Abstract
The impact toughness and phase-structural transformations in 08Kh18G8N2T steel are studied after a thermal action, which simulates the heating of the near-weld zone during electroslag welding, and subsequent thermal cycling treatment under various conditions. An increase in the impact toughness from 0.25 to 1.2 MJ/m2 is detected after thermal cycling in the temperature range 700–900°C at a rate of 1°C/s, which is associated with the redistribution of alloying elements between phases, structure fragmentation, and the morphology of excess phases.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
It seems rational to use duplex austenitic–ferritic steels to produce large structural elements of chemical apparatus operating in redox media at temperatures from –40 to 300°C [1–4]. In comparison with single-phase austenitic steels widely used under similar conditions, such steels provide structures with a higher strength and corrosion cracking resistance with significant nickel saving. Nevertheless, it should be taken into account that, in the course of welding, especially electroslag welding used for of thick metal parts [4–6], intense ferritic grain growth, change in the phase ratio, and the decomposition of unstable structures occur in duplex steels, which significantly decreases the impact toughness [3, 7–12].
One of the effective methods of increasing the impact toughness of a metal is thermal cycling treatment (TCT). The TCT of carbon and low-alloy steels is accompanied by significant structural transformations related to changes in the structure of the heat-affected zone and grain refinement, which manifests itself in decreasing the ductile–brittle transition temperature, increasing the ultimate tensile strength and the yield strength at a slight decrease in plasticity, and increasing the fatigue strength because of stress redistribution [4, 5, 9, 13–16]. Although the volume of studies of the effect of TCT on the properties of welded joints of ferritic–austenitic steels is very small, their results and accumulated experience allow us to consider TCT promising for increasing the impact toughness of the welded joints of ferritic–austenitic steels [4, 5, 8, 9].
The purpose of this work is to study the kinetics of phase and structural transformations under welding heating and the effect of subsequent TCT on the structure and properties for 08Kh18G8N2T steel are investigated
EXPERIMENTAL
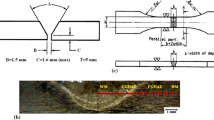
We investigated austenitic–ferritic 08Kh18G8N2T steel in the as-received state (after rolling and annealing), and its chemical composition corresponded to GOST 5632–72, namely, (wt %) 0.08 C, 18.46 Cr, 8.75 Mn, 2.66 Ni, 0.65 Si, 0.24 Ti, 0.012 S, and 0.010 P.
The imitation action of a thermal welding cycle (TWC) on the metal in the near-weld zone (NWZ) of the welded joint made by electroslag welding was carried out in a high-speed electric contact heating installation at a maximum temperature of 1300°C in the heating zone. Subsequent TCT was performed in the temperature ranges 700–900, 600–800 and 400–700°C with air cooling. The heating rate was w = 70–80°C/s for heating by a passing current and 1–2°C/s for heating in a resistance furnace. The number of cycles N was varied from 3 to 10.
Impact toughness KCV–20 was studied by performing three-point bending tests of rectangular 10 × 10 × 55 mm samples with a V-shaped notch (type XI according to GOST 9454–78), Vickers hardness HV was determined using a TPP-2 device according to GOST 2999–75, and microhardness H0.5 was determined on a PMT-3 device according to GOST 9450–76.
A structure was analyzed by optical microscopy (Leica DMi 8) and electron microscopy (UEMB- 100K), and the chemical composition of phases was determined by electron-probe microanalysis (SuperProbe-733). A structure was revealed by electrolytic etching in oxalic acid, and color tinting with Groesbeck’s reagent was used to separate phases.
RESULTS AND DISCUSSION
According to experimental, we revealed structural changes characteristic of austenitic–ferritic steels occurring in the NWZ of the welded joint. For example, the structure of 08Kh18G8N2T steel after TWC consists of large ferrite grains and acicular secondary-austenite (γ' phase) precipitates located inside these grains and along their boundaries (Fig. 1a). Thermal cycling in the temperature range 600–800 and 700–900°C leads to an increase in the amount of γ' phase in the structure (Figs. 1b, 1c), and it precipitates not only along the grain boundaries, but also in the body of the ferritic grain. The extent of ferrite fields free from the γ' phase is significantly decreased. When the upper TCT cycle temperature increases, the metal structure becomes more dispersed (see Fig. 1c).
