Abstract
The concentration regions of the phase equilibria of the components in a metallic Fe–Mg–Al–Ca–C–O melt at a temperature of 1600°C have been calculated and built for low-, medium-, and high-carbon steels by simulating the solubility surfaces of the components in a metal. The conditions of formation of calcium aluminate inclusions in the system are determined. Carbon is shown to influence the sequence of phase formation with the participation of strong deoxidizers, such as calcium, magnesium, and aluminum. The liquid metal is found to contain composition regions in equilibrium with a gaseous CO-based phase or a gaseous phase based on calcium and magnesium vapors.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
INTRODUCTION
Deoxidation is the most important operation of a metallurgical technology that enables one to ensure, to a significant degree, the quality of a melted metal by the formation of the rational composition of nonmetallic inclusions and by a decrease in the oxidation level of the metal. One of the most commonly used deoxidizers of iron-based melts is aluminum due to its comparable availability, the possibility of forming a deeply deoxidized metal, and positive influence on the initial grain size [1–3]. Calcium is also widely used as a deoxidizing and desulfurizing agent and for the modification of nonmetallic inclusions due to its high chemical activity with respect to oxygen and sulfur in liquid iron [4–7]. The application of aluminum–calcium alloys for the deoxidation of complex alloys makes it possible not only to provide a large deoxidation depth, but also to reach a globular shape of nonmetallic inclusions [8–12].
During production, a liquid metal contacts with the magnesia refractory material of the liner of steel-making units and ladles and a magnesium-containing slag, which can change the nature of the deoxidizing action of aluminum–calcium combinations. In some technological processes, special magnesium-containing deoxidizing complexes are used [13, 14].
The aim of this work is to perform thermodynamic simulation of the phase equilibria in an Fe–Mg–Al–Ca–C–O melt at a temperature of 1600°C for low-, medium-, and high-carbon steels.
SIMULATION PROCEDURE
In this work, the thermodynamic simulation was performed using the technique of constructing the solubility surfaces of metal components (SSMC) [15]. The solubility surface of metal components relates quantitative changes in the liquid metal compositions to changes in the nonmetallic inclusion compositions, which enables one to calculate the concentration regions of the thermodynamic stability of the nonmetallic phases existing in equilibrium with a metallic melt.
The SSMC coordinates of the Fe–Mg–Al–Ca–C–O system were calculated taking into account the data on the limiting solubility of elements in liquid iron at 1600°C: the oxygen solubility is 0.23 wt % [15], the magnesium solubility is 0.02 wt % [16], and the calcium solubility is 0.0463 wt % [17].
To determine possible chemical reactions in the melt of the system and the nonmetallic inclusion compositions, we performed SSMC calculations considering the following phase equilibria that take place in oxide systems: FeO–MgO, FeO–Al2O3, FeO–CaO, Al2O3–CaO, MgO–Al2O3, MgO–CaO, and FeO–MgO–Al2O3–CaO. In this case, we used the available data, according to which the melting temperature of iron oxide FeO is 1378°C [18], that of magnesium oxide MgO is 2825°C [18], that of aluminum oxide Al2O3 is 2051°C [18], and that of calcium oxide CaO is 2613°C [19].
The FeO–MgO phase diagram is characterized by unlimited solubility of the components in both solid and liquid states [20]. The data on the binary oxide phase diagrams for the FeO–Al2O3, MgO–Al2O3, FeO–CaO, Al2O3–CaO, and MgO–CaO systems were taken from [21]. For example, in the FeO–Al2O3 system, hercynite FeAl2O4 with a melting temperature of 1780°C can form. In the MgO–Al2O3 system, a magnesium oxide-based solid solution forms along with the formation of magnesia spinel MgAl2O4, with a melting temperature of 2105°C. The solubility of Al2O3 in MgO at 1600°C is lower than 0.5 wt %, which determined the possibility of SSMC calculation without considering the solubility of aluminum oxide in MgO. In the FeO–CaO system, there are solid solutions based on iron oxide and calcium oxide. The existence of two solid solutions is also confirmed in the MgO–CaO system with the minimum solubility of calcium oxide in MgO at 1600°C, which enables us to calculate SSMC without considering the formation of this solid solution. The Al2O3–CaO system does not contain solid solutions; however, there are the following four compounds observed experimentally: CaAl12O19 (Tm = 1830°C), CaAl4O7 (Tm = 1762°C), CaAl2O4 (Tm = 1602°C), and Ca3Al2O6 (Tm = 1539°C).
