Abstract
The results of field (bypass) tests of fittings made of 13KhFA steel in oilfield media with a high content of CO2, H2S, and Cl– are presented. The corrosion resistance of the branches made of 13KhFA steel is shown to be two to three times higher than that of traditionally used 09G2S steel. Intense development of bacterial corrosion in steeply curved bends is detected.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
The field pipeline systems of most deposits are now made of 13KhFA and 08KhMFA steels with a bainitic structure and high corrosion resistance in oilfield media. At the same time, pipeline branches and other fittings are still made of the traditional previously used 20, 20F, 17G2, and 09G2S steels. The low strength and corrosion properties of these steels are compensated by high metal consumption (large wall thickness) of fittings.
The branches and other fittings in pipeline systems undergo more intense corrosion-mechanical failure, since the motion direction and conditions of a transported medium change in them. In addition, the processes of forming during the production of shaped products change their structure and decrease the properties of steel, while the technologies for manufacturing shaped products are based on the principle of minimizing energy costs without taking into account reaching the necessary properties. As a result, the properties of the fittings are usually lower than those of factory-made pipes.
To ensure the operational properties of the fittings at or higher than the properties of linear sections of pipelines, it is necessary to develop and implement special-purpose production of these parts from steels of higher quality. This requires a reliable understanding of the features of the corrosion-mechanical failure of the shaped products in the composition of operating pipelines and, accordingly, pilot field tests (PFTs). An oilfield carbon dioxide media with a high chlorine content provides the most severe conditions for conducting such PFTs.
To date, the pipelines made of low-alloy chromium-containing 13KhFA steel have shown high performance in oilfield media with a high content of CO2 and H2S [1–3]. A positive effect of chromium, vanadium, and other alloying elements on the CO2 corrosion resistance of steel was noted [4–7]. Special-purpose heat treatment (double hardening with high tempering) slightly increases the corrosion resistance of 13KhFA steel in aggressive media [8, 9].
The purpose of this work is to study the mechanisms and kinetics of the corrosion-mechanical failure of the fittings of oilfield pipelines made of 13KhFA steel during operation in aggressive media.
EXPERIMENTAL
Steeply curved (with an angle of rotation of 90 deg) branches 219 mm in diameter with a wall thickness of 6 mm made of 13KhFA steel were subjected to corrosion-mechanical failure tests. The element contents in the pipe metal were as follows (wt %): 0.151 C, 0.23 Si, 0.39 Mn, 0.043 Al, 0.55 Cr, 0.008 Mo, 0.055 V, 0.06 Ni, 0.104 Cu, 0.006 Ti, 0.015 Nb, 0.016 P, and 0.009 S. The mechanical properties of the steel, which were confirmed by laboratory tests, were σu = 533 MPa, σy = 401 MPa, and δ = 32.4%, and the characteristics of its fracture toughness are given in Table 1. The resistance of steel to sulfide stress corrosion cracking (SSCC) determined in accordance with NACE TM0177 is σth/σ0.2 = 75% (σth is the threshold SSCC stress, σ0.2 is the minimum ensured yield strength), the critical stress intensity factor at the tip of a corrosion crack is KIssc = 43.1 MPa m1/2, and no hydrogen cracking cracks were detected.
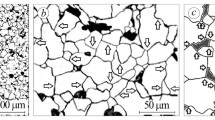
The pipes made of 13KhFA steel were subjected to double quenching (at 930 and 770°C), followed by tempering at 600°C; in comparison with conventional quenching (above temperature Ac3), this scheme provides higher plasticity, impact strength, and uniformity of properties across the pipe wall thickness. The branches were made by pulling pipe billets along a horn-shaped core using the technology including heating to ≈960°C, bending deformation by 90 deg, air cooling, subsequent double quenching (at 930 and 770°C), and high tempering at 580°C. Heating for deformation and heat treatment led to the formation of a 200-μm-thick dense scale layer and to significant decarburization of the surface to a depth of ≈100 μm. The surface layer of the branches in the initial state was represented by ferrite grains (Fig. 1a), and the base metal, by granular pearlite with ferrite crystals (Fig. 1b).
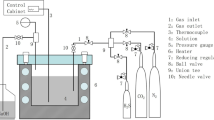
The bypass tests (Fig. 2) were carried out for 19 and 42 months under the conditions of the test bench created at the site of the oil-gathering reservoir of the Mamontovsk deposit of OOO RN-Yuganskneftegaz, the oilfield media of which are characterized by a high CO2 content and are additionally saturated with H2S and chlorine ions (Table 2). After PFT, the line was dismantled and the branches were cut and cleaned from oil products. The type, the intensity, and the character of damage to the inner surface and the composition, the structure, and the formation kinetics of corrosion products were evaluated.
The state of the inner surface of the branches was investigated by metallographic analysis. The general and local corrosion rates were determined from the change in the wall thickness and by the gravitational method. To study the phase compositions of the corrosion products, we used a DRON-3 X-ray diffractometer. The morphology, the structure, and the chemical composition of the corrosion products were studied with an XL-30 (Philips) scanning electron microscope equipped with an EDAX energy dispersive analyzer. The microbiological studies performed to detect the adhesive forms of corrosive oil biocenosis bacteria were performed in accordance with NACE Standard TMO194–2004, NACE Standard-2006, and RD 39-3-973–83. To analyze the corrosion damage across the branch thickness, we used successive grinding of the surface layers.