Electron-microscopic studies of the surface of polished sections showed that the initial metal contains excess-phase inclusions, which are located in the volume of δ ferrite grains, along interphase boundaries, and inside γ'-phase needles (Fig. 2a). After TCT in the temperature range 700–900 and 600–800°C, we detected the intermetallic σ phase, carbides, carbonitrides, and an increased amount of the ferritic δ phase, which is in good agreement with [2, 3, 5, 6, 10–13]. Although the morphology of the resulting inclusions is diverse, the following two main types can be distinguished: irregular and dendritic with well-pronounced primary and secondary dendrite arms (Fig. 2b). When the thermal action time increases, precipitated particles grow and fringes form around them (Fig. 2c).
Thermal cycling at a heating rate of 1°C /s provides a stable increase in impact toughness KCV–20 in the temperature range 700–900 and 600–800°C and weakly affects it during processing in the temperature range 400–700°C regardless of the heating rate (Fig. 3, Table 1). The most significant increase in the impact toughness (up to 1.2 MJ/m2) occurs as a result of TCT in the temperature range 700–900°C, w = 1°C/s, and N = 10 cycles. A further increase in the number of cycles weakly affects the mechanical properties.
After N = 3–5 TCT cycles in the temperature range 600–800°C and w = 1°C/s, the impact toughness increases slightly; when the number of cycles increases further, it increases rapidly. For example, after N = 10 TCT cycles, the impact toughness increases from 0.250 to 0.640 MJ/m2 (see Fig. 3, Table 1). Upon heating at a rate w = 80°C/s, thermal cycling in the temperature range 700–900°C leads to a sharp increase in the impact toughness: 5 cycles in this range give the same effect as 7 cycles in the range 600–800°C at w = 1°C/s.
After TCT according to the given conditions, the hardness and microhardness of steel are almost the same as in the as-received state but are significantly lower than after welding heating (see Table 1). It should be noted that TCT in the temperature range 700–900°C decreases hardness less than TCT at 600–800 and 400–700°C; as was noted above, the impact toughness in this case is maximal (1.2 MJ/m2). As a result of TCT, the amount of δ ferrite in steel decreases substantially, especially after treatment in the temperature range 700–900°C (see Table 1).
The revealed features of the TCT action on the steel structure are as follows: the total amount of the γ' phase increases (this phase precipitates both along ferrite grain boundaries and inside ferrite grains), the area of ferrite fields free of γ'-phase precipitates decreases significantly, the total amount of δ ferrite decreases, and the steel structure is fragmented.
The fracture surface of steel samples after TWC forms according to the mechanisms of both ductile and brittle fracture (Fig. 4). Fracture areas along interphase boundaries and ductile cleavage elements are present. Fine excess-phase precipitates of unfavorable film morphology, which initiate brittle fracture along grain boundaries, are detected.
The increase in the impact toughness and the decrease in the hardness as a result of TCT were found to be associated with the dissolution of excess film phases and their coagulation. The formation of cleavage facet surfaces is accompanied by the formation of deformation twins, i.e., according to a low-energy mechanism. The total energy intensity of brittle fracture is high largely due to fragmentation, i.e., the formation of numerous plastic ridges and valleys on the surface of brittle facets (Fig. 5).
Electron-probe microanalysis data revealed that TCT causes the redistribution of the main alloying elements between phases: nickel and manganese pass from δ ferrite to the γ' phase, and chromium, on the contrary, passes from the γ' phase to δ ferrite. For example, after TCT in the temperature range 700–900°C at w = 1°C/s and N = 10 cycles, the nickel content in ferrite decreases by 1.5 times, the manganese content decreases by 1.2, and the chromium content increases by 1.1 times. The nickel content in austenite increases by 1.2 times, the manganese content increases slightly and remains approximately at the same level, and the chromium content decreases by 1.1 times (Table 2).
The results obtained were taken into account in the production of the tanks of the chemical and petrochemical equipment in OAO Volgogradneftemash.
CONCLUSIONS
(1) TCT increases the impact toughness of austenitic–ferritic 08Kh18G8N2T steel after the embrittlement caused by welding heating.
(2) For 08Kh18G8N2T steel, TCT is optimal in the temperature range 700–900°C at a heating rate of 1°C/s and 10 cycles; as a result, impact toughness KCV–20 increases from 0.25 to 1.2 MJ/m2.
(3) The increase in impact toughness is due to changes in the composition, quantity, and morphology of excess phases, the redistribution of elements between ferrite and austenite, and the fragmentation of structural components. The total amount of the γ' phase was found to increase, and the total amount of δ ferrite was found to decrease from 35 to 15%.
(4) TCT was shown to cause the redistribution of the main alloying elements between phases: nickel and manganese pass from δ ferrite to the γ' phase, and chromium, on the contrary, passes from the γ' phase to δ ferrite.