The FeO–MgO–Al2O3–CaO phase diagram in poorly described. Handbook [21] gives data only on the total projection of the liquidus surface in the FeO–Al2O3–CaO and MgO–Al2O3–CaO systems; for the FeO–MgO–Al2O3, the positions of isotherms are shown for temperatures 1400 and 1700°C; it is found that the MgO–Al2O3–CaO system contains the ternary Ca3MgAl4O10 compound, whch exists to 1500°C. Only a continuous series of solid solutions was detected in the pseudo-binary FeAl2O4–MgAl2O4 system [22].
Thus, at 1600°C, which is characteristic of steelmaking processes, the following phases can form in the Fe–Mg–Al–Ca–C–O system as reaction products: an oxide variable-composition melt (FeO, MgO, Al2O3, CaO); solid solution |FeO, MgO|s.s.; corundum Al2O3; solid solution of spinels |FeAl2O4, MgAl2O4|s.s.; calcium oxide-based solid solution |CaO|s.s.; calcium aluminates CaAl12O19, CaAl4O7, and CaAl2O4; and gaseous reaction products {CO, CO2}, which also contain magnesium and calcium vapors. The temperature dependences of the equilibrium constants of the reactions of formation of these phases in the metallic melt of the system under study are given in Table 1.
The simulation of the phase equilibria with the participation of an oxide melt and/or the solid solutions of |CaO|s.s. oxides was performed using the theory of subregular ionic solutions, the energy parameters of which are given in Tables 2 and 3. The activities of the components of the |FeO, MgO|s.s. solid solution were calculated using the theory of regular ionic solutions; in this case, we found that the theory energy parameter was Q12 = +3000 J/mol. The |FeAl2O4, MgAl2O4|s.s. solutions were considered to be completely ionic. The metallic melt was modeled using the Wagner first-order interaction parameters (Table 4). The activities of pure solid-state aluminum oxide and calcium aluminates were taken to be 1. The activities of the gaseous phase components were determined via their partial pressures.
The simulation was performed for the following four carbon concentrations in steel: 0 wt % (equilibria for the deoxidation of pure iron), 0.1 wt % (characteristic of low-carbon steel), 0.4 wt % (medium-carbon steel), and 0.8 wt % (high-carbon steel). The limits of the Mg, Al, and Ca concentrations were as follows: for magnesium, from 10–6 wt %; for calcium, from 10–5 wt % to the limiting solubility in liquid iron; and for aluminum, from 10–5 to 0.1 wt %.
RESULTS AND DISCUSSIONS
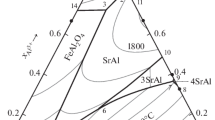
The results of simulating the isocompositional SSMC sections of the Fe–Mg–Al–Ca–C–O system at T = 1600°C are shown in Figs. 1–4. All the diagrams were plotted in log–log coordinates and the concentrations are given in wt %. In each case, the concentration of one element of the system, which is characteristic of steelmaking was fixed: in Figs. 1 and 2, magnesium concentrations of 0.0001 and 0.0005 wt % were fixed, respectively; Fig. 3 shows the data calculated at a calcium content of 0.001 wt % in liquid metal; and Fig. 4 shows the results of simulation at [Al] = 0.01 wt %.
