Abstract
The modern approaches to modeling for creating new nickel-based superalloys are analyzed. The computer calculation methods used in domestic and foreign practice and based on multifactor design of experiments are presented.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Currently, the active work on creating the fifth- and sixth-generation aviation gas turbine engines, which is being performed in advanced states, is accompanied by vigorous development of effective methods to significantly facilitate and shorten the time of production of new high-temperature alloys, the difficulties of manufacture of which have increased many times due to the sharp complication of alloying. The regions of optimum alloy compositions began to be located near the phase boundaries of various undesirable or harmful phases.
This problem is challenging due to the fact that the achieved complexity of alloying (primarily cast nickel superalloys) has led to the fact that their further development and optimization of the compositions of promising alloys are associated with ensuring simultaneous consideration of a significant number of the factors directly affecting their performance.
Naturally, the optimal solution to the problem of creating new high-temperature alloys at the present stage is possible with the active development of approaches based on modeling the relationship between the level and character of alloying with the thermodynamic, structural, and strength parameters of high-temperature metallic materials [1].
Similar problems arise on developing and optimizing new technological processes.
METHODOLOGY OF COMPUTER ANALYSIS OF ESTIMATING THE THERMODYNAMIC AND STRUCTURAL FACTORS OF NICKEL SUPERALLOYS
The development of gas turbine engine building for promising generations of engineering led to the need to intensify research to ensure a new temperature level of performance of nickel superalloys. In the fourth-generation engines, the maximum operating temperature of the materials of the specified purpose was 1000–1050°C for blades and 650–750°C for disks; according to experts for promising fifth- and sixth-generation GTEs, the operating temperature will be 1100–1150°C for blades and 800°C or more in the rim of the disks.
Thus, the problem of developing new alloys with a significantly higher temperature level of operability becomes challenging. One of the ways to solve this problem based on computer analysis of the structural and thermodynamic parameters of disk nickel superalloys is presented in [2].
It should be noted that, over the past period, the knowledge of the segregation phenomena during solidification and the understanding of the processes that cause the appearance of porosity when an alloy is heated above the temperature of complete dissolution of the γ' phase Tc.d became deeper, and methods were developed to perform homogenization and alignment of the alloy composition without the appearance of local melting phenomena.
The aforesaid makes it possible to impose requirements to ensure the lowest possible values of Tc.d of the γ' phase less rigid and to increase the concentration of the elements in alloys (W, Mo, Ta, Re, Ru) that improve the strength properties at high temperatures.
However, works in this direction are accompanied by the appearance of a number of constraining factors, since the increase in the performance characteristics of nickel superalloys and the associated complication of their composition led to a noticeable narrowing of the multicomponent region of optimal alloying. This circumstance is primarily due to the appearance of a high probability of the formation of TCP phases (Fig. 1), α phases based on Cr, W, Mo, Re, and Ta and an M23C6 carbide framework along grain boundaries (Fig. 2), and \(\gamma _{{{\text{eut}}}}^{'}\) (Fig. 3), which either causes the embrittlement of alloys and a decrease in their performance or leads to the fact that some of the effective and expensive elements do not participate in the hardening of materials. Therefore, the works aimed at creating methods for automated estimation of the structural and thermodynamic parameters of alloys, which determine the level of their performance, and developing the optimum compositions, primarily blade and disk nickel superalloys, for a new temperature level of operation are of particular importance.
It is necessary to pay attention to the fact that these works can be carried out successfully if there is an appropriate infrastructure, namely, the necessary equipment, specialists, experience in creating programs, and their effective implementation in new equipment facilities.
The developed principles for optimizing the alloying of nickel superalloys are based, among other things, on the methods and approaches created at PAO ODK-Saturn [1, 3].
ANALYTICAL CALCULATIONS OF PHASE DIAGRAMS
In the 1960s–1970s of the last century, the direction of calculating and predicting phase diagrams in materials science, which today has resulted in a rather powerful and popular direction, developed very actively. The problem of finding criteria for phase diagrams of different types has been solved since the time of van der Waals [4], who, working in the field of theoretical molecular physics, studied the behavior of molecules characterizing the state of material. In 1869, he discovered the forces of interaction between molecules, which were later called the van der Waals forces. In 1873, in his dissertation for Doctor of Philosophy, he developed a model, which uniformly describes the gaseous and liquid forms of matter, and derived an equation of state for gases and liquids. About this work, Maxwell said that it immediately put his name on a par with the most outstanding names in science.