RESULTS AND DISCUSSION
The type of failure and the determined rates of general and local corrosion of the branches made of 13KhFA steel during the test period indicate that local (pitting) form of corrosion-mechanical failure is the main type of corrosion (Fig. 3). Its rate is halved with an increase in the test time from 19 to 42 months at a slight change in the general corrosion rate (Table 3), which is thought to be caused by an increase the protective action of the corrosion products in time (they clog deeper pitting channels). It should be noted that the corrosion rate of branches made of 13KhFA steel is 2–3 times lower than that of the branches made of 09G2S steel operating in the same deposit. The branches made of 09G2S steel are characterized by intense local groove-type corrosion with numerous pits extending into the wall depth (Fig. 4).
Figures 5–9 illustrate the character of corrosion damage and the composition and morphology of the corrosion products in the 13KhFA steel branches. During layer-by-layer grinding of the surface layer, we found that corrosion damage spreads in the form of pitting channels with numerous branches in the form of smaller channels (Fig. 5). Such a “worm-type” type of damage is characteristic of carbon dioxide media containing chlorine ions [9].
The corrosion products mainly consist of iron carbonates (FeCO3), contain iron sulfides (FeS), have a high (as compared to steel)) content of alloying elements (Cr, Mo, Si, etc.), and have a carbide phase inherited from corrosive steel. Chlorine concentrates at the interface between the corrosion products and the metal (see Fig. 5–8). The predominant mechanism of failure is carbon dioxide corrosion accelerated by chlorine ions.
Bacterial corrosion develops intensely in the branches. Microbiological studies have shown the presence of a large number of bacteria with a high index of biological activity (100%) in the corrosion products. Sulfate-reducing bacteria were found in an amount of 105 cells per 1 g scrape, sulfur-oxidizing bacteria (104 cells), hydrocarbon-oxidizing bacteria (104 cells), and iron-oxidizing bacteria (103 cells). Bacteria are attached to all asperities and roughnesses of the inner surface and the branch surfaces to form a biocenosis, i.e., a community of interrelated organisms. As a result of the joint functioning of bacteria, dense layers of the products of their vital activity connected by slime form; they are represented by capsules, i.e., areas where a dangerous corrosive biocenosis occurs (Fig. 7). The capsules combine to form extended voids, as a result, promote exfoliation of the corrosion products from the branch surfaces.
CONCLUSIONS
(1) In oilfield media with a high content of CO2 and chlorine ions, the corrosion damage to 13KhFA steel is represented by a developed system of pitting channels characteristic of worm-hole corrosion.
(2) The fittings of pipelines are the places of predominant nucleation and intensive development of bacterial corrosion. The corrosion rate of the branches made of 13KhFA steel is 2–3 times lower than that of 09G2S steel, which determines the prospects of application of 13KhFA steel in the deposts of Western Siberia.
REFERENCES
V. V. Zav’yalov, Problems of the Operational Reliability of Pipelines at a Late Stage of Development of Deposits (VNIIOENG, Moscow, 2005).
A. V. Ioffe, T. V. Tetyueva, V. A. Revyakin, E. A. Borisenkova, S. A. Knyaz’kin, and T. V. Denisova, “Corrosion-mechanical failure of pipe steels during operation,” Metalloved. Term. Obrab. Met., No. 10, 22–28 (2012).
M. A. Vyboishchik, A. V. Ioffe, E. A. Borisenkova, T. V. Denisova, and A. V. Sorokin, “Corrosion damage of oil pipelines made of chromium-molybdenum-containing steels under high aggressiveness of an extracted medium,” Metalloved. Term. Obrab. Met., No. 10, 29–33 (2012).
M. Tröger, C. Bosch, H. Meuser, F. M. Knoop, H. Brauer, and J. Schröder, “New alloying concepts for increased corrosion resistance of welded linepipe steels in CO2 containing aqueous media,” in Proceedings of Conference EUROCORR 2013 (Estoril, 2013).
D. V. Edmonds and R. C. Cochrane, “The effect of alloying on the resistance of carbon steel for oilfield applications to CO2 corrosion,” Mater. Res. 8 (4), 377–385 (2005).
M. B. Kermani, J. C. Gonzales, C. Linne, M. Dougan, and R. Cocharane, “Development of low carbon Cr–Mo steels with exceptional corrosion resistance for oilfield applications,” in Proceedings of NACE Annual Conference Corrosion 2001 (2001), paper 65.
R. Nyborg and A. Dugstad, “Mesa corrosion attack in carbon steel and 0.5% chromium steel,” in Proceedings of NACE Annual Conference on Corrosion (San Diego, 1998), paper 29.
M. A. Vyboishchik, A. V. Ioffe, T. V. Tetyueva, V. A. Revyakin, and I. V. Gruzkov, “Degradation and failure of oil-and-gas pipes in media with a high content of carbon dioxide and chlorine ions,” Deform. Razrushenie Mater., No. 4, 29–36 (2020).
M. A. Vyboishchik, A. O. Zyryanov, I. V. Gruzkov, and A. V. Fedotova, “Carbon dioxide corrosion of oilfield pipes in media saturated with H2S and Cl,” Vektor Nauki Togliatti Gos. Univ., No. 2 (48), 6–17 (2019).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated by K. Shakhlevich
Rights and permissions
About this article
Cite this article
Vyboishchik, M.A., Ioffe, A.V., Kudashov, D.V. et al. Corrosion-Mechanical Failure of the Fittings of Pipeline Systems in Deposits with a High CO2 Content. Russ. Metall. 2020, 1171–1176 (2020). https://doi.org/10.1134/S003602952010033X
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S003602952010033X