Abstract
The paper presents the results of the investigation of elemental and phase composition of zinc-containing electric arc furnace dust, the product formed by the dust calcination with lime, and the residue formed by alkaline leaching of the calcined product. The phase distribution in samples is studied by X-ray diffraction analysis, Mössbauer spectroscopy, and scanning electron microscopy. It is shown that electric arc furnace dust calcination with lime followed by alkaline leaching the calcined product can be used to recycle the dust with recovery of valuable components.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
INTRODUCTION
Dust of electric arc furnaces (EAF) of steelmaking production is a valuable technical product with a high content of zinc and iron, but its recycling is still neglected in Russia. This dust is generally shipped to dumps as a waste, which leads to environmental deterioration in the regions where the dust landfills are located. Thus, the problem of processing of the generated and accumulated dust becomes more and more important from year to year.
Over the past few decades, many technologies for processing the EAF dust have been developed [1], but their industrial application is hampered by technical difficulties and a low economic efficiency. The most popular industrial method of EAF dust recycling in the world is the Waelz process [2]. This method is based on distillation recovery of zinc and lead in a rotary kiln (Waelz kiln) by heating a charge with carbonaceous reducing agent. The product collected in the off-gas cleaning system with high contents of zinc and lead, which is called Waelz oxide, and the residual product is called Waelz slag. The formed Waelz oxide is a valuable material for the production of zinc and lead. The iron-containing Waelz slag can be used in the sinter production in ferrous metallurgy.
Based on the Waelz process, the technology [3, 4] of processing the EAF dust was developed and applied in Russia. The technology has the following two stages: (i) Waelz process I is the volatilizing of zinc, lead, and halides in a Waelz kiln with carbon addition; (ii) Waelz process II is the purification of Waelz oxide from halides and lead and the removal and oxidation of the impurities that adversely affect the subsequent hydrometallurgical stages of zinc production.
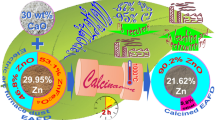
The present work looks into the possibility of the separation of zinc and lead and the removal of halides, which are harmful for the further hydrometallurgical extraction of zinc by a single-stage process. For this purpose, we propose to use the calcination of the EAF dust with lime during which the zinc-containing compounds (ferrites) that are present in the dust can transform into a highly soluble oxide form, and lead and halides can volatilize. The results of [5, 6] confirm that calcium oxide facilitates the transition of zinc ferrite to zinc oxide. After leaching the calcine, a solution for the electrolytic recovery of zinc can be formed, and the insoluble residue containing calcium and iron can be used in ferrous metallurgy.
The EAF dust and its processing products are multicomponent and complex in chemical and phase composition materials. To investigate the physicochemical basis of the proposed method of processing the EAF dust, the elemental and phase compositions of the initial and obtained products were studied. Samples were obtained in laboratory experiments by calcination of the EAF dust with lime and leaching the calcine.
EXPERIMENTAL
The calcined product was formed by the calcination of the EAF dust with 60% lime in a laboratory furnace at 1000°C for 4 hours. The amount of lime was set according to preliminary calculation to decompose more than 95% of zinc ferrite. The leaching residue was obtained by leaching the calcine in an NaOH solution.
The elemental composition of the dust, calcine, and leaching residue samples was studied by atomic emission, atomic absorption, and X-ray fluorescent spectroscopy using Jobin-Yvon Ultima 2, Therm Fisher Scientific iCE3500 and ARL QUANT’X devices, respectively. The sulfur and carbon contents were determined by a LECO CS-400 device. The phase composition of the samples was studied using an ARL X’TRA diffractometer and CuKα radiation. An analysis of iron-containing phases was carried out on an Ms‑1104Em Mössbauer spectrometer in the constant acceleration mode with a Co-57 source in an Rh matrix. After the proper preparation of the samples and carbon sputtering, the microstructure and phases of the samples were investigated by scanning electron microscopy (SEM) on a Zeiss EVO LS10 device with X-ray energy dispersive microanalysis in the backscattered electron mode.
RESULTS AND DISCUSSION
Table 1 gives the results of elemental analysis of the samples. As it follows from the elemental composition, almost all zinc passed into a solution after leaching, but 0.79% of zinc remains in the leaching residue. During the calcination of the dust with lime, the most part of lead passes into fumes. Сhlorine remains in the calcine in a trace amount.