The beginning of the second wave in the development of the theory of phase diagrams (PDs), on the crest of which we are now, is associated with the name of Soviet physicist B.Ya. Pines, who used the model of regular solutions for describing various types of PDs. Pines’s works stimulated the appearance of a number of studies devoted to an analysis and calculation of PDs (in the Soviet Union, D.S. Kamenetskaya, I.L. Aptekar’, A.L. Udovskii, et al. [4, 5]). Currently, Pines’s method is widely used both in our country and abroad. The model of regular solutions used by him made it possible to describe a number of types of PD, to give approximate criteria for them, to identify new previously unknown types, to consider the evolution of PD under pressure, to reveal boundary PDs, and to solve a number of problems of constructing specific DS. In the 1970s, L. Kaufman made a significant contribution to the development and practical implementation of the theoretical foundations of analytical construction of PDs. He published book [6] devoted to PD calculations; in 1977 he founded the international journal Calphad (Calculation of Phase Diagrams (CALPHAD), a method for calculating equilibrium PDs); and he was also the organizer of periodic international seminars on PD calculations. The wide development of PD calculations is due to the needs of materials science and the possibility of using computers. An increasingly wide range of specialists (in the electronic theory of metals, thermochemists, mathematicians, etc.) are involved in PD calculations.
Works on PD analysis and calculation in our country was actively carried out in IMET, TsNII CherMet, MISIS, IFTT RAS and other organizations.
Unfortunately, in the 1990s and in the first decade of the 2000s, the volume of research in this direction in Russia fell markedly, while the United States, England, France, Germany, Sweden, and other countries began to actively build multicomponent diagrams of state of the systems used in industry using computers and to create data banks, which significantly decreased the time and volume of experimental research in the development of new alloys with extreme properties.
Currently, the CALPHAD method is a generalizing name for all software products designed to predict the phase composition and thermodynamic state of alloys at various temperatures and to simulate the phase transformation dynamics under thermal and chemical actions on an alloy. It has been actively developing since 1997.
The CALPHAD method accumulates computational approaches to the estimation of the thermodynamic parameters of systems. Its peculiarity is the use of the ab initio principles of solid state physics. It is based on the available experimental information about the phase equilibria in binary, ternary, and quaternary combinations of elements and, today, multicomponent steels and alloys. The databases also include all thermodynamic information obtained during thermochemical and thermophysical studies. The set of thermodynamic properties of each phase is described by a mathematical model. The concept of the CALPHAD method consists in achieving a consistent description of the phase diagram of the entire system and in reliably predicting a set of stable phases and their thermodynamic properties in the phase diagram fields where experimental information is absent. Here, metastable states are estimated by modeling phase transformations.
When the phase equilibrium of a system is determined, researchers use the estimated Gibbs energy of each phase and special mathematical methods for calculating phase equilibrium.
Currently, there are several commercial software products for modeling nickel superalloys (JMatPro, MTDATA, PANDAT, ThermoCalc) and free software code, Open CALPHAD. These programs are used both in scientific research and in production. The use of these programs makes it possible to significantly decrease the time and material costs by optimizing experimental work as a result of computational prediction of the thermodynamic behavior of multicomponent systems, which would be practically impossible without the CALPHAD approach.
Databases for nickel alloys, as a rule, contain experimental binary, triple, and even quaternary phase diagrams for various combinations of the following elements: Al, B, C, Co, Cr, Cu, Fe, Hf, Mn, Mo, N, Nb, Ni, O, Pt, Re, Ru, Si, Ta, Ti, V, W, and Zr. Thermodynamic and kinetic databases along with the CALPHAD method have been successfully used in the creation and modeling of nickel superalloys for more than 40 years.
A brief summary of the capabilities of the most famous companies and their software products may be of interest.
Thermo-Calc Software Company, Sweden.
The products are Thermo-Calc, DICTRA, and TC-PRISMA.
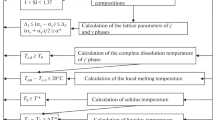
The Thermo-Calc Software package can predict the following:
the element distributions in the γ and γ' phases,
the number of phases at different temperatures,
the composition of a phase and its place inside a crystal structure,
the complete dissolution temperature of the γ' phase,
liquidus temperature,
phase diagram at a constant temperature or composition,
presence/absence of TCP phases,
minimization of the γ/γ' lattice mismatch,
the lattice parameters for a so-called disordered fcc phase,
phase and alloy densities,
nonequilibrium Scheil–Gulliver solidification curve.
The interdiffusion of thermal barrier coatings can be estimated using the DICTRA software package, which can also be used to estimate the following:
diffusion of the ordered γ' and B2 phases;
the growth and dissolution of secondary phases, such as TCP and carbides;
microsegregation during solidification;
homogenization during heat treatment.
The TC-PRISMA software package in combination with thermodynamic and kinetic databases allows one to simulate typical parallel precipitation under arbitrary heat-treatment conditions.
Examples of application fields are as follows:
parallel nucleation, growth/dissolution or coarsening of precipitates;
time evolution of particle size distribution;
the average radius of particles and their quantitative density;
the volume fraction and composition of precipitates;
the rate of formation and growth of a nucleation center;
time–temperature–precision diagrams (particle growth and dissolution);
estimation of the energy potential of multicomponent interaction.
Computherm LLC, the United States.
Its products are Pan Phase Diagram, Pan Anticipation, and PANDAT
The declared capabilities are similar to the software products of Thermo-Calc Software.
Sente Software Ltd., Great Britain.
Its product is JMatPro.