SSMCs of the Fe–Mg–Ca–Al–O–C system, [Mg] = 0.0005 wt % and [C] = (a) 0, (b) 0.1, (c) 0.4, and (d) 0.8 wt %. Points denote the experimental results [34] corresponding to the region of equilibrium of a liquid metal with a spinel solid solution.
In Figs. 1–4, region I indicates the compositions of the liquid metal in equilibrium with the inclusions of the |FeO, MgO|s.s solid solution; in region II, |FeAl2O4, MgAl2O4|s.s spinel solid solution inclusions are the nonmetallic inclusions in equilibrium with the liquid metal, which are thermodynamically stable; in region III, corundum inclusions are in equilibrium with the liquid metal; in regions IV and V, the inclusions of calcium hexa- and bialuminates, respectively; in region VI, the liquid metal compositions in equilibrium with an oxide melt of variable compositions (FeO, MgO, Al2O3, CaO) are given; in region VII, in equilibrium with calcium oxide-based |CaO|s.s solid solution; in region VIII, in equilibrium with a gaseous phase consisted of {Mg, Ca} vapors; in region IX, in equilibrium with a gaseous {Mg, Ca, CO, CO2} phase with a predominance of magnesium and calcium vapors in the composition; in region X, in equilibrium with a gaseous {CO, CO2, Mg, Ca} phase with a predominance of carbon monoxide in the composition; and in region XI, in equilibrium with a gaseous variable-composition phase. Table 5 gives the calculated gas phase compositions (total pressure in the system was Ptot = 1.013 MPa).
The available data for the Fe–Mg–Al–Ca–C–O system are contradictory and restricted. In [34], experimental results on the determination of the phase compositions of the nonmetallic inclusions formed at [Mg] = 0.0005 wt % and [C] = 0 wt % are presented (points in Fig. 2a); according to these data, the metal contains spinel inclusions (with the predominance of MgAl2O4 in the solution composition), which agrees with the results of our simulation. On the other hand, according to the calculated data in [35], magnesium oxide cannot be in equilibrium with the liquid metal at such magnesium and carbon concentrations, but this fact does not correspond to both our calculation results and the conclusions in [34].
As follows from Figs. 1–4, an increase in the carbon concentration (other things being equal) leads to a decrease in the probability of forming liquid oxide nonmetallic inclusions.
At a magnesium concentration [Mg] = 0.0001 wt % and carbon concentration [C] = 0 and 0.1 wt % (Figs. 1a, 1b), the formation of the carbon monoxide gaseous phase is thermodynamically unlikely, and there is no region X. A similar regularity can also be observed at a calcium concentration [Ca] = 0.001 wt % (Figs. 3a, 3b). The region of the liquid metal compositions in equilibrium with a carbon monooxide-based gaseous phase disappears for all fixed carbon concentrations at a magnesium concentration of 0.0005 wt % in steel (Fig. 2) or an aluminum concentration of 0.01 wt % (Fig. 4). On the other hand, the results of the calculations based the equilibrium oxygen concentration in regions X and XI in Figs. 1c, 1d, 3c, and 3d are comparable with the data for the Fe–C–O system [36], according to which the equilibrium oxygen concentration in steel is 0.00625 wt % (log[O] = –2.20) at [C] = 0.4 wt % and [O] = 0.003125 wt % (log[O] = ‒2.51) at [C] = 0.8 wt %. According to our data, the equilibrium oxygen concentration in region X in Figs. 1c and 3c ([C] = 0.4 wt %) is 0.00617 wt % (log[O] = –2.21); at a carbon concentration of 0.8 wt % in regions X (Fig. 3d) and XI (Fig. 1d), the calculated equilibrium oxygen concentration is 0.003981 wt % (log[O] = –2.4).
