, “Electronic absorption spectra of lanthanides in a molten chloride. III. Absorption characteristics of trivalent samarium, dysprosium, holmium, and erbium in various molten chlorides,” J. Alloys Compd. 441, L10–L13 (2007).
Ya. Kouji, S. Kobayashi, T. Nohira, and R. Hagiwara, “Electrochemical formation of Dy–Ni alloys in molten NaCl–KCl–DyCl3,” Electrochim. Acta 106, 293–300 (2013).
Y. Yang, M. Zhang, W. Han, P. Sun, B. Liu, H. Jiang, T. Jiang, S. Peng, M. Li, K. Ye, and Y. Yan, “Selective electrodeposition of dysprosium in LiCl–KCl–GdCl3–DyCl3 melts at magnesium electrodes: Application to separation of nuclear wastes,” Electrochim. Acta 118, 150–156 (2014).
L.-L. Su, K. Liu K., Y.-L. Liu, L. Wang, L.-Y. Yuan, L. Wang, Z.-J. Li, X.-L. Zhao, Z.-F. Chai, and W.‑Q. Shi, “Electrochemical behaviors of Dy(III) and its coreduction with Al(III) in molten LiCl– KCl salts,” Electrochim. Acta 147, 87–95 (2014).
H. Konishi, T. Nohira, and Y. Yto, “Kinetics of DyNi2 film growth by electrochemical implantation,” Electrochim. Acta 48, 563–568 (2003).
K. Yasuda, S. Kobayashi, T. Nohira, and R. Hagiwara, “Electrochemical formation of Dy–Ni alloys in molten NaCl–KCl–DyCl3,” Electrochim. Acta 106, 293–300 (2013).
S. Kobayashi, T. Nohira, K. Kobayashi, K. Yasuda, R. Hagiwara, T. Oishi, and H. Konishi, “Electrochemical formation of Dy–Ni alloys in molten LiF–CaF2–DyF3,” J. Electrochem. Soc. 159, E193–E197 (2012).
A. Saïla, M. Gibilaro, L. Massot, P. Chamelot, P. Taxil, and A. M. Affoune, “Electrochemical behaviour of dysprosium(III) in LiF–CaF2 on Mo, Ni and Cu electrodes,” J. Electroanal. Chem. 642, 150–156 (2010).
Y. Castrillejo, M. R. Bermejo, A. I. Barrado, R. Pardo, E. Barrado, and A. M. Martinez, “Electrochemical behavior of dysprosium in the eutectic LiCl–KCl at W and Al electrodes,” Electrochim. Acta 50, 2047–2057 (2005).
Y. Yang, M. Zgang, W. Han, P. Sun, B. Liu, H. Jiang, T. Jiang, S. Peng, M. Li, K. Ye, and Y. Yan, “Selective electrodeposition of dysprosium in LiCl–KCl–GdCl3–DyCl3 melts at magnesium electrodes: application to separation of nuclear wastes,” Electrochim. Acta 118, 150–156 (2014).
Z. Li, D. Nang, S. Meng, L. Gu, Y. Dai, and Z. Liu, “Electrolytic separation of Dy from Sm in molten LiCl–KCl using Pb–Bi eutectic alloy cathode,” Separation and Purification Technology 276, 119045 (2021).
A. Novoselova, V. Smolenski, and V. A. Volkovich, “Electrochemical behavior of dysprosium in fused LiCl–KCl eutectic at solid inert Mo and liquid active Ga electrodes,” J. Electrochem. Soc. 167, 112510 (2020).
V. Smolenski and A. Novoselova, “Electrochemical separation of uranium from dysprosium in molten salt/liquid metal extraction system,” J. Electrochem. Soc. 168, 062505 (2021).
A. Novoselova, V. Smolenski, V. A. Volkovich, A. Ryzhov, Y. Yan, Y. Xue, and F. Ma, “Speciation of dysprosium in molten LiCl–KCl–CsCl eutectic: an electrochemistry and spectroscopy study,” J. Electroanal. Chem. 904, 115955 (2022).
A. J. Bard and L. R. Faulkner, Electrochemical Methods, Fundamentals, and Applications (Wiley, New York, 1980).
V. A. Lebedev, Selectivity of Liquid Metallic Electrodes in Molten Halides (Metallurgiya, Chelyabinsk, 1993).
G. Kaptay, “On the tendency of solutions to tend toward ideal solutions at high temperatures,” Metall. Mater. Trans. A 43, 531–543 (2012).
HSC Chemistry 6 Software (Outotec Research Oy, Pori, Finland).
Funding
This study was supported by the Russian Foundation for Basic Research, project no. 20-03-00743.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflicts of interest.
Additional information
Translated by E. Yablonskaya
Rights and permissions
About this article
Cite this article
Novoselova, A.V., Smolenski, V.V. & Bovet, A.L. Mechanism of the Electrochemical Reduction of Dysprosium(III) Ions on Inert and Active Electrodes in Molten Cesium Chloride. Russ. Metall. 2022, 965–971 (2022). https://doi.org/10.1134/S0036029522080249
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0036029522080249