The possibilities of thermodynamic simulation are limited to a stable state of a system. However, unlike the two companies presented above, the JMatPro software package allows one to approximately estimate the strength parameters of an alloy using the well-known mechanical properties of the phase components.
Thus, today there are a sufficient number of software products that allow one to perform ab initio computer calculations of the temperature, time, and concentration parameters of the formation and annihilation of certain phases, their compositions, and interaction with other phase components of alloys.
It seems natural to ask: Are other methods of estimating phase composition and structural transformations necessary in this case? Practice shows that such methods are not only necessary, but also extremely necessary.
Of course, the methods of computer-assisted construction of phase diagrams are a very important area that needs to be actively developed. At the same time, the results of their application available today indicate that they are not yet sufficiently accurate. For comparison, we consider two Ni–Al phase diagrams, which were calculated using the ab initio principles of the theory of solids (ThermoCalc software, TCN16–TCS application software package for nickel alloys, latest version 6 in the current period (Fig. 4)) and constructed by generalizing numerous experimental data (Fig. 5) [7].
Generalized Al–Ni phase diagram based on available experimental data [7].
A comparison of the calculated (see Fig. 4) and generalized experimental (see Fig. 5) phase diagrams demonstrates the following.
(1) These diagrams are in good agreement with each other. This means that the new methods of analytical construction of phase diagrams have reached a very high level.
(2) Nevertheless, even relatively simple binary phase diagrams have noticeable differences. In particular, the calculated phase diagram (see Fig. 4) indicates a significant change in the solubility of the Ni3Al phase in Ni at temperatures from room to 1000 K, while the experimental diagram (see Fig. 5) shows that the solubility of the Ni3Al phase in Ni remains almost the same at least up to 900 K.
This circumstance is important, since the principal feature of Ni–Al alloys as high-temperature materials consists in the fact that they represent the only system characterized by high structural stability up to the operating temperatures, while the calculated diagram does not provide explanations.
In addition, the calculated PD indicates that the homogeneity range of the Ni3Al phase at room temperature narrows and almost corresponds to 25 at % Al in Ni. This result also does not coincide with the real state of affairs. Numerous experimental data suggest that, at low temperatures, there is a fairly wide homogeneity rage of the γ' phase in an alloy, varying in nickel at 72.3–76.4 at % Al.
Note that Fig. 5 does not show another compound, Al3Ni5, while Fig. 4 shows this phase formation. Therefore, it should be noted that the authors of the diagram shown in Fig. 5 say that the formation of this phase was detected in one experimental work. However, since this fact has not been confirmed in other works, they have not yet presented this compound in the phase diagram (until additional experimental results appear).
Thus, the existing software tools for modeling and calculating phase diagrams have not reached perfection to date and do not yet allow one to completely abandon the use of experimental data. At the same time, their appearance marked a breakthrough in the field of materials science and the development of high-temperature alloys. The most promising direction for the development of computational tools for modeling alloys is their integration into optimization software packages, which will be described in more detail in the next part of the work.
REFERENCES
Yu. N. Shmotin, “Methodology of designing promising gas turbine engines based on a comprehensive study of nonstationary processes and multicriteria optimization,” Doctoral Dissertation in Engineering (Moscow, 2014).
A. V. Logunov, A. V. Logachev, and A. I. Logacheva, “Software for an analysis and evaluation of the phase composition and the lattice mismatch of the gamma and gamma-bar phases and the critical values of electronic vacancies in nickel superalloys,” in Proceedings of 3rd International Engineering Conference Metaldeform–2009 (Samara, 2009), Vol. 1, pp. 100–109.
I. N. Egorov, Yu. N. Shmotin, K. S. Fedechkin, and A. A. Stepanov, “Introduction of optimization methods into the design of turbomachines,” in Proceedings of International Scientific Conference on Problems and Prospects of Engine Building Development (Samara, 2006).
I. L. Aptekar’ and L. G. Isaeva, “Analysis of possible types of phase diagrams for two-component systems,” in Phase Diagrams in Materials Science (Institute of Problems of Materials Science AN USSR, Kiev, 1979), pp. 3–12.
I. L. Aptekar’ and D. S. Kamenetskaya, “Some problems of analysis and calculation of phase diagrams,” in Phase Diagrams in Materials Science (Institute of Problems of Materials Science AN USSR, Kiev, 1979), pp. 13–35.
L. Kaufman and H. Bernstein, Computer Calculation of Phase Diagrams (Academic Press, New York, 1970).
Phase Diagrams of Binary Metallic Systems: A Handbook, Ed. by N. P. Lyakishev (Mashinostroenie, Moscow, 1996), Vol. 1, pp. 183–185.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflicts of interest.
Additional information
Translated by K. Shakhlevich
Rights and permissions
About this article
Cite this article
Logunov, A.V., Shmotin, Y.N., Khramin, R.V. et al. Computer Simulation and Optimization of the Single-Crystal Nickel Superalloy Compositions: I. Modern Computer Simulation Methods in Designing Nickel Superalloys. Russ. Metall. 2022, 588–592 (2022). https://doi.org/10.1134/S0036029522060179
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0036029522060179