Analyzing Figs. 1 and 2, we can note that an increase in the magnesium concentration in the steel, all other conditions being equal, decreases the possibility of participation of carbon in the deoxidation process. For example, at [Mg] = 0.0001 wt % and [C] = 0.8 wt % (Fig. 1d), condensed or liquid deoxidation products can form only from an aluminum concentration of 10–3 wt %; at a magnesium concentration of 0.0005 wt % and a carbon concentration of 0.8 wt % (Fig. 2d), the region of equilibrium with the carbon monoxide-based gaseous phase disappears, and gaseous phase IX (with the predominance of magnesium and calcium in the composition) is in equilibrium with liquid iron only at the maximum (close to the solubility limit) calcium concentrations.
An increase in the magnesium concentration in the liquid steel under the same conditions (Fig. 2) favors the formation of deoxidation products, such as unfavorable inclusions of oxide solid solutions (with the predominance of MgO in the composition) and spinel solid solutions [FeAl2O4, MgAl2O4]s.s with the predominance of magnesia spinel in the composition.
At the same time, if the calcium concentration in the liquid steel is 0.001 wt % (Fig. 3), then, according to the performed simulation, the formation of spinel solid solution inclusions is highly impossible thermodynamically, and the formation of particles of oxide solid solution |FeO, MgO|s.s is possible only at magnesium concentrations higher than 10–4 wt %. For all fixed carbon concentrations at a given calcium content in the liquid steel, the formation of unfavorable corundum inclusions is highly impossible; however, to form favorable calcium aluminate inclusions, aluminum concentrations higher than 10–3 wt % are necessary.
At an aluminum concentration [Al] = 0.01 wt % in the liquid steel (Fig. 4), the equilibrium region with a gaseous phase based on magnesium and calcium vapors is reduced to the solubility limits of Mg and Ca in liquid iron, and carbon is not participated in the deoxidation process. At this aluminum concentration and at the magnesium concentrations not higher than 10–4 wt %, calcium aluminate particles should form in the liquid metal as the calcium concentration increases (beginning from ~0.0001 wt % Ca).
CONCLUSIONS
We studied the phase equilibria establishing when aluminocalcium master alloys are introduced in the presence of magnesium. A thermodynamic simulation of the phase equilibria in the Fe–Mg–Al–Ca–C–O system at a temperature of 1600°C was performed for the carbon concentrations corresponding to low-, medium, and high-carbon steels. We determined the conditions of the formation of unfavorable nonmetallic inclusions, namely, oxide solid solutions with the predominance of MgO and spinel solid solutions with the predominance of MgAl2O4 in the composition, in this system. The regions of the phase equilibria in the liquid steel with favorable nonmetallic inclusions of calcium aluminates were also determined. For these inclusions to form, the aluminum concentration in the liquid metal should be about 0.01 wt %, the calcium concentration should be not less than 0.001 wt %, and the magnesium concentration should be controlled at a level of 0.0001 wt %. When the carbon concentration increases, the region of the liquid metal compositions in equilibrium with liquid oxide nonmetallic inclusions narrows and the oxygen concentration increases insignificantly due to a decrease in the activity coefficient (influence of the interaction parameters).
REFERENCES
N. A. Gokcen and J. Chipman, “Aluminum–oxygen equilibrium in liquid iron,” Trans. AIME 197, 173–178 (1953).
D. Janke and W. A. Fischer, “Desoxidationsgleichgewichte von titan, aluminum und zirconium in eisenschmelzen bei 1600°C,” Arch. Eisenhüttenwes 47 (4), 195–198 (1976).