REFERENCES
E. L. Makarov and B. F. Yakushin, Theory of Weldability of Steels and Alloys (Izd. MGTU, Moscow, 2014).
N. I. Kakhovskii, Welding of High-Alloy Steels (Tekhnika, Kiev, 1975).
M. A. Khubrikh, G. A. Sal’nikov, Zh. A. Lepilina, and I. I. Zhukova, “Kinetics of phase transformations in 08Kh18G8N2T steel during welding cycle heating,” Avtom. Svarka, No. 4, 21–23 (1986).
O. P. Bondareva, E. V. Sedov, and I. L. Gonik, “Influence of a thermal welding cycle on the crack resistance of the metal of the near-weld zone in the welded joint of ferritic–austenitic steels,” Izv. VolgGTU, Ser. Probl. Materialved., Svarki, Prochn. Mashinostr., No. 5(160), 139–142 (2015).
O. P. Bondareva, E. V. Sedov, I. L. Gonik, and O. B. Kryuchkov, “Influence of thermal aging and thermal cyclic treatment on the structure and properties of the metal of the near-weld zone of the welded joints of ferritic–austenitic 08Kh18G8N2T steel,” Tekhnol. Met., No. 6, 23–27 (2017).
V. B. Penkov, G. V. Kamenetskaya, and U. V. Poletaev, “Effect of modifying additions on the impact toughness of welded joints in low-carbon steels produced with electrodes with a basic coating,” Weld. Int. 15 (12), 983–985 (2001).
V. Poletaev and A. S. Zubchenko, “Local fracture susceptibility of the welded joints of chromium–manganese and chromium–nickel austenitic steels,” Svar. Proizv., No. 10, 11–13 (1989).
W. J. Hall, H. Kihara, V. Zut, and A. Wells, Brittle Fracture of Welded Structures (Mashinostroenie, Moscow, 1979).
O. P. Bondareva, E. V. Sedov, and I. L. Gonik, “Effect of thermal cycling treatment on structure and properties of ferritic–austenitic steel welded joint heat-affected zone metal,” Chem. Petrol. Eng. 52 (7, 8), 506–511 (2016).
C. H. Shek, K. W. Wong, J. K. Lai, and P. J. Li, “Hot tensile properties of 25Cr–8Ni duplex stainless steel containing various cellular structure after thermal treatment,” Mater. Sci. Eng., A 231, 42–47 (1997).
S. Jeong, Y. Lee, C. Park, B. Kim, J. Moon, S.-J. Park, and C. Lee, “Phase transformation and the mechanical characteristics of heat-affected zones in austenitic Fe–Mn–Al–Cr–C lightweight steel during post-weld heat treatment,” Mater. Characteriz. 177, 119–126 (2021).
S. Jeong, B. Kim, J. Moon, S.-J. Park, and C. Lee, “Influence of k-carbide precipitation on the microstructure and mechanical properties in the weld heat-affected zones in various Fe–Mn–Al–Cr–C alloys,” Mater. Sci. Eng., A 726, 223–230 (1997).
L. V. Kostyleva, A. E. Novikov, D. S. Galich, E. Yu. Karpova, and V. A. Motorin, “Improving the structure and properties of the cast parts made of medium-carbon steel by thermal cyclic treatment,” Materialoved., No. 4, 23–28 (2019).
S. A. Zinchenko, N. A. Zolotarev, and N. N. Vasilyeva, “Decreasing the degree of carbide inhomogeneity of hypereutectoid steels by thermal cyclic treatment,” Chern. Metallurg. Byull. Nauch.-Tekhn. Ekon. Inform. 76 (5), 477–482 (2020).
V. V. Glebov, Yu. I. Matveev, and A. A. Khlybov, “Effect of alloying on the mechanical and corrosion characteristics of 23Kh15N7M2 steel,” Zagot. Proizv. Mashinostr. 16 (6), 275–278 (2018).
A. N. Zhakupov and A. V. Bogomolov, “Influence of thermal cyclic treatment on the mechanical properties of low-alloy 13KhFA steel,” Vestn. Vostochno-Kazakhstanskogo Gos. Univ., No. 2, 114–119 (2018).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflicts of interest.
Additional information
Translated by K. Shakhlevich
Rights and permissions
About this article
Cite this article
Bondareva, O.P., Sedov, E.V. & Kryuchkov, O.B. Effect the Thermal Cycle of Welding and Thermal Cycling Treatment on the Properties and Fracture of 08Kh18G8N2T Steel. Russ. Metall. 2022, 385–390 (2022). https://doi.org/10.1134/S0036029522040073
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0036029522040073