Figure 1 shows the X-ray diffraction patterns of the dust, calcine, and leaching residue. Table 2 presents the Mössbauer spectra parameters of the samples. The results of these studies point out that zinc in the EAF dust is both in the form of ferrite and oxide, and zinc in the form of ZnFe2O4 is more than in ZnO. In addition, the EAF dust contains magnetite, calcite, graphite, sodium, and potassium chlorides. Unlike the EAF dust sample, the formed calcine contains more zinc in the form of ZnO than in ZnFe2O4. Mössbauer spectroscopy of the iron-containing phases shows that 66% of iron is in the form of ZnFe2O4 in the dust sample and only 19% in the calcine sample. The main phase of calcine is dicalcium ferrite. This finding indicates that, during the calcination, there is a transition of zinc from ferrite to oxide form according to the general reaction
According to X-ray diffraciton analysis, a small amount of zinc remains in the leaching residue is in the form of ferrite. Dicalcium ferrite Ca2Fe2O5 transforms into the hydrated calcium ferrite Ca3Fe2(OH)12 in leaching. In the doublet with wide lines of the Mössbauer spectrum of the leaching residue, it is impossible to quantitatively calculate the iron distribution ratio between Ca3Fe2(OH)12 and ZnFe2O4.
Figure 2 shows SEM microphotographs of the EAF dust sample. Table 3 gives the compositions of the particles shown in Fig. 2. As the microphotographs show, the EAF dust sample contains conglomerates of submicron particles and a great number of slag particles with a wide range of compositions. In addition, large particles of magnetite, wustite, and graphite are detected. The submicron particles collected in conglomerates substantially consist of zinc and iron distillates oxidized in the off-gas cleaning system. Zinc both in ferrite and in oxide forms is mainly as submicron constituents. Zinc oxide particles of 1–3 μm in size are also detected (Table 3, no. 9).
The calcination of the EAF dust with lime at 1000°C leads to a significant increase in the particle size in the obtained calcine sample. Except for calcium silicates and alumosilicates with various compositions, the extensive regions of Ca2Fe2O5 (Table 3; nos. 11, 13) that surround small ZnFe2O4 sections (Table 3, nos. 10, 14) are present in the microphotographs of the calcine sample. Numerous particles of zinc oxide few microns in size are also detected (Table 3, no. 16). The relative positions of unreacted zinc ferrite and obtained dicalcium ferrite are characteristic of a solid-phase interaction, where the layer contact between raw materials is broken after the formation of a continuous product, which leads to incomplete reaction. Therefore, it is necessary to thoroughly mix the reagents for more complete reaction (1) and increasing its rate. In addition, reaction (1) can proceed more fully with an increase of the quantity of lime.
The leaching residue sample contains many submicron conglomerated particles based on Ca3Fe2(OH)12 of various sizes. Dicalcium ferrite, quartz, and other phases are present in the form of singular grains up to 100 μm in size. The remaining zinc ferrite is detected both in submicron conglomerates and in particles up to 15 μm in size (Table 3; nos. 23, 24).
CONCLUSIONS
During the calcination of the EAF dust with lime, most zinc was found to pass from ferrite to soluble zinc oxide. The most part of lead passes into fumes and the chlorine is removed. The zinc content in the residue after the alkaline leaching of the calcined product is less than 1%.
Thus, the calcination of the EAF dust with lime and the subsequent alkaline leaching can be used to recycle the EAF dust to produce intermediate products for the production of zinc and lead and an iron-containing material for ferrous metallurgy.
REFERENCES
I. F. Kurunov, “Environmental aspects of industrial technologies for recycling sludge and dust that contain iron and zinc,” Metallurgist 55 (9–10), 634–639 (2012).
A. M. Pan’shin, L. I. Leont’ev, P. A. Kozlov, et al., “Technology of processing the electric arc furnace dust in OAO Severstal’ in the Waelz plant of OAO ChPZ,” Ekolog. Prom. Rossii, No. 11, 4–6 (2012).
P. A. Kozlov, The Waelz Process (Ore and Metals Publishing House, Moscow, 2003).
P. A. Kozlov, “Implementation of the recycling of the technical wastes of metallurgy production,” Tsvetn. Met., No. 2, 45–52 (2014).
A. B. Peltekov and B. S. Boyanov, “Study of solid state interactions in the systems ZnFe2O4–CaO, ZnFe2O4–MgO and zinc cake with CaO and MgO,” J. Min. Metall. B 49 (3), 339–346 (2013).
R. Dimitrov and B. Boyanov, “Investigation of solid state interactions in the systems ZnO–α-Fe2O3, ZnFe2O4–CuO, ZnFe2O4–CaO,” Rud.-Metal. Zb. 31 (1), 67–80 (1984).
ACKNOWLEDGMENTS
The work was carried out according to state assignment no. 007-00129-18-00.
Author information
Authors and Affiliations
Corresponding author
Additional information
The article was translated by the authors.
Rights and permissions
About this article
Cite this article
Yakornov, S.A., Panshin, A.M., Grudinsky, P.I. et al. Method of Electric Arc Furnace Dust Processing by Calcination with Lime with Following Alkaline Leaching. Russ. Metall. 2018, 1282–1287 (2018). https://doi.org/10.1134/S0036029518130268
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0036029518130268