Abstract
The leaching of the major mineral (potassium alunite) from alunite ores with a potassium hydroxide solution is studied. The process occurs with the retention of other minerals (dickite, hematite, quartz) in the leaching residues. The kinetics of leaching of potassium alunite from alunite raw materials is studied by the powder dissolution method in the temperature range of a potassium hydroxide solution 333–368 K at a KOH concentration of 52.96–243.8 g/L. The activation energy of the process is calculated using the experimental results obtained (Ea = 73.14 kJ/mol). The calculated energy indicates that the leaching of alunite from alunite ores is controlled by a surface chemical reaction, and the reaction order with respect to KOH is 1.02. The process under study is described by a first-order kinetic equation.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
INTRODUCTION
In the present time, more than 90% of primary aluminum are produced from bauxites. However, bauxite resources in nature are being exhausted because of the vigorous development of the aluminum industry and they would not provide the further development of this field. The alunite ore in Azerbaijan (Zaglikskoe deposit) is considered to be a potential source for manufacturing both alumina and potassium fertilizers.
The complex technology for the processing of alunites from the Zaglikskoe deposit has been developed for several years at the Nagiev Institute of Catalysis and Inorganic Chemistry (Academy of Sciences of Azerbaijan) and Institute of Geology and Geophysics (Academy of Sciences of Azerbaijan). These alunites are considered as the most promising raw materials for the development of a new alumina production.
Many diverse methods for alunite rock processing are described in the literature [1–8]. The problem of the efficient processing of alunite ores, especially alunites from the Zaglikskoe deposit, remains challenging up to presently. Many studies were carried out on the processing of alunite ores from various deposits with the recovery of valuable products. The processing methods can be divided into acid [1–4] and alkali [5‒8] methods. Serious problems were found for acid leaching related to iron removal and separation products, which disproves the selectivity of acid leaching. Unlike acid leaching, alkali leaching has many advantages. It is known that alunite is readily dissolved in caustic potash (caustic soda) at the temperatures higher than 60°C. In this case, aluminum goes to the solution in the form of potassium (sodium) aluminate, as well as alkaline metal sulfates. The alkaline agents are highly reactive toward potassium alunite thus preventing other metals in the residue from leaching.
In spite of the technological processes of alumina recovery from alunite ores of the Zaglikskoe deposit, which were studied and described in rather detail, quantitative characteristics of the alunite dissolution kinetics are lacking. The study of these characteristics could give information for controlling the process and apparatus design. The dissolution rate of alunite from various deposits with a KOH solution was studied [7, 8]. The authors [7] proposed alunite leaching from alunite tailings with a highly concentrated solution of KOH (10–15.7 mol/L) at 50–90°C. It is shown that aluminum is completely leached from the ores within 30 min during alunite leaching at 90°C and the KOH concentration higher than 13.5 mol/L. The apparent activation energy calculated from the experimental data is 44.48 kJ/mol, and the reaction order with respect to KOH is 1.76. Unlike the published data [7], the leaching kinetics of alunite was considered for pure natural alunite (36.9% Al2O3) [8]. The effect of the KOH concentration on alunite dissolution was determined for a concentration of 1.4 mol/L. At 95°C the reaction ceases within the time less than 20 min. The reaction rate was shown [7] to be controlled by the surface chemical reaction, the reaction order with respect to KOH was 1.327, and the activation energy was 94.18 kJ/mol. Published data on the kinetics of alkali leaching of alunite from the Zaglikskaya alunite ore are scarce.
Taking into account the aforesaid, the aim of in this work is to study the kinetics of direct alunite leaching with an alkali solution from the alunitized rocks that were not subjected to annealing.
EXPERIMENTAL
Experiments on studying the leaching kinetics of the powdered material were carried out by the dissolution method [9, 10]. This method is based on the dissolution of a dispersed solid mixed with a solvent in open or closed vessels.
The average chemical composition of the alunite ore from the Zaglikskoe deposit is the following (%): 19.2 Al2O3 (alunite), 2.5 Al2O3 (dickite), 20.0 SO3, 1.38 Na2O, 3.72 K2O, 41.4 SiO2, 5.05 Fe2O3, 0.2 CaO, 0.18 P2O5, 0.12 MgO, 0.53 TiO2, and 6.5 H2O.
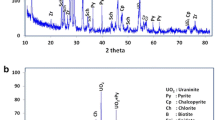
The X-ray diffraction (XRD) pattern of the alunite ores from the Zaglikskoe deposit is presented in Fig. 1. The major minerals composing the alunite ores are potassium (sodium) alunite KAl23(SO4)2(OH)6, kaolinite (dickite) Al4(Si4O10)(OH)8, hematite Fe2O3, and quartz SiO2. Thus, aluminum in the ore exists in alunite and dickite.