M. K. Paek, J. M. Jang, Y. B. Kang, et al., “Aluminum deoxidation equilibria in liquid iron: part I. Experimental,” Metall. Mater. Trans. B. 46 (4), 1826–1836 (2015). https://doi.org/10.1007/s11663-015-0368-0
K. Mineura, I. Takahashi, and K. Tanaka, “Deoxidation and desulfurization of pressurized liquid high nitrogen stainless steels with calcium,” ISIJ Intern. 30 (3), 192–198 (1990). https://doi.org/10.2355/isijinternational.30.192
K. Taguchi, H. Ono-Nakazato, D. Nakai, et al., “Deoxidation and desulfurization equilibria of liquid iron by calcium,” ISIJ Intern. 43 (11), 1705–1709 (2003). https://doi.org/10.2355/isijinternational.43.1705
H. Fujiwara, M. Tano, K. Yamamoto, et al., “Solubility and activity of calcium in molten iron in equilibrium with lime and thermodynamics of calcium containing iron melts.” ISIJ Intern. 35 (9), 1063–1071 (1995). https://doi.org/10.2355/isijinternational.35.1063
M. Imagumbai and T. Takeda, “Influence of calcium treatment on sulfide- and oxide-inclusions in continuous-cast slab of clean steel—dendrite structure and inclusions,” ISIJ Intern. 34 (7), 574–583 (1994). https://doi.org/10.2355/isijinternational.34.574
K. Taguchi, H. Ono-Nakazato, T. Usui, et al., “Complex deoxidation equilibria of molten iron by aluminum and calcium, ISIJ Intern. 45 (11), 1572–1576 (2005). https://doi.org/10.2355/isijinternational.45.1572
Y. Higuchi, M. Numata, S. Fukagawa, et al., “Inclusion modification by calcium treatment,” ISIJ Intern. 36 (S), S151–S154 (1996). https://doi.org/10.2355/isijinternational.36.Suppl_S151
G. M. Faulring and S. Ramalingam, “Inclusion precipitation diagram for the Fe–O–Ca–Al system,” Metall. Trans. B. 11 (1), 125–130 (1980). https://doi.org/10.1007/BF02657.181
E. Kh. Shakhpazov, A. I. Zaitsev, N. G. Shaposhnikov, I. G. Rodionova, and N. A. Rybkin, “Physicochemical prediction of the types of nonmetallic inclusions. Complex deoxidation of steel with aluminum and calcium,” Russ. Metall. (Metally), No. 2, 99–107 (2006).
A. B. Akhmetov, G. D. Kusainova, A. A. Kuszhanova, et al., “Effect of calcium modification on the Hadfield steel structure and the morphology of nonmetallic inclusions formed in it,” Elektrometallurgiya, No. 3, 8–12 (2017).
V. I. Zhalybin and G. S. Ershov, “On recovery of liner magnesium during smelting aluminum-alloyed steel,” Izv. Akad. Nauk SSSR, Ser. Met., No. 1, 49–53 (1966).
V. I. Zhuchkov, S. V. Lukin, and I. V. Shilina, “Deoxidation of steel with calcium–magnesium–silicon ferroalloys,” Izv. Vyssh. Uchebn. Zaved., Chern. Met., No. 12, 69–71 (1977).
G. G. Mikhailov, B. I. Leonovich, and Yu. S. Kuznetsov, Thermodynamics of Metallurgical Processes and Systems (MISiS, Moscow, 2009).
Y. Du, J. R. Zhao, C. Zhang, et al., “Thermodynamic simulation in the Fe–Mg–Si System,” J. Min. Metall. Sect. B. 43 (1), 39–56 (2007). https://doi.org/10.2298/JMMBo701039D
M. Berg, J. Lee, and D. Sichen, “Study on the equilibrium between liquid iron and calcium vapor,” Metall. Mater. Trans. B. 48 (3), 1715–1720 (2017). https://doi.org/10.1007/s11663-017-0946-4
O. Kubaschewski and C. B. Alcock, Metallurgical Thermochemistry (Pergamon, Oxford, 1979).
H. A. Wriedt, “The Ca–O (calcium–oxygen) system,” Bull. Alloy Phase Diagr. 6 (4), 337–342 (1985). https://doi.org/10.1007/BF02880517
P. Wu, G. Eriksson, A. D. Pelton, et al., “Prediction of the thermodynamic properties and phase diagrams of silicate systems: evaluation of the FeO–MgO–SiO2 system,” ISIJ Intern. 33 (1), 26–35 (1993). https://doi.org/10.2355/isijinternational.33.26
Slag Atlas, Ed. by V. D. Eisenhüttenleute (Stahleisen, Düsseldorf, 1995).