During leaching, the target mineral (potassium alunite) was dissolved with a solution of potassium hydroxide, whereas dickite, hematite, and quartz are retained in the leaching residue (red mud) (Fig. 1b). Two diffraction lines characteristics of the indicated minerals are pronounced in the XRD patterns.
The main reaction of alunite leaching is described by the following equation:
When studying the leaching kinetics of alunite with a potassium hydroxide solution, it was accepted that all leached alunite passed to the solution via reaction (1).
Leaching was carried out in a temperature-maintained glassware with stirring at variable parameters of the process (potassium hydroxide concentration in solution CKOH, temperature T, and stirring time τ). The experimental procedure was as follows. A weighed sample of the ore was stirred in a KOH solution, during which excess alkali providing a high alumina to caustic ratio should be achieved in all cases. The solutions with the following concentration CKOH (g/L) were prepared for the study: 52.96 (5%), 110.9 (10%), 174.6 (15%), and 243.8 (20%).
A glass beaker containing 100 mL solution with one of the indicated concentrations was placed in a thermostat, and samples were taken after a given temperature of the solution was reached or a certain time was elapsed. The aluminum content was determined by photocolorimetry, and the content of \({\text{SO}}_{4}^{{2 - }}\) ions was also monitored in the solution.
Preliminary experiments showed that, for a stirrer speed >700 rpm, the rate of alunite transition to a solution was independent of the stirring rate. Therefore, the leaching kinetics of alunite was studied at a stirrer speed of ~700 rpm.
RESULTS AND DISCUSSION
A milled material with various granulometric compositions was used for leaching. The surface of this material changes during leaching, which results in a change in the leaching rate along with the reagent concentrations. If reaction (1) is taken to be irreversible and not accompanied by the formation of a solid product shell, the rate of alunite leaching v from the ores can be expressed as a rate of decrease of the leached alunite content in the solid phase [9],
where α and (1 – α) are the fractions of alunite passed to the solution and remained in the solid phase with the surface area S, respectively; k′ is the rate constant of leaching (1); and n is the reaction order with respect to the potassium hydroxide concentration CKOH.
To take into account the change in the solid phase surface in time, the surface area was assumed to change during leaching proportionally to the relative fraction of the leached metal in the solid phase in the power β; that is S = S0(1 – α)β, S0 is the initial specific surface area (50 dm2/g). In this case, Eq. (2) for the leaching rate is reduced to the form
where the rate constant is k′′ = k′S0.
The experimental data obtained at different concentrations of the solvent showed that the relative fraction of alunite in the solid phase decreased in time via the exponential law [5]
where k is the coefficient determined experimentally as the slope of the straight line in the ln(1 – α)–τ coordinates. After taking logarithm and differentiating Eq. (4), we have
A comparison of Eq. (5) with Eq. (3) allows us to find that β = 1. Then, we have
It is seen from the data in Fig. 2 that the amount of alunite passed to the solution changes proportionally to the alkali concentration. An increase in the potassium hydroxide concentration in the solution favors faster dissolution of alunite.
The dependence of the leaching rate constant of alunite on the potassium hydroxide concentration in the solution is shown in Fig. 3. Refined rate constants k in Eq. (6) were as follows: 0.003388, 0.00616, 0.00964, and 0.01271. It is seen from the plot in Fig. 3 that the dependence of k′ on the KOH concentration in a range of 52.2–237.3 g/L is rectilinear; that is, the process proceeds via a first-order reaction law. This corresponds to the real change in the potassium hydroxide concentration in the industrial leaching process and is expressed by the equation
where \(k_{0}^{'}\) = 4 × 10–4 (see Fig. 3), and k′′ = 5 × 10–5 at T = 298 K.
The dependence of ln k on ln CKOH is shown in Fig. 4. The slope of the curve indicates a reaction order of 1.02 with respect to the KOH concentration.
To study the temperature effect on the process of alunite dissolution, experiments were carried out at temperatures of 333, 343, 353, and 368 K, and the potassium hydroxide concentration during leaching was 110.9 g/L. The results of these experiments (Figs. 5a, 5b) show that the dissolution rate curve increases proportionally to the temperature rise.
The given below rate constants for alunite dissolution at different temperatures were calculated as the slopes of straight lines 1–4 (see Fig. 5b):
T, K | 333 | 343 | 353 | 368 |
|---|---|---|---|---|
k′, min–1 | 0.0066 | 0.02613 | 0.0421 | 0.0843 |
The determined temperature dependence of k′ is adequately described by an Arrhenius equation (Fig. 6).
In this case, the activation energy can be graphically determined from the slope of the straight line by the equation
where δ is the slope of the straight line to the abscissa, R is the gas constant, and ξ is the ratio of scales on the axes of abscissa and ordinate.
The temperature dependence of the rate constant for alunite dissolution in each chosen scale \(\left| {\tan \delta } \right|\) = 1.91 is shown in Fig. 6. If the ratio of scales on the abscissa and ordinate is (1 : 1.01 × 10–3) : (1 : 0.2) = 2 × 103, we have Ea = 2.303 × 8.314 × 1.91 × 2 × 103 = 73 142.0824 J/mol = 73.14 kJ/mol.
Thus, the apparent activation energy Ea calculated from the experimental data is 73.14 kJ/mol. The temperature coefficient of the dissolution rate of alunite in KOH solutions in the temperature range 333–368 K was determined from the experimental data and turned out to be low, 1.1 on average.
Apparent activation energy Ea was also calculated from four conventional equations. The results of the theoretical calculations made it possible to derive the temperature dependence of the rate constant of alunite leaching in a KOH solution, k′ = 1.721 × 108exp(–7863/T).
The activation energy of the reaction is \(E_{{\text{a}}}^{{{\text{alunite}}}}\) = 7863R = 7363 × 8.314 = 65.372 kJ/mol.
The determined values of activation energy and temperature coefficient suggest that the leaching of alunite from the alunite ores with a KOH solution in a temperature range of 333–368 K is controlled by a surface chemical reaction.
CONCLUSIONS
(1) The rate constants were calculated on the basis of the studies of alunite leaching with potassium hydroxide solutions with concentrations of 52.2, 109, 170.7, and 237.3 g/L at 333 K and were found to be 0.003388, 0.00616, 0.00964, and 0.01271, respectively. The dependence of the process rate on the KOH concentration was constructed using these data, and this curve is rectilinear.
(2) The temperature dependence of the leaching rate of alunite obeying the Arrhenius law was studied, and the apparent activation energy was determined (Ea ≈ 73.14 kJ/mol). According to the published data, the obtained activation energy is characteristic of surface chemical reactions.
REFERENCES
K. W. Loast, “Recovery of aluminium from alunite using acid leaching to purity the residue for Bayer leach,” VP Patent 4.031.182, 1977.
L. J. Froisland, M. L. Wouden, and D. D. Harbuck, “Acid sulfation of alunite,” in United States Bureau of Mines Report (1989), R19222.
W. Zhao, X. Yao, S. P. Zhong, Y. Y. Zhu, X. Yang, L. Yi, G. Li, J. Song, H. Yu, R. Ruan, and T. Qi, “Extraction of Al and K salts from associated alunite tailings by an acid calcination—water leaching method,” J. Clean. Prod. 107, 792–798 (2015).
M. Özdemir and H. Cetisli, “Extraction kinetics of alunite in sulfuric acid and hydrochloric acid,” Hydrometallurgy 76, 217–224 (2005).
G. Z. Nasyrov, E. I. Zemlyanskaya, and I. V. Ravndonikas, “Process for alunite treatment,” US Patent 4.117.077, 1978.
M. Ozacar and L. Sengil, “Optimum conditions for leaching calcined alunite ore in strong NaOH,” Can. Metall. 38 (4), 249–255 (1999).
M.-J. Luo, C.-L. Liu, J. Xue, P. Li, and J.-G. Yu, “Leaching kinetics and mechanism of alunite leaching from alunite tailings in highly concentrated KOH solution,” Hydrometallurgy 174, 10–20 (2017).
M. Mohammadi and M. M. Salarirad, “Kinetics of direct leaching of natural alunite in KOH,” Ind. Eng. Chem. Res. 52 (40), 1459–1465 (2013).
A. I. Zelikman, T. M. Vol’dman, and L. V. Vel’yavskaya, Theory of Hydrometallurgical Processes (Metallurgiya, Moscow, 1975).
V. V. Dolivo-Dobrovol’skii, “Some regularities of the dissolution of solids,” Zap. Leningrad. Gorn. Inst. Khim. Metallurg. Obogashch. 42 (3), 3–23 (1963).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated by E. Yablonskaya
Rights and permissions
About this article
Cite this article
Geidarov, A.A., Alyshanly, G.I., Gulieva, A.A. et al. Kinetic Laws of the Dissolution of Alunite from Alunite Ores with an Alkali Solution. Russ. Metall. 2020, 933–937 (2020). https://doi.org/10.1134/S0036029520090050
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0036029520090050