A. Ono, “Fe–Mg partitioning between spinel and olivine,” J. Japan Assoc. Min. Petr. Econ. Geol. 78, 115–122 (1983).
G. G. Mikhailov, L. A. Makrovets, and L. A. Smirnov, “Thermodynamic simulation of the interaction processes of lanthanum with components of iron-base metallic melts,” Izv. Vyssh. Uchebn. Zaved., Chern. Met. 58 (12), 877–883 (2015).
G. G. Mikhailov and D. A. Zherebtsov, “On the interaction of calcium and oxygen in liquid iron,” Mater. Sci. Forum 843, 52–61 (2016). doi: 10.4028/www.scientific.net/MSF.843.52
T. Fuwa and J. Chipman, “The carbon–oxygen equilibria in liquid iron,” Trans. AIME 218, 887–891 (1960).
N. Satoh, T. Taniguchi, S. Mishima, et al., “Prediction of nonmetallic inclusion formation in Fe–40 mass % Ni–5 mass % Cr alloy production process,” Tetsu-to-Hagané 95 (12), 827–836 (2009).
J. H. Park and H. Todoroki, “Control of MgO ⋅ Al2O3 spinel inclusions in stainless steels,” ISIJ Intern. 50 (10), 1333–1346 (2010). https://doi.org/10.2355/isijinternational.50.1333
H. Itoh, M. Hino, and S. Ban-Ya, “Thermodynamics on the formation of non-metallic inclusion of spinel (MgO ⋅ Al2O3) in liquid steel,” Tetsu-to-Hagané 84 (2), 85–90 (1998).
Steelmaking Data Sourcebook. Japan Society for the Promotion of Science. The 19th Committee on Steelmaking (Gordon & Breach, New York, 1988).
L. J. Wang, Y. Q. Liu, Q. Wang, et al., “Evolution mechanisms of MgO ⋅ Al2O3 inclusions by cerium in spring steel used in fasteners of high-speed railway,” ISIJ Intern. 55 (5), 970–975 (2015).
H. Prox, M. Hino, and S. Ban-Ya, “Assessment of Al deoxidation equilibrium in liquid iron.” Tetsu-to-Hagané 83 (12), 773–778 (1997).
G. K. Sigworth and J. f. Elliott, “The thermodynamics of liquid dilute iron alloys,” Metal Science 8, 298–310 (1974).
Yu. V. Balkovoi, P. A. Aleev, and V. K. Bakanov, First-Order Interaction Parameters in Iron-Based Melts: Review (Chermetinformatsiya, Moscow, 1987).
T. Kimura and H. Suito, “Calculation deoxidation equilibrium in liquid iron,” Metall. Mater. Trans. B. 25 (1), 33–42 (1994). https://doi.org/10.1007/BF02663176
T. Zhang, Y. Min, C. Liu, et al., “Effect of Mg addition on the evolution of inclusions in Al–Ca deoxidized melts,” ISIJ Intern. 55 (8), 1541–1548 (2015). https://doi.org/10.2355/isijinternational.ISIJINT-2014-691
V. I. Yavoiskii, Theory of Steelmaking Processes (Metallurgiya, Moscow, 1967).
Funding
This work was supported by the Government of the Russian Federation (decision no. 211 of March 16, 2013), agreement no. 02.A03.21.0011.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated by Yu. Ryzhkov
Rights and permissions
About this article
Cite this article
Mikhailov, G.G., Makrovetz, L.A., Samoilova, O.V. et al. Phase Equilibria in the Liquid Steel Deoxidized with Aluminum and Calcium in the Presence of Magnesium. Russ. Metall. 2020, 640–648 (2020). https://doi.org/10.1134/S0036029520060130
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0036029520060130